- LOGIN
- MemberShip
- 2026-03-09 20:47:41
- Product
- Pharmacies express discontent over Xigduo distribution
- by Kang, Hye-Kyung Feb 05, 2026 07:48am
- ChatGPT-generated image.As the distribution of a widely prescribed pharmaceutical product has shifted from a manufacturer-led model to a wholesaler-led model, supply blind spots have emerged, leaving pharmacies that do not have a direct contract with the wholesaler unable to secure the drug.Pharmacies claim they are effectively forced to enter direct trading agreements with the specific distributors to receive products, facing unprecedented inconvenience.The product in question is AstraZeneca's diabetes drug, Xigduo XR (metformin hydrochloride, dapagliflozin propanediol monohydrate).Until last year, HK Inno.N was in charge of its sales and supply. However, as of January 1 this year, sales and supply were transferred to AstraZeneca Korea. In the process, a large domestic wholesaler (Wholesaler A) secured exclusive pharmacy distribution rights for the product.The problem is that since Company A secured the pharmacy distribution rights, supply to other wholesalers became impossible. In other words, pharmacies can only receive the drug if they have a direct trading relationship with Wholesaler A.One pharmacist said, “None of our four primary wholesalers can source the product from Wholesaler A, and it shows as out of stock across online wholesale platforms. For pharmacies like ours that do not have a direct trading relationship with Wholesaler A, securing inventory is impossible. It is unfair for a single wholesaler to effectively monopolize the sale and supply of a prescription drug.The pharmacist added that the product should at least be made available to other wholesalers through secondary wholesale channels.Another pharmacist noted, “When we checked why Xigduo XR was unavailable, we found that it could only be purchased through Wholesaler A. According to the sales representative, distribution to other wholesalers will only take place after distribution rights and inventory are fully reorganized.”In response, Wholesaler A stated that the policy is unavoidable given current supply conditions, citing limited inventory as a key constraint.A representative from Company A stated, “Due to limited market inventory, we are prioritizing supply to our contracted pharmacies. Secondary wholesale distribution is physically not feasible at this time.”The official added, “We currently have trading relationships with approximately 80% of pharmacies nationwide. Pharmacies with direct trade agreements can secure inventory as long as they meet the minimum order requirement.”However, this leaves around 20% of pharmacies facing significant hurdles, as initiating a new trading relationship requires meeting an initial minimum order threshold.Critics point out that pharmacies with low order volumes or long-standing relationships with other primary wholesalers are unlikely to open a new account solely to secure Xigduo XR—particularly when doing so may require purchasing several million wons worth of products upfront to meet initial order requirements.
- Company
- Kwangdong and MSD form pneumococcal vaccine alliance
- by Hwang, byoung woo Feb 05, 2026 07:48am
- The vaccine alliance between Kwangdong Pharmaceutical and MSD Korea is expanding into the adult pneumococcal market. Beyond their HPV vaccine collaboration, the partnership now includes the adult-specific 21-valent pneumococcal vaccine, marking an evolution in the companies' preventive portfolio partnership.Kwangdong Pharmaceutical is shifting its business focus toward prescription pharmaceuticals and vaccines by extending its existing vaccine sales infrastructure into the adult preventive care domain.On the 4th, Kwangdong Pharmaceutical announced that it had entered into a domestic co-promotion agreement with MSD Korea for Capvaxive, an adult-only 21-valent pneumococcal protein-conjugated vaccine. The two companies will jointly handle domestic marketing and distribution of Capvaxive starting from its launch in the first quarter of 2026.This collaboration builds on the partnership established through the co-promotion of the HPV vaccines Gardasil and Gardasil 9, which the two companies have jointly marketed and distributed in Korea since 2024.Given their existing cooperative relationship, the combination of Kwangdong’s sales infrastructure and MSD’s global vaccine portfolio is expected to generate significant synergies in the adult prevention market.Adult-only 21-valent design… broader serotype coverage expands protectionCapvaxive is a newly designed vaccine aimed at preventing invasive pneumococcal disease (IPD) and pneumococcal pneumonia in adults. It reflects the latest epidemiological characteristics of adult pneumococcal disease to address unmet needs.It prominently features the addition of eight unique serotypes—15A, 15C (deOAc15B), 16F, 23A, 23B, 24F, 31, and 35B—providing the broadest serotype coverage among currently approved pneumococcal protein-conjugated vaccines in Korea.According to the Ministry of Food and Drug Safety (MFDS) approval, Capvaxive is indicated for the prevention of invasive disease and pneumococcal pneumonia caused by pneumococcal serotypes (3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15B, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F, and 35B) in adults aged 18 years and older.Having accumulated over 25 years of experience supplying pneumococcal vaccines to adults in Korea, MSD Korea plans to fully implement adult-tailored prevention strategies through this partnership.The company stated that it will work with Kwangdong Pharmaceutical to ensure that more adults benefit from expanded serotype-based protection.The current direct competitor is Pfizer's 20-valent pneumococcal protein-conjugated vaccine, Prevnar 20.With Chong Kun Dang handling domestic sales for Prevnar 20 for adults, marketing competition between the co-promotion partners is expected to intensify.For Kwangdong Pharmaceutical, the agreement represents more than a simple product addition, as it represents part of its broader effort to build experience in the vaccine business.Having already established preventive vaccine sales and marketing systems through the co-promotion of Gardasil and Gardasil 9, the company is now positioned to extend that infrastructure to adult pneumococcal vaccines.Sung-won Choi, CEO of Kwangdong Pharmaceutical, said, “This collaboration will further strengthen our vaccine portfolio and establish a foundation to address broader preventive healthcare needs. Leveraging our differentiated sales infrastructure and expertise, we will support the successful market entry of Capvaxive and continue to enhance our competitiveness in the domestic vaccine market.”Industry observers view this collaboration as an extension of Kwangdong Pharmaceutical’s ongoing strategic shift.While maintaining stable cash flows from its beverage and OTC businesses, the company is increasingly expanding its portfolio into high-value-added areas such as prescription drugs and vaccines.According to the recently disclosed quarterly report (cumulative for Q3 2025), Kwang Dong Pharmaceutical's sales structure is gradually shifting toward a pharmaceutical-centric portfolio.Reviewing sales and order status, the combined sales of prescription drugs (ETC) and vaccines reached KRW 140.5 billion. This represents approximately 18.3% of the company’s total revenue (KRW 768.5 billion).
- InterView
- [Reporter's View] Medical AI must read 'NULL' values
- by Hwang, byoung woo Feb 05, 2026 07:48am
- When we discuss medical AI, we often think of lines of data, more detailed records, and more meticulous numbers. It is widely believed that a data set with complete electronic medical records and no missing values is a shortcut to improving performance.However, the medical field is different. In medical data, 'NULL' values are not simply omitted during data processing. It is rather an outcome of a clinical decision made by doctors who observe patients and then state 'the testing is no longer needed.When a patient is stable, it is common practice in the field to avoid frequent blood draws and imaging. In other words, unrecorded silence itself is the most powerful data proving the patient's stable condition.It means that the data's nature is not limited to recorded values but also includes unrecorded contexts.An issue often arises when a NULL space is mechanically filled. AI loses context when it adjusts a NULL value to man-made numbers to improve data analysis efficiency.It falls into the trap of Domain Shift, a phenomenon where the distribution of the training environment and the actual clinical field diverges. While it is a technical choice to create a clean table, the context of medical data is compromised in the process.In fact, research results showing that AI's prediction accuracy drops by nearly 10% when data is forcibly adjusted while ignoring clinical prescription patterns are highly suggestive.This is because the AI model experiences a form of "cognitive dissonance" when encountering numbers forcibly inserted where NULL should be. Ultimately, this results in erasing the subtle signals sent by medical staff to save patients.At this point, the perspective on Medical AI must change as well. A good Medical AI is not a model that uses complex algorithms, but one that reflects the field's decision-making structure without distortion. The sense of understanding the flow of the medical system is becoming more important than the technology of matching numbers.Now, as the government discusses the early introduction and expansion of Medical AI, the question of "what philosophy governs the data?" becomes as important as "how much data was collected?" Filling in NULL is not absolute. Sometimes, respecting the blank itself is the way to preserve the performance of Medical AI.It is now time to consider whether we are erasing the silence left by medical staff to save patients in the name of data refinement. What Medical AI needs now is not the technology to fill in numbers, but the insight to hear the voices of medical staff contained within those NULL values.
- Company
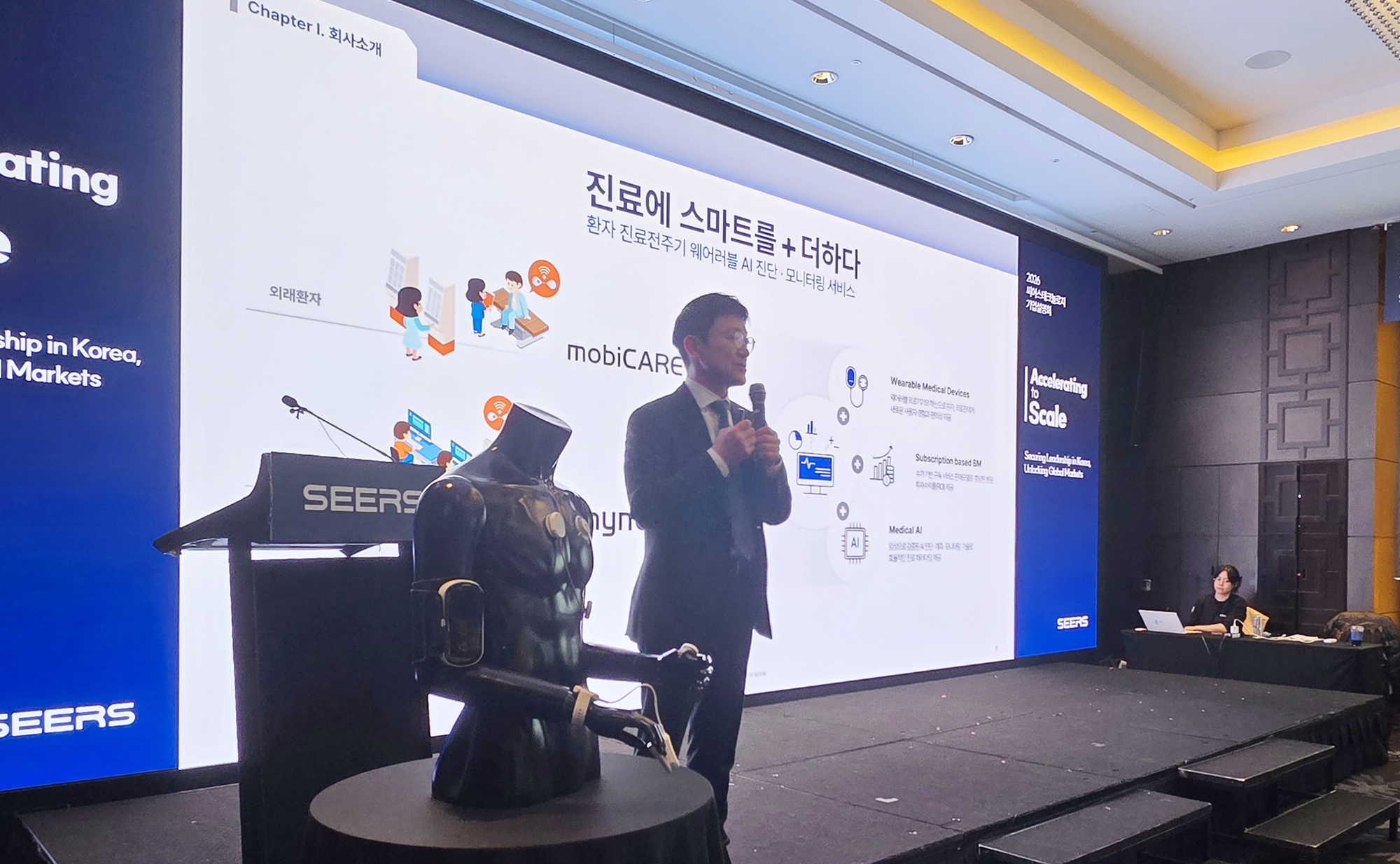
- Sears turns profit, seeks expansion from Middle East
- by Hwang, byoung woo Feb 05, 2026 07:47am
- Seers Technology, having solved the long-standing profitability challenge in Korea’s medical AI industry, has now proposed building ‘global smart wards’ as its next step.Leveraging its integrated healthcare model that starts from inpatient monitoring and extends to outpatient and home care, Seers plans to accelerate its global market expansion from the Middle East.At an investor relations (IR) event held in Yeouido on the 4th, Seers unveiled its achievement of turning an annual profit in 2025 and its blueprint for a quantum leap as a healthcare platform company.“Workflow improvements felt by HCPs, the key to profitability”Young-shin Lee, Founder & CEO of Seers TechnologyAt the event, CEO Young-shin Lee cited “hospital workflow optimization” as the core driver behind the company’s return to profitability.Lee said, “Hospitals are not just places for treatment, but spaces where patients are continuously monitored and managed. To extend real-time monitoring beyond intensive care units to general wards, we focused on workflow improvements that reduce medical staff's workload.”Seers’ inpatient monitoring platform, thynC, continuously collects and analyzes vital signs from all patients across the entire hospital wards.Once installed at the bedside, the platform is combined with a subscription-based service, which the company believes will create a virtuous cycle of recurring revenue from existing beds.Lee explained, “thynC is not a one-time installation; its structure builds recurring revenue over time from existing beds. From 2028, new deployments and renewals will overlap, driving full-scale profit leverage.”Seers reported 2025 revenue of KRW 48.2 billion, a 595% increase from KRW 8.1 billion in 2024. Operating profit also turned positive, swinging from a KRW 8.7 billion loss to a KRW 16.3 billion profit.By 2028, which is when Lee believes the profit leverage is expected to fully materialize, the company projects the number of hospitals using its platform to nearly double to around 400.The company views this structure as closer to an operational platform than simple equipment sales. It is a model that enhances hospital operational efficiency itself by linking patient biometric data with nursing records, electronic medical records (EMRs), and ward workflows.Middle East as a “model transplantation hub,” not just a sales market… medical fees 3–5 times higherA major focus of the briefing was Seers’ overseas strategy, particularly in the Middle East and North Africa(MENA).Seers has positioned the UAE as a strategic foothold for its global expansion and is collaborating with PureHealth, the largest state-owned healthcare group in the Middle East.Lee noted, “If the reimbursement for a one-day arrhythmia test in Korea is around KRW 60,000, in the Middle East it is three to five times higher. The scale of inpatient beds in the MENA region is also larger than in Korea, and the number of chronic disease patients is significantly higher, resulting in a fundamentally different revenue structure.”Seers’ approach is not simply product supply. The company plans to leverage PureHealth’s hospital network, insurance, and distribution infrastructure to deploy an integrated wearable AI healthcare model within local systems. This model encompasses ▲arrhythmia screening based on mobiCARE, ▲inpatient monitoring via thynC, and ▲remote patient monitoring (RPM) for home care. Proof-of-concept (PoC) programs for the full product portfolio are currently underway in stages.Lee emphasized, “Selling a few pieces of equipment overseas is unlikely to yield meaningful results. Our goal is to replicate the ‘hospital-outpatient-home care integrated model that has been validated in Korea.The company has set a target to raise the proportion of overseas sales to around 50% by around 2029 through this phased expansion strategy.Lee particularly conveyed the company's determination to penetrate the market based on technological specialization, noting that global giants like Philips and GE Healthcare already dominate the international arena.He stressed, “The Middle East market is a battleground where all global leaders compete. Seers distinguishes itself by internalizing the entire process—from materials to sensors and AI algorithms. We will secure references by winning against them to enter the US and European markets.”Additionally, Seers is pursuing a collaborative model linking real-time patient data accumulated via wearable devices to the pharmaceutical industry. The vision is to combine medication information with biometric data to expand into the realm of clinical and real-world data (RWD) applications.Lee concluded, “By achieving a 50% overseas revenue share by 2029, we aim to become a global wearable AI healthcare platform company. Our first-ever profitability milestone is only the starting line. We will continue to build a sustainable revenue structure while completing our global expansion.”
- Opinion
- [Reporter's View] Is reimb criteria restricting patient access?
- by Son, Hyung Min Feb 05, 2026 07:47am
- The same question is asked whenever the reimbursement criteria for a new drug are unveiled. "Is regulation aligning with the disease treatment flow?"Concerns are mounting about the reimbursement criteria for Ozempic, a GLP-1-based diabetes drug. Despite the goal of its criteria to suppress prescriptions for obesity, the criteria may narrow treatment access.Professionals in clinical practice are continually raising issues. They assert that the government's goal in reducing unregulated prescriptions for obesity treatments can be acknowledged. Yet if this goal is applied too broadly, it could undermine effective diabetes treatments.The government limited Ozempic reimbursement recipients by considering factors such as body mass index (BMI), blood sugar levels, and prior medication history. The problem is that the criteria were designed to focus on reducing the risk of abuse rather than reflecting patient condition variables.According to the reimbursement criteria for Ozempic, the drug can be prescribed as part of a triple therapy including metformin and sulfonylurea (SU), or in combination with insulin to Type 2 diabetes patients whose glycated hemoglobin (HbA1C) levels remain at 7% or higher despite at least 2 to 4 months of combination drug therapy. Following the initial treatment, when patients experience substantial improvement in blood sugar levels, their treatment scheme can be switched to a dual combination therapy (metformin + Ozempic).Additionally, reimbursement is applied to the combination therapy of Ozempic plus basal insulin (±metformin) if the glycated hemoglobin (HbA1c) level remains at 7% or higher even after administering basal insulin monotherapy or metformin combination for more than 2-4 months, or if the HbA1c remains at 7% or higher following the combination therapy of Ozempic and metformin (±SU).In current clinical practice, combination therapies centered on DPP-4 inhibitors and SGLT-2 inhibitors are widely used, and there is a trend toward increasingly excluding SU due to the risk of hypoglycemia and specific patient characteristics.Despite this, requiring patients to return to sulfonylureas and essentially forcing a "treatment failure" to meet reimbursement requirements feels less like a typical clinical pathway and more like a regulatory mandate that mandates failure.Another concern is that even non-reimbursement prescriptions are not permitted for patients who fail to meet the reimbursement criteria. While the intent is to block use for obesity purposes, the result is the elimination of even the minimum leeway to adjust treatment strategies based on individual patient characteristics. This has resulted in backlash, with accusations that the government has arbitrarily and preemptively defined the therapeutic needs of patients.A bigger problem is the high probability that these criteria will be applied identically to other GLP-1 class diabetes treatments to be released in the future. If the structure of the criteria is finalized, with even innovative new drugs having their reimbursement status judged through a single frame of BMI, blood sugar, and prior treatment conditions, the domestic reimbursement policy will inevitably tilt further toward regulation. This serves as a warning that South Korea's clinical practices may continue to diverge from international treatment guidelines.In the case of migraine treatments, international and domestic guidelines recommend calcitonin gene-related peptide (CGRP) class agents as first-line medications.However, because Korean insurance criteria are excessively stringent, there are cases where patients must wait until their pain worsens or repeat multiple medication failures to meet reimbursement requirements. Consequently, new drugs that were granted reimbursement to increase treatment accessibility have become even more out of reach for patients.Everyone acknowledges that institutional mechanisms to prevent misuse and abuse are necessary. However, a structure in which regulations are created first without clear standards ultimately result in uncertainty and disadvantages patients.If the purpose of regulation is to aid patient treatment, what is needed now is not simple control, but a sophisticated design that places the patient at the center.
- Policy
- Will the NA pass the 'mandatory INN prescription' bill?
- by Lee, Jeong-Hwan Feb 04, 2026 06:51am
- While the simplified post-notification system for substitution drugs through the government's electronic network was implemented on February 2, the pharmaceutical industry is increasingly focusing on the "limited International Nonproprietary Names (INN) prescription" bills currently pending in the National Assembly.Members of the ruling party on the Health and Welfare Committee have expressed a consensus on the need to accelerate the review of these bills, noting that the Lee Jae Myung administration has chosen mandatory INN prescription for national essential medicines as a national task to resolve supply instabilities.As of February 3, three bills related to limited INN prescription, introduced by Rep. Kim Yoon and Rep. Jang Jong-tae of the Democratic Party, are pending in the National Assembly.Rep. Kim Yoon's Pharmaceutical Affairs Act Bill proposes a legal definition for supply-unstable drugs and allows the Minister of Health and Welfare to recommend the use of INN for national essential and supply-unstable drugs.This method is considered less coercive as it does not mandate health professionals to use INN prescription during patient care and prescription.Rep. Jang Jong-tae's Medical Service Act & Pharmaceutical Affairs Act Bill requires the MOHW to designate supply-unstable drugs after deliberation by a Supply Management Committee. It mandates physicians to use INN instead of brand names when prescribing these designated drugs.Notably, Rep. Jang's proposal is considered highly coercive because it includes a penalty of up to one year in prison or a fine of up to KRW 10 million for physicians who violate the INN mandate.Some ruling party members on the Health and Welfare Committee state that if a legislative subcommittee is held this month, these bills should be officially adopted for review. They argue there is no reason to delay, especially given President Lee's recent criticisms regarding the slow pace of legislation.Rep. Kim's bill has been pending for over a year since its introduction in December 2024.The pharmaceutical industry is showing significant attention as the MOHW and the Health Insurance Review and Assessment Service (HIRA) began the implementation of the simplified post-notification system for substitution drugs, formulations, and dosages on February 2.An official from the Health and Welfare Committee stated, "The ruling and opposition party mangement groups are currently coordinating the schedule for the February legislative subcommittee," and added, "If the schedule is confirmed, there is a high possibility that the INN prescription bills will be placed on the agenda, as they are drawing significant attention from both the proposed offices and the committee members."
- Policy
- Merck’s mirdametinib drug receives GIFT designation
- by Lee, Tak-Sun Feb 04, 2026 06:51am
- Gomekli (mirdametinib) was approved by the US FDA in February last yearMerck’s neurofibromatosis treatment candidate mirdametinib has been designated for the Ministry of Food and Drug Safety’s (MFDS) expedited review support program, GIFT (Global Innovative products on Fast Track).As a result, the drug’s entry into the Korean market is expected to accelerate.According to the MFDS, mirdametinib was designated as a GIFT product on the 20th of last month.GIFT is an expedited review program launched in September 2022 to support rapid product development and provide patients with faster access to new treatment options.The program targets innovative medicines, including those for life-threatening diseases, rare diseases with no existing treatment alternatives, and new drugs developed by certified innovative pharmaceutical companies.The MFDS designates GIFT products based on a comprehensive evaluation of factors such as innovative therapeutic effects, contribution to addressing public health needs, and the developer’s efforts.Once designated, the review period is reduced by at least 25% (from 120 working days to 90 working days).The program also applies rolling reviews, under which submitted data are reviewed on an ongoing basis, and provides close communication between regulators and developers through product briefings and supplementary meetings. In addition, regulatory consulting and other support measures are offered to facilitate rapid commercialization.Mirdametinib is Korea’s 64th GIFT product. The proposed indication submitted to the MFDS is for the treatment of ‘pediatric and adult patients aged 2 years and older with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas.’Mirdametinib is a selective inhibitor of mitogen-activated protein kinase kinases 1 and 2 (MEK1/2). It exerts antitumor activity by blocking MEK activity, thereby inhibiting the phosphorylation of Extracellular-regulated kinase 1 and 2 (ERK1 and ERK2) within the Mitogen-activated protein (MAP) pathway.It received Fast Track designation from the US FDA on February 11 last year and was approved by the EMA on July 17 last year. In Korea, it was designated as an orphan drug on December 11 last year.Mirdametinib was originally developed by the U.S. biotech company SpringWorks Therapeutics. In April 2023, SpringWorks was acquired by Germany-based Merck for USD 3.9 billion, giving Merck global commercial rights to the drug.Once launched, mirdametinib is expected to compete with the existing neurofibromatosis therapy Koselugo (selumetinib, AstraZeneca).Koselugo, which is also indicated for neurofibromatosis type 1, has been reimbursed by Korea’s national health insurance for patients aged 3 years and older since January 2024.Neurofibromatosis is a type of neurocutaneous syndrome characterized by abnormalities affecting both the skin and the central nervous system. Among its various subtypes, neurofibromatosis type 1 is the most common. Depending on the location of the neurofibromas, patients may exhibit brain tumor symptoms, and spinal involvement can lead to scoliosis. As of 2024, there are 6,490 known patients in South Korea.With recent increases in cases of reduced reimbursement for Koselugo, calls for improving reimbursement criteria are growing among neurofibromatosis patients and medical professionals.
- Company
- Pharma companies cut distribution margins in succession
- by Son, Hyung Min Feb 04, 2026 06:51am
- Generated using AIThe pharmaceutical distribution industry is mounting strong opposition as pharmaceutical companies successively cut distribution margins in the wake of the government's drug price system reform.According to industry sources on the 4th, one multinational pharmaceutical company has reportedly lowered distribution margins on certain products by approximately 5% compared with previous levels.In addition, several small and mid-sized domestic pharmaceutical companies are said to have begun implementing or reviewing margin cuts in the range of 1% to 4%.The distribution industry is concerned that these unilateral, unconsulted adjustments could destabilize the industry ecosystem.A distribution company official stated, “While we understand that pharmaceutical companies face increased burdens due to the drug price reform, reducing margins without discussion with distributors undermines the mutually beneficial structure.”A senior official from the Korea Pharmaceutical Distribution Association also pointed out, “Indiscriminate margin reductions shake the foundation of normal business operations. With logistics costs and labor expenses already significantly increased, further reductions are difficult to withstand.”In some quarters, calls have even emerged to reconsider handling products from pharmaceutical companies that have reduced margins. Industry sentiment is rapidly cooling, with distributors warning that accumulated profitability deterioration, combined with margin cuts, could directly lead to bankruptcy risks.Meanwhile, pharmaceutical companies maintain that this is an unavoidable adjustment to withstand the government's pressure for drug price reductions. However, the distribution industry is strongly protesting, claiming its survival is threatened, leading to an escalation of conflict between the two sides.Against this backdrop, the Korea Pharmaceutical Distribution Association is scheduled to hold its general assembly on the 4th to discuss response measures. Industry observers are watching closely to see whether the association will outline strategies such as ▲strategic negotiation with pharmaceutical companies ▲collective responses, including refusal to handle products ▲policy recommendations directed at the government.An association official emphasized, “The pressure structure between pharmaceutical companies and distributors must not be repeated due to changes in the drug pricing system. When designing policies, the government must reflect the realities of the distribution structure, and a consultative body involving pharmaceutical companies, distributors, and the government is necessary.”Ho-young Park, Chair of the Korea Pharmaceutical Distribution Association (right), also expressed concern at a New Year's press briefing last month about the potential reduction in distribution margins arising from drug price cuts.
- Company
- Why new drugs are still not reimbursed within 150 days
- by Eo, Yun-Ho Feb 04, 2026 06:50am
- Questions continue to surround the effectiveness of Korea’s pilot program for parallel approval, assessment, and price negotiation.The Ministry of Health and Welfare has operated the parallel ‘approval–assessment–negotiation’ pilot program since 2023 to improve access to treatments for life-threatening severe and rare intractable diseases. The program’s core objective is to shorten the time required for new drugs to be listed on the National Health Insurance (NHI) reimbursement scheme by running regulatory approval, reimbursement assessment, and price negotiations in parallel. The stated goal is to reduce the reimbursement listing timeline, which used to take a maximum of over 300 days, to 150 days.As of 2026, expectations for the Approval-Evaluation-Negotiation pilot project were high, but the results appear to be less than satisfactory.The first pilot program, launched in 2023, concluded after nearly two years, which was longer than originally planned. Among the drugs included, Bylvay (odevixibat), the last to secure reimbursement status, took more than a year to listing.Similarly, the drugs selected for the second pilot project in December 2024—▲‘ Winrevair(sotatercept)’, ▲‘Rimqarto (anbal-cel)’, ▲‘Fintepla (fenfluramine)’—have not shown significant progress even though nearly a year has passed since the program began.At the start of the new year, the government announced plans to strengthen support for rare and severe intractable diseases, stating the intent to “reduce the reimbursement listing period for rare disease treatments, which previously took over a year, to 100 days through streamlining reimbursement appropriateness evaluations and negotiations.” However, skepticism remains within the pharmaceutical industry, questioning, “Given that even the ‘150 days’ target of the pilot project was rarely met, is this really feasible?”If the existing cost-effectiveness evaluation method remains unchanged, for chronic rare and intractable diseases requiring lifelong treatment, the longer a patient survives, the longer they must continue taking the medication. This means that while the drug improves survival and quality of life, the associated drug costs also increase.This structure makes it inherently disadvantageous to demonstrate cost-effectiveness and, in extreme cases, creates a dilemma in which a patient’s earlier death would paradoxically improve cost-effectiveness outcomes.A representative example is Winrevair, a pulmonary arterial hypertension (PAH) therapy included in the second pilot program. Winrevair is the first approved activin signaling inhibitor (ASI) in this therapeutic area, where drug development is particularly challenging. Unlike existing therapies focused on vasodilation, Winrevair improves vascular remodeling, the fundamental cause of the disease.Consequently, no comparable therapeutic alternative is available. If Winrevair is evaluated within the existing economic assessment framework, it would be compared to treatments developed two decades ago. This situation naturally delays the listing process. This challenge is not unique to Winrevair but is one commonly faced by drugs included in the parallel pilot program. Nearly 200 days have already passed since Winrevair received approval from Korea’s Ministry of Food and Drug Safety.Moreover, pulmonary arterial hypertension is a rare, severe, and chronically progressive disease. The longer patients receive sustained treatment to reach a low-risk group and maintain a favorable condition, the longer the drug is used. This creates the irony that proving cost-effectiveness becomes difficult precisely because the drug ‘keeps patients alive longer’. This is why flexible application of the ICER threshold is necessary.A representative from a multinational pharmaceutical company commented, “The parallel approval–assessment–negotiation pilot program was introduced to recognize the value of innovative medicines, yet it continues to apply conventional comparative evaluation criteria. The system was designed to improve access to innovative therapies for rare and severe intractable diseases, but it lacks evaluation standards capable of reflecting that innovation.”
- Policy
- Novartis withdraws all statin agents from the Korean mkt
- by Lee, Tak-Sun Feb 03, 2026 10:32pm
- Product photo of LescolNovartis is withdrawing all its statin agents used to treat hyperlipidemia from the Korean pharmaceutical market.Analysis suggests that the product's competitiveness has weakened due to other statin agents.According to the Ministry of Food and Drug Safety (MFDS) on the 2nd, Novartis Korea reported discontinuing supply of 'Lescol XL Tab (fluvastatin sodium),' the company's only statin-containing drug.Novartis stated, "In accordance with our global strategy and supply planning, we are discontinuing the supply of Lescol XL extended-release Tablet."The supply discontinuation date is set for July 31. Novartis noted, "Following the discontinuation of supply, please consider substituting with other statin-based products of the same mechanism of action (e.g., rosuvastatin, atorvastatin, simvastatin, pravastatin, etc.)."Lescol XL Extended-Release Tablet is currently the only statin product from Novartis that remains approved.Novartis entered the statin market in Korea in 1994, after obtaining MFDS approval for Lescol capsule. However, the product failed to achieve high performance as it lost out in competition with other statin products.Consequently, five of the six approved fluvastatin items were removed from the approval list in 2022 and 2023 due to withdrawals.Lescol XL was the only remaining product. However, following the latest report of supply discontinuation, fluvastatin will be completely withdrawn from the Korean market. There are currently no separate generic items available.Lescol XL Tab is indicated for ▲reduction of risk for cardiovascular disease, ▲hyperlipidemia ▲an adjunct to diet to reduce elevated total cholesterol, LDL-cholesterol, apo-B protein, and triglyceride levels and to increase HDL-cholesterol in pediatric and adolescent patients (boys: aged 9 to 16; girls: aged 10 to 16 and post-menarche) with heterozygous familial hypercholesterolemia who do not respond adequately to dietary therapy.Based on 2024 UBIST data, outpatient prescription amount totaled KRW 2.8 billion, a 23.7% decrease from the previous year. The sales volume is considered small when compared to Viatris' Lipitor (atorvastatin calcium trihydrate), a statin agent, which recorded KRW 188.6 billion in prescription sales during the same period.The underperformance is considered the most significant factor in this withdrawal from the Korean market.