- LOGIN
- MemberShip
- 2025-12-21 08:32:26
- Company
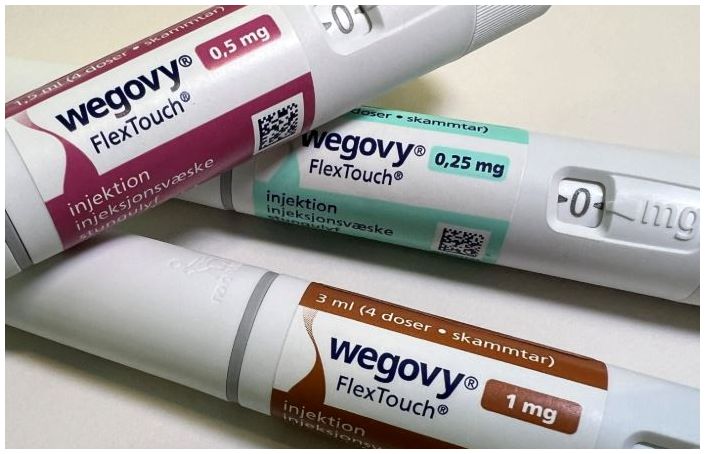
- 'Wegovy' dominating the South Korean obesity drug market
- by Chon, Seung-Hyun May 27, 2025 06:17am
- Novo Nordisk's Wegovy has dominated the South Korean obesity treatment market, establishing a monopolistic competition with over 70% market share. In just six months since its launch in Korea, Wegovy generated a sensation, surpassing KRW 100 billion in cumulative sales. Wegovy's success has expanded the obesity drug market to its largest size ever, while sales of Saxenda, which previously led the market, sharply declined. According to pharmaceutical market research firm IQVIA, on May 26, the obesity drug market reached KRW 108.6 billion in the first quarter of this year, a 162.3% increase compared to KRW 41.4 billion in the same period last year. This is the first time the quarterly obesity drug market size has exceeded KRW 100 billion. Quarterly Obesity Treatment Market Size and Key Product Sales. Legend: Bars-obesity market, Black-Wegovy, Purple-Qsymia, Green-Saxenda (Unit: KRW 100 million, Source: IQVIA) Novo Nordisk's Wegovy clearly stood out. In the first quarter, Wegovy's sales recorded KRW 79.4 billion, accounting for a dominant 73.2% of the entire obesity drug market. Wegovy, which was approved by the Ministry of Food and Drug Administration (MFDS) in April 2023, is a GLP-1 analogue containing semaglutide, known to reduce HbA1c. Novo Nordisk developed Wegovy as a once-weekly obesity treatment using semaglutide after observing weight loss effects in patients during clinical trials of GLP-1 class diabetes drug candidates. Wegovy gained immense popularity immediately after its domestic launch in October last year. Despite its high price, prescription demand surged due to its significant weight loss efficacy. Wegovy's sales reached KRW 60.3 billion in the fourth quarter of last year, propelling it to the top of the obesity drug market. The obesity drug market size was KRW 47.4 billion in the third quarter of last year, but it soared by 97.9% to KRW 93.8 billion in just one quarter following Wegovy's launch. Wegovy held a 63.4% market share in the obesity drug market in the fourth quarter of last year, and its share has continued to rise this year. Under six months since its domestic release, Wegovy's cumulative sales reached KRW 139.8 billion, exceeding KRW 100 billion. Wegovy is thriving globally due to its groundbreaking weight loss effects. Wegovy's sales last year reached DKK 58.26 billion (approximately KRW 11.7 trillion), an 85.7% increase from DKK 31.343 billion in 2023. Demand for Wegovy surged to the point of shortages after its launch in the U.S. market. Even before its domestic launch, Wegovy gained global fame for shortages, having been rumored as the weight loss secret of international celebrities like Tesla CEO Elon Musk. Despite its high price of around KRW 500,000, Wegovy gained explosive interest immediately after its domestic release, leading to supply shortages. Even with the suspension of non-face-to-face prescriptions, demand for Wegovy continued to rise. Initially, Wegovy was actively prescribed through non-face-to-face medical consultations. When concerns were raised that Wegovy was being indiscriminately prescribed via non-face-to-face consultations regardless of a person's weight or obesity status, health authorities suspended non-face-to-face prescriptions for obesity treatments from December 16 of last year. The introduction of Wegovy has led to a reduction in sales of Saxenda and Qsymia, which previously dominated the obesity drug market. The presence of Novo Nordisk's Saxenda has significantly declined in the obesity drug market. Saxenda's first-quarter sales were KRW 4.2 billion, down 72.3% from KRW 15.1 billion in the same period last year. Compared to its KRW 24.2 billion sales in the second quarter of last year, its sales have sharply declined to less than 20%. Saxenda, launched in Korea in 2018, was the world's first obesity drug approved as a GLP-1 analog. Saxenda's active ingredient, liraglutide, is identical to Victoza's, prescribed for type 2 diabetes patients, differing only in dosage and administration. The analysis suggests that Wegovy, a GLP-1 class drug similar to Saxenda, has further taken Saxenda's market. With reduced domestic supply since Wegovy's launch, there are rumors of Saxenda's production being discontinued. After becoming the obesity treatment market leader with sales of KRW 42.6 billion immediately after its launch in 2019, Saxenda maintained its lead for five consecutive years until 2023. Saxenda's sales reached KRW 66.8 billion in 2023, accounting for 37.5% of the obesity treatment market that year. However, following the introduction of Wegovy, Saxenda's sales sharply declined, and its market share in the first quarter of this year was only 3.8%. The first-quarter sales of Alvogen Korea's Qsymia decreased by 3.9% year-on-year to KRW 8.6 billion. Launched in late 2019, Qsymia is a combination drug containing 'phentermine' and 'topiramate'. Alvogen Korea secured domestic marketing rights from U.S.-based Vivus in 2017. Alvogen Korea entered a co-promotion agreement with Chong Kun Dang in late 2019 and began full-scale sales in Korea. Qsymia recorded KRW 10.2 billion in sales in the third quarter of last year but fell to 9.3 billion KRW in the fourth quarter, when Wegovy was launched, and has further decreased this year.
- Company
- Re-evaluation possibility of 'Bylvay' gathers attention
- by Eo, Yun-Ho May 26, 2025 05:57am
- Product photo of Ipsen KoreaAttention has been drawn to when 'Bylvay Cap,' the first medicine chosen for the 'Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations,' will be re-evaluated. Bylvay (odevixibat), Ipsen Korea's treatment option for progressive familial intrahepatic cholestasis (PFIC) in patients aged 3 months or older, received a decision of reevaluation at the Health Insurance Review and Assessment Service (HIRA)'s Drug Reimbursement Evaluation Committee (DREC) meeting held in April. After that, the drug had not been considered for the DREC review in May so it will likely be considered for the upcoming review. As part of implementing the first concurrent approval-evaluation-negotiation pilot project since October, the government selected two medicines, including 'Qarziba (dinutuximab)' for treating rare disease in children and Bylvay, as the first drugs for the pilot project. The concurrent approval-evaluation-negotiation pilot project conducts the Ministry of Food and Drug Safety (MFDS)'s approval, HIRA's drug evaluation, and drug pricing simultaneously, expediting the insurance listing process, including approval, drug evaluation, and the Ministry of Health and Welfare (MOHW) reporting. However, Bylvay not being approved for reimbursement has raised questions about the validity of the expedited listing of selected drugs. Furthermore, Bylvay was challenged at the stage of setting the reimbursement criteria. It has been reported that the company poorly took the expert advice while setting the reimbursement criteria because the expert advice gathering process was reduced to merely a 'formality.' In response, the HIRA stated, "To facilitate DREC's efficient evaluation, HIRA operates a small committee for setting reimbursement criteria before a review. At the small committee meeting, HIRA was reviewed in depth by gathering advice from experts and academics regarding clinical usefulness and cost-effectiveness. It was not indeed a thorough process rather than merely a formality." It is to be watched whether Bylvay, which was selected for the concurrent approval-reimbrusement-drug price pilot project, is to be included in the reimbursement list. Meanwhile, the efficacy of Bylvay was demonstrated through the Phase 3 ASSERT study involving children and adolescent patients aged 17 years and below. The study results demonstrated that Bylvay reduced itchiness statistically significantly compared to a placebo, meeting the primary endpoint. Furthermore, Bylvay statistically improved the average serum bile acid concentration at the primary endpoint target time-points, at week 20 and week 24, than the placebo. This effect of Bylvay continued through 24 weeks of treatment.
- Policy
- Moderate-risk trial granted for 'Amtagvi' for melanoma
- by Lee, Jeong-Hwan May 26, 2025 05:56am
- The government has approved a moderate-risk clinical study to treat refractory melanoma patients using the T-cell therapy, Amtagvi. The government also approved a high-risk clinical study that administer multi-virus antigen-specific immune T-cells to pediatric and adolescent patients with resistant·refractory multi-virus infections who underwent allogeneic hematopoietic stem cell transplantation. On May 23, the Ministry of Health and Welfare (MOHW) announced these decisions as part of the resolution results of the 5th Advanced Regenerative Medical and Advanced Biopharmaceutical Review Committee. The Committee reviewed a total of four clinical study plans (two high-risk, two moderate-risk) from institutions, including Catholic University of Korea Seoul St. Mary's Hospital and Samsung Medical Center. Of these, two were approved, and two were deemed unsuitable. Approval of 'Amtagvi' clinical study for melanoma patients The approved project is a moderate-risk clinical study to evaluate the safety and efficacy of tumor-infiltrating lymphocytes (TIL) derived from the patient's own body, in refractory (intractable) melanoma patients who have failed immune checkpoint inhibitor therapy. Melanoma is a malignant tumor originating from melanin cells in the skin. Although it accounts for 1-4% of skin cancers, it has the highest mortality rate. Tumor-infiltrating lymphocytes (TIL) are lymphocytes present in the tumor microenvironment that recognize and destroy tumor cells. They have a high potential to overcome tumor heterogeneity and tumor cell immune evasion, leading to tumor cell death. The TIL therapy 'Amtagvi' is the world's first approved T-cell therapy for solid tumors. Clinical trials of TIL therapy are currently underway for various solid tumors both domestically and internationally. This study aims to evaluate safety by confirming adverse reactions and to assess efficacy by checking objective response rates and progression-free survival, following the administration of TILs manufactured using a domestically developed method. This is to provide a new treatment option using TIL for domestic melanoma patients who have failed most existing treatments. Approved for T-cell therapy in multi-virus infected patients The study was a high-risk clinical study that involved administering multi-virus antigen-specific immune T-cells (VST) to pediatric and adolescent patients who have undergone allogeneic hematopoietic stem cell transplantation and show resistance· refractoriness to standard treatment for multi-virus infections or related infectious diseases. Graft-versus-host disease (GVHD), which primarily occurs during allogeneic hematopoietic stem cell transplantation, is a major severe complication where donor immune cells attack the patient's tissues. To manage this, strong immunosuppressants are used, and as a side effect, the patient's overall immunity is weakened, making them susceptible to infections. This leads to the reactivation of latent viruses within the patient's body, causing infections. Existing antiviral drugs find it difficult to suppress multiple viruses simultaneously, can lead to resistance with long-term use, and have high kidney·liver toxicity. This study aims to simultaneously suppress multiple viruses using VSTs and prevent long-term recurrence through immune memory. The director of the Minister of Food and Drug Safety (MFDS) informed the committee that the research data submitted by the researchers were valid through a rapid and combined review of the high-risk clinical study. Following the procedure, regenerative clinics will conduct the clinical study after receiving approval notification from the MFDS. Kim Woo-ki, Secretary-General of the Advanced Regenerative Medical and Advanced Biopharmaceutical Review Committee, explained, "Based on a re-deliberation decision regarding clinical study plans that required adjustments, the committee granted final approval so researchers can submit supplements."
- Policy
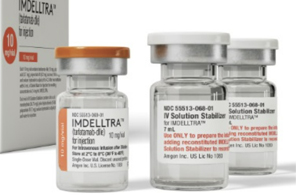
- Amgen’s SCLC drug tarlatamab soon to be approved in KOR
- by Lee, Hye-Kyung May 26, 2025 05:55am
- The approval of Amgen's new drug for small cell lung cancer, tarlatamab (U.S. brand name Imdelltra), is imminent in Korea. According to industry sources on the 23rd, the Ministry of Food and Drug Safety has completed its safety and efficacy review for tarlatamab. The MFDS's completion of the review means a new drug approval will follow soon. Tarlatamab was designated as a “GIFT” item by the MFDS in January last year and underwent a fast-track review upon the submission of the new drug application. In the United States, the FDA designated tarlatamab as breakthrough therapy in October 2023 and granted accelerated approval on May 16, 2024, for the treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC) whose disease has progressed during or after platinum-based chemotherapy. Tarlatamab is a bispecific antibody that recognizes antigens in both tumor cells and T cells (immune cells). This allows for the drug to induce T cells to attack tumor cells even when the tumor cells try to avoid them. This drug demonstrated efficacy in the Phase II DeLLphi-301 trial. The trial evaluated the efficacy of tarlatamab in patients with SCLC who had failed two or more prior lines of treatment. The patients that enrolled in the trial received tarlatamab 10 mg every two weeks. The team recruited and randomized 220 patients who had failed first-line treatment for small cell lung cancer at 56 centers in 17 countries around the world, to find a new treatment strategy that would maximize the effectiveness of tarlatamab, which is currently under development while maintaining patient safety. Results showed that the group of subjects who received tarlatamab 100 mg every 2 weeks achieved an objective response rate of 40%, with a median response duration of 9.7 months. The median overall survival was 14.3 months. Currently, available treatments for small cell lung cancer in Korea include the immunotherapy drug 'Imfinzi (durvalumab)' and the chemotherapy drug ‘Zepzelca (lurbinectedin).'
- InterView
- ‘SGLT2i+TZD promising for diabetics with fatty liver risk’
- by Kim, Jin-Gu May 26, 2025 05:55am
- A large-scale epidemiological study conducted on a Korean population has found that metabolic dysfunction-associated steatotic liver disease (MASLD), which commonly accompanies diabetes, increases the risk of cardiovascular disease and mortality. MASLD is not merely a liver disease but a cause that exacerbates overall metabolic disorders, making it a focus of recent attention. As a result, proactive treatment strategies to address it early are gaining prominence. Professor Cheol-Young Park of the Endocrinology Department at Gangnam Samsung Hospital said, “Combining SGLT-2 inhibitors and TZD-class diabetes medications early on could be a promising treatment option for diabetes patients with MASLD. This combination therapy not only improves blood sugar control but also regulates lipid metabolism and insulin resistance, while also mitigating the side effects of each medication.” A follow-up study on 500,000 diabetes patients revealed that those with MASLD had a 1.2-fold increased risk of death. Professor Cheol-Young Park, along with Professor Kyung Soo Kim from Bundang Cha Hospital, Professor Sangmo Hong of Hanyang University Guri Hospital, and Professor Kyung-do Han of Soongsil University, conducted a large-scale epidemiological study to examine the correlation between MASLD, cardiovascular disease, and mortality in patients with Type 2 diabetes. MASLD is a condition previously known as non-alcoholic fatty liver disease (NAFLD). It refers to the accumulation of fat in the liver in the presence of metabolic abnormalities (such as diabetes, hyperlipidemia, and obesity), regardless of alcohol consumption. It is not merely a liver disease but is considered a manifestation of systemic metabolic issues and is associated with cardiovascular disease, kidney disease, and certain cancers. Professor Cheol-Young Park’s team conducted a follow-up study on 500,000 patients with type 2 diabetes among 7.7 million participants in the National Health Insurance Service's health screening program in 2009. The results showed that patients with diabetes and MASLD had a 1.37 times higher risk of developing major cardiovascular diseases such as myocardial infarction and ischemic stroke. The risk of death from all causes also increased significantly by 1.21 times. This trend was consistently observed even in those with mild NAFLD (grade 1). In patients with NAFLD but without diabetes, the absolute risk of developing cardiovascular disease within 5 years was 1.23% to 1.42%. In contrast, patients with NAFLD and diabetes had a significantly higher absolute risk of developing cardiovascular disease within five years, ranging from 3.34% to 4.66%. The risk of death also showed a similar trend. Professor Cheol-Young Park explained, “This study shows that MASLD does not simply affect liver health, but also has a significant impact on overall cardiovascular outcomes. In particular, the results showing that patients with diabetes and NAFLD have a higher risk of cardiovascular disease and death highlight the importance of treating both conditions together.” “SGLT2i+TZD combination, a promising option for treating MASLD in patients with diabetes” Regarding these complex metabolic disorders, Professor Park proposed the SGLT-2 inhibitors and TZD drug combination as a promising treatment strategy. The two drugs have complementary effects in alleviating insulin resistance and reducing fat accumulation in the liver through different mechanisms. TZD class drugs promote the differentiation of subcutaneous adipocytes, redistribute fat from visceral depots, suppress hepatic glucose production, and enhance glucose uptake in muscle and adipose tissue, thereby improving insulin sensitivity. SGLT-2 inhibitors induce energy loss by excreting glucose through the kidneys, thereby indirectly promoting fat oxidation and reducing liver fat. Professor Park explained, “TZDs redistribute fat outside the liver to reduce fat accumulation within the liver, while SGLT-2 inhibitors reduce blood glucose levels and encourage the use of fat as an energy source. When used in combination, they regulate the metabolic environment through different pathways, producing a synergistic effect in improving MASLD.” Furthermore, Professor Park emphasized that the combination of the two drugs has the potential to offset each drug’s side effects. TZD-class drugs are associated with side effects such as weight gain and edema. In this context, the weight-reducing and diuretic effects of SGLT-2 inhibitors can partially offset these side effects. Additionally, by fundamentally improving insulin resistance, they reduce the burden on insulin-secreting cells (β-cells), thereby alleviating hyperinsulinemia and potentially contributing to reduced insulin dependence in the long term. Professor Park added, “Combination therapy is an integrated strategy that can regulate not only blood sugar levels but also lipid metabolism, insulin resistance, weight, and lipid status. It is necessary to consider this treatment approach early on for patients with both diabetes and MASLD. “MASLD, a key risk factor for cardiovascular disease… Early use of combination therapy required to improve prognosis” Professor Park explained that considering the high prevalence of MASLD in Korea, early combination therapy is important for patients with both diabetes and MASLD. He emphasized that since MASLD is not merely an indicator of liver health but also increases the risk of metabolic disorders and cardiovascular disease, an integrated management approach is required from early on. MASLD is deeply associated with various metabolic abnormalities such as insulin resistance, dyslipidemia, visceral obesity, and hypertension. These metabolic abnormalities interact synergistically to negatively impact the cardiovascular system. For example, insulin resistance accelerates fat accumulation in the liver while also causing dyslipidemia through increased triglycerides, reduced HDL cholesterol, and changes in LDL cholesterol particle size. Visceral obesity increases systemic inflammation by secreting inflammatory cytokines from fat cells, while hypertension increases atherosclerosis and cardiac burden, making it a direct risk factor for cardiovascular disease. Professor Park stressed, “The factors do not exist independently. When one worsens, it has a cascading effect on the others. MASLD originates at the core of this metabolic imbalance and should be recognized not just as a simple liver disease but as the starting point of a systemic disease.” Professor Park noted, “For patients with MASLD accompanying diabetes, strategies that not only control blood sugar but also reduce liver fat accumulation and improve the overall metabolic environment should be used. The combination of SGLT-2 inhibitors and TZDs could be an effective approach that can simultaneously target these complex goals.”
- Company
- Pfizer’s Lorviqua is granted reimbursement in Korea
- by Whang, byung-woo May 26, 2025 05:54am
- Lorviqua (lorlatinib), a treatment for ALK-mutated non-small cell lung cancer (NSCLC), has been approved for reimbursement as a first-line treatment, heralding a tectonic shift in the field. Experts saw this as a positive development in addressing unmet patient needs and improving access to treatment. In this sense, the presence of third-generation treatments for ALK-positive metastatic non-small cell lung cancer is expected to grow further. On the 21st, Pfizer Korea held a press conference to commemorate the expanded reimbursement of Lorviqua (lorlatinib), a first-line treatment for ALK-positive metastatic non-small cell lung cancer (NSCLC), highlighting the significance of the milestone. Ji-Youn Han, Professor of Hematology and Oncology at the National Cancer Center Lorviqua is a third-generation ALK tyrosine kinase inhibitor (TKI) designed to be effective against ALK mutations and to effectively penetrate the blood-brain barrier (BBB). In May 2022, it was granted reimbursement as a first-line treatment, 3 years after the indication was expanded to include ALK-positive metastatic non-small cell lung cancer as a first-line treatment. Patients with ALK-positive non-small cell lung cancer account for over 80% of all lung cancer cases and are characterized by relatively young age and a history of minimal or no smoking. Lorviqua’s efficacy as a first-line treatment was confirmed in the global Phase III CROWN trial. According to the 5-year follow-up results of the global Phase III CROWN clinical trial, Lorviqua demonstrated an 81% reduction in the risk of disease progression or death compared to the crizotinib group in patients with no prior treatment experience. Also, the median progression-free survival (PFS) for Lorviqua was not reached at 60.2 months of follow-up, regardless of brain metastasis status, while the median PFS for crizotinib was 9.1 months at 55.1 months of follow-up. According to a 5-year analysis of the CROWN trial, this is the longest progression-free survival rate achieved among ALK-positive non-small cell lung cancer treatments to date. Ji-Youn Han, Professor of Hematology and Oncology at the National Cancer Center who presented at the event, said, “Among non-small cell lung cancer, which accounts for the majority of lung cancer cases, treatment for ALK-positive non-small cell lung cancer, a major genetic mutation, has progressed from the first-generation crizotinib to the third-generation Lorviqua. According to the five-year follow-up analysis of the CROWN study, this is the treatment with the longest median progression-free survival (mPFS) among ALK-positive NSCLC treatments to date.” Han added, “There are studies showing that 25-30% of patients with ALK-positive metastatic non-small cell lung cancer do not receive later-line treatment and that the main cause is rapid clinical deterioration due to tumor progression. The reimbursement of Lorviqua as a first-line treatment is significant in that it addresses unmet needs and greatly improves access to treatment.” Furthermore, the efficacy and safety profile of Lorviqua was consistently demonstrated in studies involving patients in Asia, including Korea. In a study of Asian patients, after 5 years of follow-up, the median PFS for Lorviqua was not reached, while the median PFS for crizotinib was 9.2 months. Furthermore, Lorviqua demonstrated a 99% reduction in the risk of disease progression and death compared to crizotinib at the 5-year mark. However, some have expressed concerns about the risk of central nervous system side effects associated with Lorviqua. One percent of patients who experienced cognitive impairment as an adverse reaction after receiving Lorviqua discontinued treatment. Nevertheless, given that Lorviqua demonstrates superior efficacy compared to existing treatments, Han believes it can become the preemptive choice as a first-line treatment. He stated, “Based on the data, Lorviqua shows positive results in patients with over 5 years of follow-up, so there seems to be no room for second-generation treatments. While there may be cases where the drug is replaced due to CNS side effects, using Lorviqua, which maintains PFS for over 5 years, as a backup and using second-generation treatment first is something patients would not agree to.” In fact, Lorviqua was included in the 2025 National Comprehensive Cancer Network (NCCN) guidelines (recommendation level: Category 1), the 2024 American Society of Clinical Oncology (ASCO, recommendation level: Strong), and the 2023 European Society for Medical Oncology (ESMO, recommendation level: Tier I-A) guidelines as one of the first-line treatments for ALK-positive metastatic non-small cell lung cancer.
- Company
- Accomplishments of the approval-drug price pilot project
- by Eo, Yun-Ho May 23, 2025 05:52am
- Winrevair·Fintepla·Limcato expected to be commercialized With the drugs that were reviewed for approval and drug prices simultaneously about to be commercialized, the industry gathers attention. The Ministry of Health and Welfare (MOHW) has been running the 'Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations' since 2023 to improve treatment access for life-threatening severe·rare diseases. The project conducts approval, reimbursement evaluation, and drug price negotiations simultaneously, aiming to shorten the time required for new drugs to be included in the National Health Insurance list. The first pilot project is at the final stage, showing accomplishments. Three drugs were selected in the second pilot project, which recently completed the selection of items. Typically, it takes approximately 330 days for a new drug to be introduced into the market. This pilot program aims to significantly reduce the time for ▲Product Approval (Ministry of Food and Drug Safety, MFDS) for 120 days ▲DrugReimbursement Evaluation (Health Insurance Review & Assessment Service, HIRA) for 150 days ▲ Drug Price Negotiation (National Health Insurance Service, NHIS) for 60 days. The two drugs selected for the first pilot program (2023) are either already on the reimbursement list or nearing their evaluation process's completion. Following this, ten drugs were submitted for the second pilot program, launched in 2024, and three new drugs were selected. These three are 'Winrevair (sotatercept),' a pulmonary hypertension treatment from MSD Korea; 'Fintepla (fenfluramine),' a Dravet syndrome treatment from UCB Pharma Korea; and 'Limcato,' a large B-cell lymphoma treatment from the Korea-based company Curocell. All three drugs are currently expected to be commercialized in Korea this year. Among them, Winrevair is receiving particular attention. This drug is a first-in-class innovative new drug with a novel mechanism, marking its appearance 20 years after 'Sildenafil', which targeted the NO-sGC-cGMP pathway, in 2005. As of 2023, the number of pulmonary hypertension patients in Korea is approximately 3,600. The average age of these patients is in their 40s, a demographic that plays a crucial role in society and family. Although the 5-year survival rate has significantly improved compared to the past, 3 out of 10 Korean pulmonary hypertension patients still die within 5 years. Furthermore, most patients experience significant difficulties performing daily activities such as housework, childcare, and light outings. Pulmonary hypertension is a rare, intractable, and progressive disease, where delaying the worsening of the condition directly impacts patients' quality of life and survival. To date, no cure through drug treatment has been discovered, and the mechanism of existing drugs primarily aims to alleviate symptoms by relaxing thickened pulmonary arteries. It remains to be seen how quickly drugs undergoing the concurrent approval-evaluation process, including Winrevair, will be listed. The selection criteria for the second pilot program considered drugs that met all of the following conditions: ▲ drugs for which approval and reimbursement decisions can be applied by June 2025 ▲ drugs with sufficient efficacy intended for treating life-threatening diseases with a life expectancy of less than one year, or for rare diseases ▲drugs for which no existing treatment is available or that show clinically significant improvement over current treatments ▲drugs that have been designated for or are eligible to apply for, MFDS's Global Innovative Products on Fast Track (GIFT) program. The MOHW announced December 2024 that it had selected three drugs for the second pilot program. This selection considered factors such as disease severity, availability of alternative drugs, urgency, treatment efficacy, and expert opinions from the submitted applications.
- Company
- CKD-Novartis new drug advances to the next phase trial
- by Chon, Seung-Hyun May 23, 2025 05:52am
- Chong Kun Dang (CKD)'s new drug candidate out-licensed to Novartis is entering the next clinical stage. CKD has secured its first milestone payment of KRW 7 billion, 1 year and 6 months after the technology export contract. CKD announced on May 22 that it expects to receive a milestone payment of USD 5 million (KRW 7 billion) from Novartis, upon the achievement of a stage-based milestone for CKD-510. As Novartis submitted its Investigational New Drug (IND) application for a Phase 2 clinical trial of CKD-510 to the U.S. Food and Drug Administration (FDA), the condition for the milestone payment, as per the contract, was met. CKD is set to receive an additional milestone payment for the first time since the CKD-510 technology export. CKD-510 is a new drug candidate that CKD out-licensed to Novartis in November 2023. It was a blockbuster technology export agreement with a non-refundable upfront payment of USD 80 million. The maximum milestone payment, contingent on development and approval stages, reaches up to USD 1.225 billion. CKD-510 is a new drug candidate researched and developed by CKD. It is an HDAC6 inhibitor developed using a highly selective non-hydroxamic acid (NHA) platform technology. In preclinical studies, the efficacy of the candidate was confirmed in various HDAC6-related diseases, including cardiovascular diseases. Its safety and tolerability were demonstrated in Phase 1 clinical trials conducted in Europe and the United States. CKD has completed the European Phase 1 trial of CKD-510 for Charcot-Marie-Tooth (CMT) disease. CMT is a hereditary peripheral neuropathy where motor and sensory nerves are damaged due to gene mutations, making normal walking and daily life difficult. It is a rare disease, but there is currently no definitive treatment. CKD has strategized to derive optimal drugs for various diseases based on the fundamental structure of HDAC6 inhibitors. CKD is developing new drugs applying its HDAC6 platform technology to CMT disease, Huntington's disease, Alzheimer's disease, hematologic cancers, and autoimmune diseases. Novartis enters a Phase 2 clinical trial for the first time since in-licensing CKD-510. However, Novartis has not disclosed the target indication for CKD-510.
- Policy
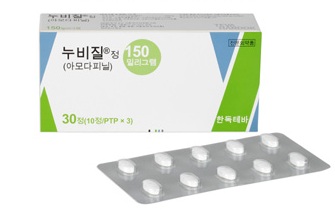
- Myung In’s Nuvigil first generic listed for reimb in Korea
- by Lee, Tak-Sun May 23, 2025 05:52am
- Myung In Pharm’s first generic version of the narcolepsy drug Nuvigil (amodapine) is expected to be sold in earnest following its reimbursement listing in June. Currently, narcolepsy treatments are primarily composed of two ingredients: modafinil and amodafinil. With Mitsubishi Tanabe Pharma's ' 'Wakix (pitolisant)' recently withdrawing from the Korean market, fierce competition among existing treatments is anticipated. Currently, modafinil and armodafinil are primarily used for the treatment of narcolepsy. With Mitsubishi Tanabe Pharma Korea's recent withdrawal of 'Wakix (pitolisant)' from the Korean market, the competition among existing treatments is expected to intensify further. Amidst this situation, Myung In Pharm lowered the price of its generic product at a price below the calculated price and announced plans for aggressive market entry. According to industry sources on the 22nd, two dosages (150 mg and 250 mg) of its Amonil Tab, the first generic version of Nuvigil, will be listed for reimbursement on June 1. This drug is the first generic version of armodafinil, the active enantiomer of Provigil (modafinil). It works by activating dopamine in the brain to promote wakefulness and is characterized by its improved duration of action compared to the original drug. The original amodafinil drug is Teva-Handok’s Nuvigil. Nuvigil was approved for reimbursement in Korea in June 2018 and is competing with existing modafinil preparations. Last year, the amount of outpatient prescriptions for the drug amounted to KRW 1.1 billion, according to UBIST. Narcolepsy is a neurological disorder characterized by excessive daytime sleepiness, hallucinations when falling asleep or waking up, sleep paralysis, and sleep attacks. It is designated as a rare and intractable disease in Korea due to the low number of patients. The prevalence rate in Korea is estimated to be 0.002–0.18%, and it primarily occurs during adolescence or early adulthood before the age of 30. Due to the small number of patients, there are few medications available. For modafinil, there are two products: JW Pharmaceutical's Provigil Tab 200mg and Hanmi Pharmaceutical's Modanil Tab 200mg. For amodafinil, there are only two brands: Nuvigil Tab and the newly approved Amonil Tab. Even Wakix, a new drug that was approved in 2021 and listed for reimbursement that same year, withdrew from the Korean market last year due to the small number of patients and low drug prices. From the perspective of pharmaceutical companies, the domestic market for narcolepsy treatments is not so attractive. Nevertheless, Myung In Pharm is showing enthusiasm, lowering its drug’s price during reimbursement listing. Amonil 150mg is priced at KRW 1,206 per tablet, which is about 59% of the price of the original Nubizil 150mg at KRW 2,027. The price of Amonil 250mg is also significantly cheaper at KRW 1,766, compared to Nubizil 250mg at KRW 2,968. Amonil Tab is used for excessive daytime sleepiness associated with adult narcolepsy, with a recommended dosage of 150 mg once daily in the morning. The maximum daily dose is 250 mg. Therefore, attention is now focused on whether Myung In Pharm, which has been making notable strides in the central nervous system treatment area, will also establish a strong presence in the narcolepsy treatment market with its first generic version of Nuvigil.
- Company
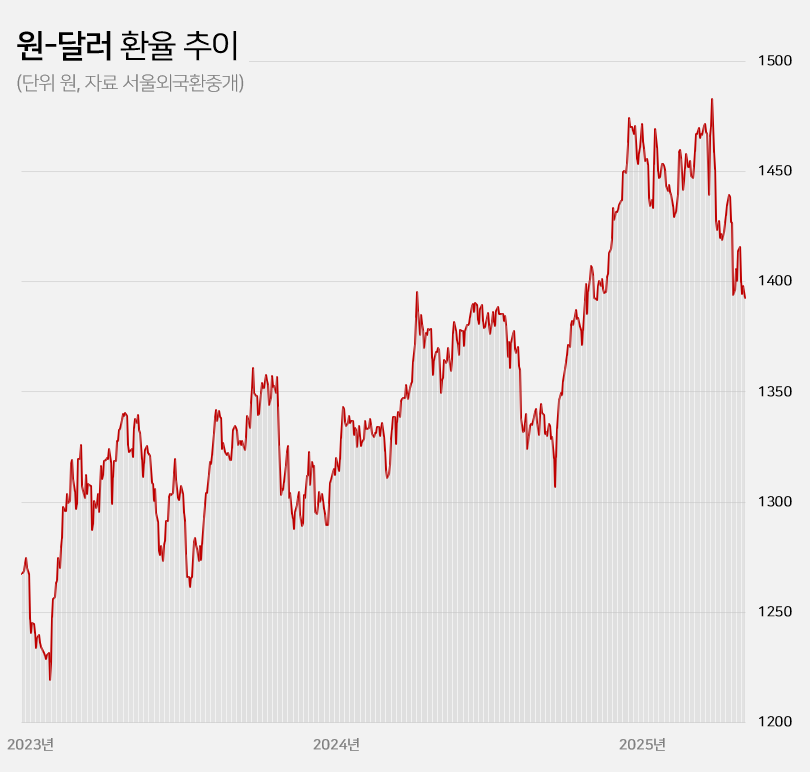
- Won-dollar rate lowest in 6mths... industry mixed
- by Kim, Jin-Gu May 23, 2025 05:51am
- With the won-dollar exchange rate falling below KRW 1,400, pharmaceutical and biotech companies are experiencing a mix of anticipation and concern. If the prolonged high exchange rate returns to previous levels, API imports and overseas clinical trial costs are expected to decrease, leading to an improvement in the cost structure. On the other hand, some predict that the asset value of pharmaceutical and biotech companies with a high proportion of drug exports will decline. Won-dollar exchange rate falls below KRW 1,400... Will the burden of API purchases and overseas clinical trials decrease? According to industry sources on the 22nd, the won-dollar exchange rate closed at KRW 1,392.60 on the 21st, down KRW 5.40 from the previous trading day. This is a 6.1% (KRW 90.30) decrease from the KRW 1,482.90 on the 10th of last month when concerns over the U.S.’s mutual tariffs were at their peak. The won-dollar exchange rate has remained high for a long time since entering the KRW 1,300 range in March 2023. It rose further to over KRW 1,350 from the middle of last year and broke through KRW 1,400 after the martial law crisis at the end of last year. With mutual tariffs by the US adding to the concern, the exchange rate soared to exceed KRW 1,480 at the beginning of last month. However, the won-dollar exchange rate has been falling this month amid expectations that trade tensions between the US and China will ease. In particular, the exchange rate has remained in the KRW 1,300 range for 4 consecutive trading days since the 16th, raising expectations that the prolonged high exchange rate trend will return to normal levels. KRW to USD exchange rate The pharmaceutical industry is hopeful that the decline in the won-dollar exchange rate will continue, leading to an improvement in the cost structure. Over the past 2 years, the rise in the won-dollar exchange rate has had a negative impact on the cost structure of domestic pharmaceutical and biotechnology companies. Due to their high dependence on imported APIs, the exchange rate increase directly led to higher manufacturing costs. Additionally, since these companies purchase APIs from China and India, with a high import dependency, they were significantly affected by the rise in the won-dollar exchange rate. As of 2023, the self-sufficiency rate for domestically produced APIs for pharmaceuticals stands at 25.4%. Among these, imports of APIs from China account for 30.5% of the total, while those from India make up 15.2%. Together, these two countries account for nearly 50% of the total API imports. Although manufacturing costs rose due to the increase in the won-dollar exchange rate, unlike other consumer goods, the price of finished drugs cannot be arbitrarily set by companies. As a result, the deterioration of the cost structure in the pharmaceutical industry has become more pronounced over the past 2 years. Additionally, the burden of clinical trial costs conducted overseas has steadily increased during the prolonged high exchange rate environment. Most clinical trials targeting the US and European markets are conducted locally. When the won-dollar exchange rate rises, global clinical trial costs also increase accordingly. In this situation, there are expectations that a decline in the won-dollar exchange rate will reduce the cost of importing APIs and the burden of global clinical trials, ultimately improving cost burdens. In the long term, improvements in the cost structure are expected to contribute positively to performance recovery. Asset value decline is inevitable for companies with high export ratios... Samsung Biologics to lose KRW 91.6 billion if exchange rate falls by 10% However, companies with high export ratios, such as Samsung Biologics and Celltrion, are expected to see their asset values decline due to the decline in the won-dollar exchange rate. These companies earn a large amount of assets in dollars overseas, so when the exchange rate rises, their asset values increase. Conversely, when the exchange rate falls, their asset values decline. Samsung Biologics had an overseas sales ratio of 96.5% in the first quarter. Of the KRW 1.2983 trillion in sales rendered in the first quarter, KRW 1.2528 trillion came from overseas. As the proportion of overseas sales is high, its performance is expected to be greatly affected by fluctuations in the exchange rate. Samsung Biologics explained in its quarterly report that a 10% increase or decrease in the won-dollar exchange rate would result in a KRW 91.6 billion increase or decrease in pre-tax income. Considering how the current won-dollar exchange rate has fallen by 1.5% compared to the average exchange rate (KRW 1,452.66) at the time of the quarterly report, the recent decline in the exchange rate is estimated to have reduced asset value by approximately KRW 14 billion. Samsung Biologics has seen an increase in cash and cash equivalents over the past 2 years due to the impact of high exchange rates. The figures were KRW 12.2 billion in 2023 and KRW 41.6 billion last year. This was due to the average won-dollar exchange rate rising from KRW 1,291.95 in 2022 to KRW 1,307.90 in 2023 and KRW 1,363.09 in 2024. The same goes for Celltrion and SK Biopharm. Given their high proportion of overseas sales, they are relatively more affected by fluctuations in the exchange rate. Celltrion saw its cash and cash equivalents increase by KRW 52.4 billion last year due to exchange rate changes. The company explains that, assuming all other variables remain constant, an 8% change in the won-dollar exchange rate would result in a KRW 33.8 billion increase or decrease in pre-tax profit. For SK Biopharm, the change in cash and cash equivalents due to exchange rate fluctuations last year amounted to KRW 3.6 billion. Based on the exchange rate at the end of last year, a 10% change in the exchange rate would result in an increase or decrease of KRW 14.1 billion in the company's pre-tax profit. It is expected that the fluctuation in pre-tax profit due to exchange rate changes will widen further this year if sales of Xcopri increase in the United States.