- LOGIN
- MemberShip
- 2026-03-09 20:47:42
- Policy
- First matching-form Ofev generic listed at half price
- by Jung, Heung-Jun Feb 24, 2026 03:55pm
- A generic version of the chronic fibrotic interstitial lung disease treatment Ofev Soft Cap (nintedanib esylate) with the same formulation will be listed for reimbursement for the first time.With its reimbursement entry at a price more than 50% lower than the original, full-scale market competition is expected.According to industry sources on the 20th, Ildong Pharmaceutical’s Cuninta Soft Cap 100 mg and 150 mg will be added to the March reimbursement list.The substance patent for Boehringer Ingelheim Korea's Ofev Soft Capsules expired in January last year. Subsequently, generic companies have successively launched their versions.However, unlike the original drug, those products obtained approval in tablet formulations. From July last year, Yungjin Pharm, Ildong Pharmaceutical, Daewoong Pharmaceutical, Kolon Pharmaceutical, and others entered reimbursement. Whan In Pahrm also received approval for Ofenib Tab, but the drug is yet to be listed for reimbursement.Until now, no reimbursed generic matching Ofev’s soft capsule formulation has been available. Ildong Pharmaceutical is expected to be the first to list a soft capsule generic, Cuninta Soft Cap, following its release of Cuninta Tab.Cuninta Tab 150 mg was previously listed at KRW 13,500, roughly half the price of Ofev Soft Cap, which was priced at KRW 26,220.Although generics designated as orphan drugs could receive equivalent pricing to the original, manufacturers pursued lower prices to strengthen market competitiveness.The reimbursement ceiling prices for Cuninta Soft Cap 100 mg and 150 mg are set at KRW 8,500 and KRW 13,500, respectively. Notably, the lower dose represents about 40% of the originator’s 100 mg price of KRW 20,960.Where earlier competition strategies relied on tablet differentiation and lower pricing, manufacturers can now seek prescription expansion based on identical formulation at half the cost.Because switching prescriptions within the same formulation is expected to face less patient resistance, Ildong Pharmaceutical is anticipated to intensify its competitive positioning against Ofev.Given that Ofev entered reimbursement relatively late, 8 years after domestic approval, Ofev is expected to face intensifying competition from the later entrant generics.
- Policy
- Government to establish bioequivalence standards for complex generics
- by Lee, Jeong-Hwan Feb 24, 2026 03:55pm
- The government is moving forward with the development of guidelines, including bioequivalence evaluation standards for complex generic drugs.This initiative aims to support the rapid development of complex generics as market entry for new technology and new concept drugs increases.Formulations targeted for new assessment criteria and testing methodologies include oral agents, injectables, inhalation products, and ophthalmic preparations.On the 20th, the National Institute of Food and Drug Safety Evaluation (NIFDS) under the Ministry of Food and Drug Safety (MFDS) announced the launch of a research project titled the “New Technology and New Concept Drug Guideline Development Project”.To develop bioequivalence assessment standards and testing method guidelines for complex generics, the Ministry of Health and Welfare (MOHW) will establish a multi-layered advisory framework involving industry, academia, and regulatory experts. Policy decisions will be made through an expert committee following internal review for stage-by-stage examination and verification.The research will define the concept of complex generics and conduct comparative analyses of domestic pharmaceutical industry demand alongside guideline frameworks from major global regulatory authorities to create new guidelines.Guidelines currently operated by the US FDA, European EMA, and Japan PMDA will be analyzed. Priorities for new guideline development will be determined based on technological impact, industry needs, and regulatory necessity.Subsequently, it will present general evaluation principles applicable to all dosage forms of complex generics and publish a reference manual to support development.In addition, bioequivalence guidelines specific to complex generics will be developed. It plans to create equivalence evaluation guidelines for oral, injectable, inhaled, and ophthalmic combination generics, validate them through a separate expert advisory body, including the Ministry of Food and Drug Safety (MFDS), and then finalize them.For this year, oral formulations selected for guideline development include pentosan polysulfate sodium products, ferric citrate hydrate capsules, and mesalamine extended-release tablets.Injectable products include dexamethasone extended-release implants and paliperidone palmitate long-acting injections. Inhalation products cover budesonide, formoterol fumarate inhalation aerosols, fluticasone propionate inhalers, and fluticasone propionate/salmeterol xinafoate inhalation powders.Ophthalmic formulations include brinzolamide suspension eye drops and prednisolone acetate suspension eye drops.Notably, authorities plan to establish a collaborative framework with the task force supporting rapid drug development guidelines. Once completed, newly developed guidelines will be promoted to industry and academic stakeholders, alongside implementation activities such as conferences.NIFDS stated, “We plan to develop more than 10 bioequivalence guidelines for complex generics and publish a project report. Through technological support for complex generics, we aim to accelerate the timely commercialization of high-quality generics while strengthening review reliability and transparency through science-based guidelines.”
- Company
- Fintepla’s reimbursement listing process draws attention
- by Eo, Yun-Ho Feb 24, 2026 03:55pm
- Attention is focused on the insurance reimbursement process for ‘Fintepla’, a drug included in Korea’s approval-evaluation-negotiation parallel pilot program.The reimbursement application for UCB Pharma Korea’s Dravet syndrome drug Fintepla (penfluramine) has been submitted to the Health Insurance Review and Assessment Service's (HIRA) Pharmacoeconomic Evaluation Subcommittee and is currently undergoing subsequent procedures.Approved domestically last December, Fintepla was designated as an orphan drug and was selected as a candidate for the second phase of the government's ‘Approval-Evaluation-Negotiation Parallel Pilot Program’. Amid ongoing debates about the effectiveness of the pilot program, it remains to be seen whether Fintepla can smoothly complete the listing procedures, including review by the Drug Reimbursement Evaluation Committee.Dravet syndrome is an ultra-rare, pediatric, intractable disorder that typically manifests in infancy. According to experts, approximately 80% of cases are associated with SCN1A mutations.It typically manifests around 12 months of age, with up to 15% of patients dying during infancy or adolescence. Patients face elevated risks of both physical and neurodevelopmental comorbidities, including motor impairment, language delay, autism spectrum disorders, intellectual disability, and ADHD.Caregivers also endure high caregiving stress and low quality of life, including the burden of 24-hour care, career disruptions, and loss of income.Frequent, long-term seizures in Dravet syndrome patients not only degrade the quality of life for both patients and caregivers but also carry a risk of sudden unexpected death in epilepsy (SUDEP). Therefore, seizure reduction or elimination is the central treatment objective for the condition.However, conventional antiepileptic drugs often show limited efficacy, and certain therapies may even exacerbate seizures, leaving considerable unmet medical need in the domestic treatment landscape. Fintepla is being evaluated as a treatment option that not only reduces seizure frequency but may also achieve near-complete seizure control in some patients.Fintepla’s clinical value has been demonstrated through 3 randomized Phase III trials (Study 1–3).An integrated analysis combining data from 119 patients enrolled in Study 1 and participants recruited in Study 3 showed that the Fintepla treatment group showed a reduction in monthly convulsive seizure frequency (MCSF) by 62.3% and 64.8%, respectively. Notably, near-complete seizure elimination was observed exclusively in the Fintepla treatment groups.Study 2, a 15-week trial consisting of a six-week baseline, three-week titration, and 12-week maintenance phase, randomized patients 1:1 to receive Fintepla or placebo alongside standard therapy with stiripentol (plus clobazam and/or valproate). Results showed that 54% of patients in the Fintepla combination group achieved at least a 50% reduction in MCSF from baseline, compared with only 5% in the placebo group.
- Policy
- Backlash mounts to drug price reduction reform plan
- by Lee, Jeong-Hwan Feb 24, 2026 03:55pm
- The Korean pharmaceutical industry continues criticizing that the background of the Minister of Health and Welfare (MOHW)'s drug pricing system reform, which includes 'calculation criteria for impact of drug price reductions' and 'preferential drug price criteria,' is unreasonable.The MOHW calculated simulation results for losses incurred from drug price reductions based on total sales amount. However, the view is that the calculation standard itself is flawed, as sales amount includes all accounting elements involved in the manufacturing and production of medicines.Furthermore, the criticism is that preferential drug price clauses based on whether a company is certified as an Innovative Pharmaceutical Company or on its ratio of new drug Research and Development (R&D) expenditure to sales are inappropriate because they are arrived at unilaterally, without proper agreement between the government and the industry.On the 22nd, the pharmaceutical industry voiced concerns about MOHW's unilateral push for drug pricing reform, stating, "The justification for the price reduction is unclear, and such administration significantly undermines trust in the government, leading to results that run counter to the fostering of the global pharmaceutical industry."On the 20th, the Health Insurance Policy Deliberation Committee (HIPDC) subcommittee excluded the drug pricing system reform plan, including the price reduction for currently listed generics, from its agenda.The MOHW cited insufficient gathering of industry opinions and said it would continue communicating with the industry until next month's (March) HIPDC meeting.Despite the MOHW’s decision to defer or postpone the agenda, the pharmaceutical industry remains skeptical about the possibility of revisions to the reform plan. This is due to the observation that the MOHW has not shown a proactive negotiation attempts thus far.Korean pharmaceutical companies cite the MOHW's methodology for calculating the impact of drug price reductions as the biggest issue.It is reported that the MOHW, based on simulation results analyzing the financial impact after price reductions using the sales revenue of several domestic pharmaceutical companies operating the generic business, concluded that revenue losses would be minimal even if the generic price calculation rate were lowered to 40%.Furthermore, the ministry believes that its reform plan is sufficiently valid and consistent, having derived results showing that certified innovative pharmaceutical companies or those with a high proportion of new drug R&D relative to sales would remain relatively unaffected by the price reductions.Korea's domestic pharmaceutical companies maintain that if the MOHW simulates the impact of price reductions based solely on total sales revenue or simple operating profit, an accurate evaluation is impossible.The reason is that a simulation based on sales revenue calculates figures by including every accounting element without exception. In contrast, one based on operating profit excludes selling, general, and administrative (SG&A) expenses. This evaluation method is impossible to reflect the innovation of pharmaceutical companies that have substantially contributed to the development of the domestic industry.In particular, while the MOHW has stated multiple times that the purpose of this drug pricing system reform is to foster the pharmaceutical industry rather than reduce health insurance drug expenditures, the pharmaceutical industry argues that reducing generic drug prices significantly while claiming to foster the industry is contradictory.The criticism is that the ministry's administrative goal, which it seeks to achieve through price reductions, is unclear.An official from domestic Pharmaceutical Company A pointed out, "The sales revenue evaluation, which is the standard for the MOHW’s impact simulation, is flawed as it includes all accounting elements," and added, "They must divert from single standards like sales revenue, perform a value evaluation considering various factors, and then proceed with simulations in a direction that grows the pharmaceutical industry and the drug market."Then added, "The MOHW changed the health insurance listing method for drugs from a negative list to a positive list system," and added, "This implies that the governemtn will make value judgments on drug prices. Therefore, all-at-once reduction of drugs listed through this system means the government considers existing medicines to have no value and intends to suppress the market."An official from domestic Pharmaceutical Company B also expressed, "The MOHW can analyze the reduction in pharmaceutical companies' revenue quite precisely through health insurance claim amounts or supply history reports. It is puzzling why they designed the drug price reduction and the pricing system reform plan with a simulation that excludes such big data when it is available for use."The official stated, "The pharmaceutical industry will be able to trust the government again only if the MOHW discloses transparent data regarding the evidence and standards used to design the drug pricing system reform and shows a willingness to negotiate," and added, "For now, meetings between the MOHW and the pharmaceutical industry may become a superficial formality, aimed at filing a quota of superficial mutual consultations."
- Policy
- Imjudo + Imfinzi comb to enter reimb list next month
- by Jung, Heung-Jun Feb 23, 2026 09:16am
- AstraZeneca Korea's liver cancer treatment, Imjudo (tremelimumab), will be included in the reimbursement list next month. This follows three months after the Drug Benefit Evaluation Committee (DBEC) approved combination therapy with Imfinzi.It is reported that a ceiling price of KRW 16.5 million has been set for this newly listed high-cost drug. Treatment access to hepatocellular carcinoma treatment is expected to improve significantly.According to industry sources on the 20th, AstraZeneca Korea's Imjudo Injection (0.3g/15mL) will be reimbursed starting in March.In November last year, the DBEC approved the reimbursement appropriateness of Imjudo as 'first-line treatment of adult patients with advanced or unresectable hepatocellular carcinoma in combination with durvalumab.'The listing process proceeded smoothly as drug price negotiations with the National Health Insurance Service (NHIS) began in December. Following the conclusion of price negotiations, insurance coverage is scheduled to begin in March.Imjudo was a drug for which requests for rapid listing were made during the Ministry of Health and Welfare (MOHW)'s National Assembly audit last year. At that time, the National Assembly inquired about plans to expand the reimbursement evaluation method, given the drug's distinct single-dose administration.According to the MOHW's response at the time, an application for health insurance coverage for Imjudo was filed in March last year, and the DBEC conducted a review of the drug cost comparison criteria in September of the same year.However, the DBEC delivered a reconsideration decision regarding the combination therapy with Imfinzi last September. After that, Imjudo's appropriateness for reimbursement was recognized during the DBEC reconsideration in November.Analysis suggests that the government's designation of a flexible application of the Incremental Cost-Effectiveness Ratio (ICER) value for Imjudo, second since the ADC anticancer drug 'Trodelvy (sacituzumab govitecan)', facilitated the smooth winding up of the drug price negotiations.
- Company
- Two years after Forxiga exit… Jardiance 34%↑, Dapa.N↑
- by Kim, Jin-Gu Feb 23, 2026 09:16am
- Two years after the withdrawal of the SGLT-2 inhibitor ‘Forxiga (dapagliflozin)’ from the Korean market, ‘Jardiance (empagliflozin)’ is strengthening its dominant position in this market, expanding its prescription performance by over 30%.Among the flood of generics launched following Forxiga’s patent expiration, HK inno.N’s Dapa.N has shown particularly strong growth. Industry observers attribute this largely to the product’s successful succession of indications from the originator.Two years postexit… Jardiance prescriptions rise from KRW 58.1billion to 77.7billionAccording to the pharmaceutical market research firm UBIST on the 21st, outpatient prescriptions for SGLT-2 inhibitor monotherapies reached KRW 164.9B last year. This represents a 17.9% increase compared with KRW 139.8 billion in 2023, just before Forxiga’s market withdrawal.The market experienced substantial shifts surrounding Forxiga’s exit. AstraZeneca Korea decided to withdraw Forxiga from the Korean market at the end of 2023. The following year, it halted the domestic supply of new inventory, leaving only existing stock circulating in the market. In April 2024, the company voluntarily withdrew marketing authorization, followed by full reimbursement delisting in December, and completed its full withdrawal from the market.Forxiga recorded KRW 55.5 billion in prescriptions just before its market exit in Korea. At the time, fierce marketing and sales competition unfolded in the SGLT-2 monotherapy market among competing brands and generic manufacturers to capture the over KRW 50 billion annual gap left by Forxiga. Analysts note that this competitive dynamic contributed to nearly 18% market expansion over the two-year period.During this period, Jardiance’s prescription sales grew significantly. Jardiance's prescription sales, which were KRW 58.1 billion in 2023, increased to KRW 77.7 billion last year, marking a 33.8% growth. In this process, Jardiance strengthened its dominant market position. As of last year, Jardiance’s market share in the SGLT-2 monotherapy segment reached 47.2%, approaching half of total prescriptions.A potential variable is the entry of Jardiance generics. Following the expiration of Jardiance’s substance patent last October, 23 generic versions entered the market. The market penetration of Jardiance generics this year could potentially influence the original drug’s dominant position going forward.Another original product, Daewoong Pharmaceutical’s Envlo (enavogliflozin), also saw a significant increase in prescriptions around the time of Forxiga's withdrawal. Since its launch in May 2023, prescriptions increased from KRW 3.2 billion to KRW 10.6 billion in 2024, then to KRW 11.8 billion last year.Forxiga generics mark KRW 74.6 billion combined... ‘Dapa.N’ sales surge with inherited indicationsForxiga generics have also successfully filled the void left by the original. Notably, HK Inn.N’s Dapa.N, which inherited Forxiga’s indications, has recently shown a particularly pronounced upward trend in prescription sales.Forxiga generics were launched en masse in 2023 following the expiration of the original product's substance patent. A total of 86 companies obtained generic product approvals, with 65 of them launching products.Within the overall SGLT-2 inhibitor market, prescription sales for dapagliflozin-containing products increased slightly from KRW 73.4 billion in 2023 to KRW 74.6 billion last year. The 2023 structure, which consisted of KRW 55.5 billion for the original Forxiga + 17.2 billion won for generics, was replaced by KRW 74.6 billion entirely from generics last year.Among the generics, HK inno.N’s Dapa.N has demonstrated particularly strong prescription growth. In April 2024, AstraZeneca Korea withdrew Forxiga’s marketing authorization while simultaneously transferring clinical data to the company, enabling Dapa.N to inherit Forxiga’s indications.Immediately after the indication succession, Dapa.N’s prescription growth was limited. However, the upward trend accelerated from the fourth quarter of 2024. Prescriptions, which stood at KRW 2.4 billion in 2024, surged more than fourfold to KRW 10.5 billion last year. Analysts suggest that the declining domestic supply of Forxiga shifted prescription demand toward Dapa.N.Excluding Dapa.N, other Forxiga generics showed mixed performance. Last year, Daewoong Bio’s ‘Forxidapa’ recorded KRW 6.0 billion, Hanmi Pharmaceutical’s ‘Dapalon’ KRW 5.8 billion, and Boryung’s ‘Trudapa’ KRW 5.6 billion. Additionally, Aju Pharm’s ‘Daparil’, Dong-A ST’s ‘Dapapro’, Chong Kun Dang’s ‘Exiglu’, and Daewon Pharm’s ‘Dapawon’ exceeded KRW 3.0 billion in prescriptions.In contrast, most companies recorded prescriptions below KRW 1.0 billion. Of the 65 companies that launched products, 49 reported annual prescription performance under KRW 1.0 billion. This suggests that only about one in four generic companies entering this market achieved the expected commercial outcomes.
- Policy
- Recall of GSK's antiviral 'Valtrex Tab 500mg' over impurities
- by Lee, Tak-Sun Feb 23, 2026 09:16am
- Antiviral medication 'Valtrex Tab 500mg,' used to treat shinglesA recall has been initiated for certain batches of GlaxoSmithKline's antiviral medication, Valtrex Tab 500mg (valacyclovir hydrochloride), after nitrosamine impurities were detected above the permissible limit.This is the first recall of a valacyclovir-containing formulation due to impurities.The Ministry of Food and Drug Safety (MFDS) announced on the 20th that it has issued an operator-led recall order for specific manufacturing units of Valtrex Tab. 500mg that exceeded the Acceptable Daily Intake (ADI) of the nitrosamine impurity N-nitroso-N-ethyl-valacyclovir.The affected batch numbers are V53B (expiry 2027-04-24) and YR9K (expiry 2027-06-04).This product received domestic approval in 2005 and is indicated for ▲Shingles ▲Treatment of initial and recurrent genital herpes infections ▲Suppression of recurrent genital herpes infections ▲Reduction of transmission of genital herpes when used as suppressive therapy in combination with safer sex practices ▲Prophylaxis of cytomegalovirus (CMV) infection following kidney transplantation ▲Ccold sores ▲Treatment of chickenpox in immunocompetent pediatric patients aged 2 to under 18 years.As of 2024, the import performance of the drug totalUSD 4,137,731 (approximately KRW 6 billion). The ADI for N-nitroso-N-ethyl-valacyclovir in valacyclovir components is set at 400ng per day.The announced impurity is N-nitrosamine, a potential carcinogen and mutagen that can be generated at the ethylamine site of valacyclovir. It is known to form in trace amounts during the synthesis of the Active Pharmaceutical Ingredient (API) itself or through reactions during storage.Currently, 17 valacyclovir products are approved for sale in the Korean market. Attention is now focused on whether the recall of the original product will expand to include generic versions.
- InterView
- [Desk View] Need for transparency toward drug price reform
- by Chon, Seung-Hyun Feb 23, 2026 09:15am
- The Ministry of Health and Welfare (MOHW) has reportedly delayed a decision on the drug pricing reform agenda at the Health Insurance Policy Deliberation Committee. In November last year, the MOHW reported to the HIPDC a plan to lower the price calculation rate for generics and patent-expired drugs from the current 53.55% to 40%, announcing a final decision in February and implementation by July of this year. While it was expected that the reform plan would be finalized at the HIPDC subcommittee held on the 20th to initiate the institutional reform, the process has been delayed by at least a month.The industry appears relieved by the MOHW's decision to defer the HIPDC discussion. Expectations are emerging that the ministry may have felt burdened by the prospect of forcing through the reform while receiving strong opposition from the pharmaceutical sector. On the 10th, the Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA) unanimously adopted a resolution during its board of directors meeting, urging the deferment of the resolution and implementation of the drug pricing system reform.Through its resolution, the KBPMA Board of Directors urged the government to ▲ delay the vote and implementation of the large-scale drug price reduction plan by the Health Insurance Policy Deliberation Committee ▲an impact assessment on how these cuts would affect public health and employment ▲the abolition of the market-linked actual transaction price implementation plan ▲ support measures to help small and medium-sized pharmaceutical companies upgrade their business structures ▲a formal governance structure between the government and industry to regularly discuss drug pricing policies and industrial growth.Labor organizations have also voiced opposition against the government regarding the reform. On the 29th, the Federation of Korean Trade Unions (FKTU) issued a statement warning, "The government must transparently disclose the basis and financial effects of the drug pricing system reform and immediately establish a social discussion structure where the opinions of stakeholders are reflected," adding, "We will not remain passive regarding any attempts to rollback labor conditions or increase job insecurity under the guise of this policy."The Korean Democratic Pharmaceutical Union (KDPU), primarily composed of labor unions from multinational pharmaceutical companies, also formalized its opposition last month by holding a picket protest in front of the Health Insurance Review and Assessment Service (HIRA) in Seocho-dong, Seoul.Critics point out that the government has not disclosed specific details since announcing the reform, further fueling anxiety among pharmaceutical companies.The MOHW has not released a specific position regarding the industry's demands for deferment or cancellation of the reform. An industry official stated, "The MOHW has not once presented a specific figure for the generic price reduction since reporting the reform plan in November last year."If the generic price standard is adjusted from 53.55% to 45%, the maximum price of a generic is mathematically calculated to drop by 16.0%. If the reform standard is set at 40%, the price drops from KRW 53.55 KRW to KRW 40, increasing the reduction rate for the maximum generic price to 25.3% compared to the previous standard. Given that the profit margin for a single generic product would drop by more than 20%, pharmaceutical companies' losses would inevitably be substantial. However, because specific reduction rates have not been presented, pharmaceutical companies are unable to estimate loss scenarios resulting from the reform.A detailed roadmap for whether price cuts will apply to currently listed drugs has also not been disclosed. If the reformed pricing system is applied to currently listed drugs, pharmaceutical companies' losses will be even greater. For example, if the price of a product with annual sales of KRW 10 is reduced from 53.55% to 40%, KRW 2.5 billion in annual revenue would evaporate.The MOHW plans to sequentially implement the reformed pricing system, starting with listed generics that have maintained a calculation standard of over 50% for more than 13 years since the blanket price reduction in 2012. The vision is to adjust approximately 3,000 items over three years, starting from the second half of next year, and sequentially reduce 1,500 items that have maintained a rate of 45% or higher starting from the second half of 2027.According to this scenario, the targets for price reduction differ across generic products with the same ingredient, depending on their market entry timing. For example, a total of 156 items have been approved for the single-agent antiplatelet agent clopidogrel, with approvals ranging from 2005 to 2021. The clopidogrel generic market formed, with 19 items approved in 2005 and 29 in 2006. From 2014 to 2018, 5 to 9 generics entered the market each year, and in 2019, new approvals surged to 17. At that time, as the government set out to reform the pricing system, including tiered pricing and criteria, there was a flood of new approvals.If the government pursues price reductions for generics listed before 2012, it is estimated that 64 items approved between 2005 and 2011 would be subject to price cuts, while 92 items approved from 2012 onwards would be excluded. In this case, a very strange situation would arise where different pricing systems apply to the same product. Issues of equity would inevitably surface, as the system would disadvantage only specific products and companies.The industry also raises the possibility that targets for price reductions could be categorized by ingredient based on when the generic market opened. This is a scenario in which, if even one generic were listed before 2012, all drugs containing that ingredient would be categorized as targets for price reduction. In this case, the losses pharmaceutical companies would incur from price cuts would be even greater. If the criteria requirements, such as bioequivalence tests, are also applied, the scale of losses could expand exponentially.In its press release announcing the drug pricing system reform, the MOHW problematized the 'generic-centered industrial ecosystem.' The justification is that to create a virtuous cycle of an innovative ecosystem through R&D activation, an urgent overhaul of the drug pricing system is needed to balance appropriate compensation for value. The view is that only when pharmaceutical companies move away from a generic-centered business model and focus on new drug development can South Korea become a pharmaceutical powerhouse.However, the ministry has yet presented any measures regarding the anxiety over the threat to pharmaceutical companies' survival. Communication is necessary. Deferring the resolution process for the drug pricing reform by a month or two, to observe the industry's response, does not mean the efforts at communication are recognized. The government must specifically disclose its policy goals and content and engage in substantive communication with the industry. If the government’s policy is justified and legitimate, it should at least make an effort to persuade companies. The communication process must also be transparently disclosed. Under the President Lee Jae Myung administration, which makes Cabinet meetings and business reports public, we hope that policies will not be pursued in secret.
- Company
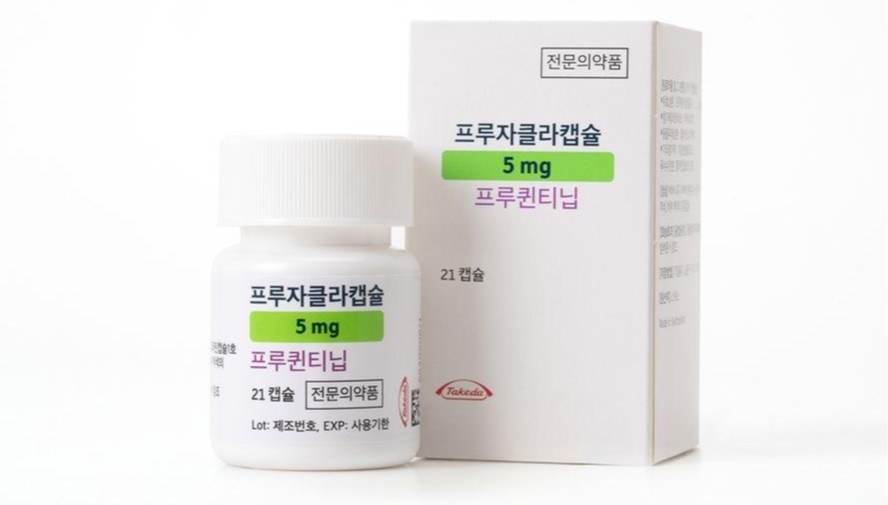
- Fruzaqla may be prescribed in general hospitals in Korea
- by Eo, Yun-Ho Feb 23, 2026 09:15am
- The new colorectal cancer therapy Fruzaqla may be prescribed at general hospitals in Korea.According to industry sources, Takeda Pharmaceutical Korea's colorectal cancer treatment Fruzaqla (fruquintinib), which selectively inhibits vascular endothelial growth factor receptor (VEGFR)-1,2,3, has passed the Drug Committee (DC) reviews of major medical institutions nationwide, including Korea’s ‘Big 5’ teritary hospitals - Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary’s Hospital, Asan Medical Center, and Severance Hospital.Approved in Korea in March last year, Fruzaqla was previously designated as a drug for the Global Innovative Products on Fast Track (GIFT) program as an innovative oncology therapy.Specifically, the drug is indicated for 'patients with metastatic colorectal cancer (mCRC ) who have previously received fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, and have been treated with, or are not candidates for, available therapies including anti-VEGF agents, anti-EGFR agents (for RAS wild-type disease), and trifluridine/tipiracil or regorafenib.However, Fruzaqla is yet to be reimbursed in Korea. Takeda submitted its reimbursement application to health authorities last year, and the listing process is currently ongoing. This is why whether Fruzaqla will secure coverage and enable broader patient access is gaining attention.Meanwhile, Fruzaqla’s clinical efficacy was demonstrated in the Phase III FRESCO and FRESCO-2 trials.Clinical findings showed that Fruzaqla extended median overall survival (mOS) by 2.7 months to 9.3 months in patients with previously treated metastatic colorectal cancer compared to placebo, while reducing the risk of death by 35%.In addition, as an oral medication taken once daily without complex dietary restrictions, Fruzaqla is expected to positively impact both treatment efficacy and patient quality of life.Dong-Hoe Koo, Professor of Oncology at Kangbuk Samsung Hospital, said, “Fruzaqla exhibits high drug specificity and avoids unnecessary targets. This enables efficient VEGFR inhibition and sustained drug exposure. The potential for combination strategies with existing therapies warrants further clinical investigation.”
- Company
- ‘Enhertu sets new standard in breast cancer treatment’
- by Son, Hyung Min Feb 23, 2026 09:15am
- Enhertu is setting a new standard in HER2-positive breast cancer. With its treatment scope expanding beyond conventional HER2-positive and HER2-low populations to include ultra-low HER2 expression, the therapy is being viewed as a potential turning point in treatment strategy, particularly for HR+/HER2- low-expression metastatic breast cancer patients whose options were previously limited after endocrine therapy failure.Professor Seok-Ah Im, Department of Hematology-Oncology, Seoul National University HospitalOn the 20th, Daiichi Sankyo Korea and AstraZeneca Korea held a press conference at The Plaza Hotel in Jung-gu, Seoul, to commemorate the indication expansion of the antibody-drug conjugate (ADC) Enhertu (trastuzumab deruxtecan).The newly approved indication added last month is Enhertu as monotherapy for the treatment of adult patients with unresectable or metastatic breast cancer exhibiting HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2 ultra-low expression (IHC 0 with membrane staining), who have previously received one or more endocrine therapies in the metastatic setting.Enhertu is considered a therapy with broad potential across multiple solid tumors. While first-generation ADCs such as Roche’s Kadcyla (trastuzumab emtansine) remained largely confined to breast cancer indications, second-generation ADCs have successfully secured diverse indications. Enhertu, in particular, has demonstrated efficacy across various solid tumor types, including breast cancer, non-small cell lung cancer, and colorectal cancer.ADCs are novel anticancer drugs created by linking an antibody that binds to a specific target antigen on the surface of cancer cells with a cytotoxic drug via a linker. The advantage of ADCs is that they leverage the antibody's selectivity for its target and the drug's cytotoxic activity to ensure the drug acts selectively only on cancer cells, thereby enhancing therapeutic efficacy while minimizing side effects.Hormone receptor-positive (HR+) / HER2-negative (HER2-) breast cancer represents the most common subtype, accounting for approximately 70% of all breast cancers. Although generally associated with a more favorable prognosis relative to other subtypes, patients who are unsuitable for endocrine therapy or develop resistance often face limited treatment options, with chemotherapy remaining the primary alternative. The PFS achievable with first-line therapy is only about 6 months, indicating a high unmet clinical need.The basis for the expanded indication is the Phase III DESTINY-Breast06 trial.The trial enrolled 866 adult patients with metastatic HR-positive breast cancer who were HER2-low or HER2-ultra-low, had previously received endocrine therapy, and had no prior chemotherapy history in the advanced or metastatic setting.In this study, HER2 ultra-low expression was defined as faint and incomplete HER2 staining on the cell membrane observed in 10% or fewer tumor cells (IHC 0 for membrane staining; in this study, IHC >0 and <1+).Patients were randomized 1:1 to receive either Enhertu or the physician’s choice chemotherapy (capecitabine, nab-paclitaxel, or paclitaxel).Results demonstrated that Enhertu significantly extended median PFS to 13.2 months, compared with 8.1 months in the chemotherapy arm, based on blinded independent central review (BICR).Enhertu also achieved an objective response rate (ORR) of 57.3%, nearly 1.8 times higher than the 31.2% observed in the control group. Complete responses (CR), absent in the chemotherapy cohort, were observed in approximately 3% of patients in the Enhertu group.In terms of safety, adverse events were consistent with prior Enhertu studies. However, one Grade 5 interstitial lung disease (ILD) event associated with drug administration occurred..Professor Seok-Ah Im, Department of Hematology-Oncology at Seoul National University Hospital, said, “Enhertu demonstrated a median progression-free survival exceeding 1 year while maintaining patient quality of life, suggesting a fundamental shift in treatment strategy. Following failure of endocrine therapy and CDK4/6 inhibitors, Enhertu has become the global standard of care.”Clinical benefit extends from low to ultra-low expression… redefining HER2 treatment standardsProfessor Gyeong Yeop Kong, Department of Pathology at Asan Medical CenterThe subtype accounts for approximately 20–25% of breast cancer cases and tends to progress more rapidly and aggressively than other subtypes.Prior to Enhertu’s introduction, HER2 classification relied primarily on immunohistochemistry (IHC), categorizing tumors as HER2-negative or HER2-positive.IHC testing categorizes protein expression as 0, 1, 2, or 3, with 1 classified as HER2-negative and 3 as HER2-positive. Cases with a score of 2 are determined via in situ hybridization (ISH) analysis.However, Enhertu demonstrates efficacy even in patients with low or ultra-low expression (IHC scores 0 or 1), establishing itself as a new standard treatment option across the entire HER2 expression spectrum.Professor Gyeong Yeop Kong of the Department of Pathology at Asan Medical Center said, “The expansion of the HER2 expression spectrum to include not only low-expression but also ultra-low-expression cases provides clinical justification for considering a significant proportion of metastatic breast cancer patients as candidates for HER2-targeted therapy.”He added, “Re-testing may be considered even for HR-positive patients initially diagnosed as HER2 IHC 0. Pathology reporting systems must evolve to enable accurate identification of ultra-low expression populations.”