- LOGIN
- MemberShip
- 2025-12-21 06:21:02
- Company
- Interest in Lotte Biologcs’ partner Ottimo Pharma rises
- by Kim, Jin-Gu Jun 20, 2025 06:05am
- Interest is growing in Ottimo Pharma, a biotech company that has signed a contract manufacturing agreement for its antibody-drug with Lotte Biologics. Founded in 2017, this UK-based biotech venture owns a new drug candidate called ‘Jankistomig’. The drug candidate bifunctional antibody targeting solid tumors, and the company plans to submit an Investigational New Drug (IND) application to the US Food and Drug Administration (FDA) within this year. Lotte Biologics announced on the 19th that it has signed an antibody-drug contract manufacturing agreement with Ottimo Pharma at the BIO INTERNATIONAL 2025 (Bio USA) event. The contract covers the production of drug substance (DS) for Ottimo Pharma's antibody drug Jankistomig at the Syracuse Bio Campus in New York. Ottimo Pharma was founded in 2017 in Kent, England, under the name Ultrahuman Eight Limited. In October last year, the company changed its name to Ottimo Pharma. At that time, Medicxi Ventures UK, a UK-based life science venture capital, participated as an initial investor. Medicxi led Ottimo Pharma's Series A investment round. Ottimo Pharma successfully secured USD 140 million (approximately KRW 190 billion) in Series A investment in December last year. To date, Ottimo Pharma’s only pipeline is Jankistomig. This candidate drug works by simultaneously inhibiting PD-1 and VEGFR2. The drug candidate was known to be designed based on camrelizumab, developed by China's Jiangsu Hengrui Pharmaceuticals. It is designed to reduce VEGF-related side effects while providing immune checkpoint inhibition effects. Jankistomig is in the preclinical stage and is being tested in the UK for solid tumors. There is no clinical trial number registered on ClinicalTrials.gov, a clinical trial registration site. This suggests that no official clinical trials have been initiated in any country, including the US and the UK. The company announced last year that it had secured Series A investment and would submit an Investigational New Drug (IND) application to the US FDA by the end of this year. At the time, Ottimo mentioned its development of other pipelines in addition to Jankistomig, but did not disclose specific substance names or stages.
- Company
- New pneumococcal vaccine expected to be launched
- by Whang, byung-woo Jun 19, 2025 06:04am
- As 'Capvaxive,' a 21-valent pneumococcal conjugate vaccine (PCV21) developed by MSD, is anticipated to receive marketing authorization in South Korea, competition in the market is likely to heat up. Product photo of CapvaxiveAccording to pharmaceutical industry sources, MSD has filed with the Ministry of Food and Drug Safety (MFDS) for marketing authorization of Capvaxive. It is expected to be approved by the second half of 2025. Capvaxive is a vaccine designed to prevent adults from serotype that causes most of the invasive pneumococcal disease (IPD). The safety and immunogenicity of Capvaxive were cofirnmed based on the Phase 3 STRIDE clinical trial, comparing Capvaxive to PCV20 in adults aged 18 years and above who have no prior history of pneumococcal vaccination. Capvaxive was found to be nonequivalent to PCV20 regarding 10 serotypes (3, 6A, 7F, 8, 10A, 11A, 12F, 19A, 22F, 33F) that are commonly included in PCV20. 10 out of 11 serotypes (9N, 15A, 16F, 17F, 20A, 23A, 23B, 24F, 31, 35B) that are included in Capvaxive but not in PCV20 were demonstrated to be superior to PCV20. Capvaxive was approved in the United States in June 2024 based on these study results, and it also obtained European approval in March. There is growing attention on whether Capvaxive will obtain Korean approval during the second half of this year, as it will be the third consecutive year a new pneumococcal vaccine is approved in South Korea. In late 2023, MSD's 15-valent vaccine, 'Vaxneuvance,' was expedited for inclusion in the pediatric National Immunization Program (NIP). Then, a year later, in October 2024, Pfizer's 20-valent vaccine, Prevenar 20, won MFDS approval. As the 21-valent vaccine, which is the higher serotype vaccine, is expected to be introduced in less than a year, competition is likely to get intense. If there are no setbacks to the approval process for Capvaxive, it is expected to be launched at the very end of the first half of next year. For instance, Vaxneuvance was launched in late April, and Prevenar 20 was launched in June exclusively for adults aged 18 years and above. The market is also highly likely to be competitive, with Vaxneuvance and Prevenar 20 competing for the pediatric NIP and Prevenar 20 and Capvaxive competing for the adult NIP. Regarding this, MSD Korea is expected to employ a marketing strategy that differentiates its vaccine portfolio for pediatric (15-valent) and adult (21-valent) populations. Indeed, MSD previously announced the use of tailored strategies for pediatric and adult populations during its Vaxneuvance launch 1st-anniversary media seminar. In the long term, Pfizer's Prevenar 20, with its first-mover advantage, will be competing directly with MSD's 21-valent Capvaxive, which includes a higher number of serotypes. Capvaxive will reportedly be preventing approximately 84-85% of adult IPD. This estimate is higher than the coverage for Prevenar 20. In this case, Pfizer is expected to defend its position by highlighting the performance and extensive clinical experience of Prevenar 20. Currently, Pfizer emphasizes that Prevenar 20 offers safety and convenience based on the well-established technology of Prevenar 13, validated through long-term pediatric and adult vaccinations, and with its 20-serotype coverage. Additionally, potential competition against Sanofi-SK bioscience is another variable. Although their commercialization timeline is the latest, if they succeed in developing a 21-valent vaccine, another equally strong competitor will emerge. Notably, the Sanofi vaccine is being developed for pediatric use, suggesting that the company will employ a future strategy to cover all age groups, from infants and young children to adults.
- Company
- K-Bios face string of clinical failures in H1
- by Son, Hyung Min Jun 19, 2025 06:04am
- A series of clinical trial failures for new drug candidates under development by Korean biotech companies in the first half of the year have raised concerns about the feasibility of future technology exports. Orum Therapeutics halted a clinical trial due to safety concerns, while Genexine and Bridge Biotherapeutics both failed to demonstrate statistical significance in their respective Phase II trials for glioblastoma and idiopathic pulmonary fibrosis. Stem cell therapy developers such as Anterogen and SCM LifeScience are also struggling to prove efficacy in clinical settings. According to industry sources on the 19th, Orum Therapeutics recently suspended its Phase 1 trial of ORM-5029. ORM-5029 was the company’s only pipeline in clinical trials targeting human epidermal growth factor receptor 2 (HER2), a major biomarker for solid tumors. The company received IND approval for ORM-5029 from the U.S. FDA in 2022. However, a severe adverse event (sAE) occurred during the trial, upon which the company reported it to the FDA. Due to toxicity issues, administration had to be halted even at low doses. ORM-5029 is a Degrader Antibody Conjugate (DAC) candidate. DACs combine Targeted Protein Degradation (TPD) mechanisms with Antibody Drug Conjugates (ADCs) and are expected to offer higher safety due to the use of TPD, which are small-molecule degraders. Orum emphasized that the sAE was limited to only the ORM-5029 substance and that there were no issues with the company's technology or platform itself. Orum plans to focus its resources on its blood cancer candidate ORM-1153, which also utilizes the company’s DAC platform. The company explained that it has shown strong GSPT1 degradation and robust anti-proliferative effects in blood cancer cell lines. Genexine and Bridge Biotherapeutics fail Phase II trials Genexine and Bridge Biotherapeutics both faced setbacks in Phase II trials. In March, Genexine's GX-I7 (Interleukin-7), an immune-oncology drug candidate, failed to demonstrate efficacy in glioblastoma mulifrome (GBM) patients. GX-I7 is a new drug candidate that maximizes immune anticancer effects by inducing T-cell amplification in the body. GBM is a type of glioma, a malignant tumor that originates in the brain. Despite surgery and chemotherapy, the five-year survival rate for GBM is only 5%, with an average survival time of about a year. The Phase II trial for GX-I7 enrolled 20 patients with recurrent or progressive glioblastoma, and evaluated a combination of the GX-I7 and bevacizumab (Avastin), a VEGF inhibitor used as a targeted therapy. Bevacizumab inhibits angiogenesis to prevent tumor growth, and its combination with existing anticancer drugs was expected to enhance therapeutic efficacy. However, no significant improvement was observed in the primary endpoints of progression-free survival (PFS) and overall survival (OS). Meanwhile, Bridge Biotherapeutics announced in April that its top-line data analysis results showed that its idiopathic pulmonary fibrosis (IPF) candidate BBT-877 failed to demonstrate a statistically significant improvement in the primary endpoint of forced vital capacity (FVC) change at 24 weeks. BBT-877 is an innovative novel drug candidate that selectively inhibits the novel target protein autotaxin. Autotaxin is a protein known to bind to intracellular receptors and be involved in pathological mechanisms such as fibrosis and tumorigenesis. BridgeBio previously secured global exclusive rights to BBT-877 from LegoChem Bio (now LigaChem Bio) in 2017. In May, BridgeBio received a recommendation from the IDMC to continue the clinical trial. The Phase 2 clinical trial of BBT-877 was conducted in 5 countries - South Korea, the United States, Australia, Poland, and Israel - to evaluate the efficacy, safety, and tolerability of the drug in patients with idiopathic pulmonary fibrosis (IPF). A total of 129 patients participated, and the study results showed that changes in FVC were observed in both the drug group and the placebo group; however, there was no statistically significant difference between the two groups. Bridge Biotherapeutics licensed out BBT-877 to Boehringer Ingelheim in 2019 in a deal worth up to KRW 1.5 trillion. Upon transferring BBT-877, which was in Phase 1 clinical trials, the company received approximately KRW 600 billion in upfront and milestone payments (short-term milestones). In late 2019, following the completion of Phase I clinical trials for BBT-877, BridgeBio paid approximately KRW 50 billion to LigaChem Bio as milestone revenue sharing. However, in 2020, Boehringer Ingelheim returned the rights to BBT-877 due to potential toxicity issues. BridgeBio determined that the toxicity issues were caused by high-dose drug administration in additional experiments and decided to develop the candidate on its own, but failed to demonstrate its efficacy in trials. stem cell therapy developers also struggling with commercialization Stem cell therapy developers are also facing commercialization hurdles. SCM LifeScience failed to achieve statistical significance in Phase 2 clinical trials of its stem cell therapy candidate SCM-CGH. This is the company's second failed attempt at commercialization following the failure of its acute pancreatitis clinical trial in 2022. The trial, which targeted patients with steroid-resistant or steroid-dependent chronic graft-versus-host disease, was conducted from 2017 to 2024 at 11 hospitals in South Korea, including Seoul St. Mary's Hospital. The results of the Phase II clinical trial of SCM-CGH showed no statistically significant difference in the primary efficacy endpoint, the overall response rate (ORR) at 12 weeks. Upon closer examination, the ORR at 12 weeks was higher in the placebo group than in the SCM-CGH group, and the results were not statistically significant. Anterogen failed to demonstrate the efficacy of its stem cell therapy ALLO-ASC-DFU in the U.S. Phase III clinical trial. In the trial, ALLO-ASC-DFU recorded a complete wound closure rate of 46%, which was lower than the 60% in the control group that was treated with hydrogel sheets. The therapy had garnered attention as a treatment for diabetic foot ulcers (DFU), but its failure to meet the key primary endpoint has significantly reduced the likelihood of its FDA approval. Anterogen is conducting further analyses to revise its development strategy.
- Company
- KOR-JPN jointly launches Healthcare Distribution Alliance
- by Son, Hyung Min Jun 19, 2025 06:03am
- (From the left) Jun-Jae Hyeon (CEO, Dongwon Healthcare), Jun-ho Hyun (CEO, Dongwon Pharmaceutical Wholesale), Seung-Uk Eom (CEO, Boksan Nice), and Seongwook Cho (Country Manager, Suzuken Korea) Three pharmaceutical distribution companies in Korea and Japan have joined forces to launch the Healthcare Distribution Alliance to lead the domestic market by introducing advanced overseas models. The alliance aims to go beyond simple logistics agreements – it seeks to build an innovative cooperation structure where companies can share capital and operational know-how, and combine each company's strengths to transform the pharmaceutical distribution market. Jun-Jae Hyeon (CEO, Dongwon Healthcare), Jun-ho Hyun (CEO, Dongwon Pharmaceutical Wholesale), Seung-Uk Eom (CEO, Boksan Nice), and Seongwook Cho (Country Manager, Suzuken Korea) recently met with reporters to explain the alliance's goals. Eight affiliates of Dongwon Pharmaceutical Group, Boksan Nice, and Suzuken have signed a business partnership agreement and established an organizational framework for cooperative operations at the alliance level. As part of the collaboration, the companies also entered into a capital partnership, with Suzuken acquiring a 33.6% stake in Gyeongnam Dongwon Pharmaceutical, and Boksan Nice acquiring a 3.4% stake in Gyeongnam Dongwon Pharmaceutical. This alliance goes beyond simple logistics cooperation by sharing capital and strategy direction of the companies. With the direct participation and investment of Suzuken, a major Japanese pharmaceutical distribution company, the alliance aims to pursue a long-term model that pursues the maximization of distribution productivity, supply chain stability, and function as part of a social infrastructure. CEO Seung-Uk Eom said, “We decided to pursue this alliance to survive in the rapidly changing pharmaceutical distribution industry and create growth opportunities through innovation. We aim to realize economies of scale through the alliance between Dongwon Pharmaceutical, Boksan Nice, and Suzuken and maximize productivity in the pharmaceutical distribution market while driving innovation for mutual growth.” Industry observers expect synergy from the partnership. Dongwon Pharmaceutical Group and Boksan Nice each reported over KRW 1 trillion in annual sales last year. Suzuken, one of Japan's top three pharmaceutical distributors, posted annual revenue exceeding JPY 2 trillion (approx. KRW 19 trillion) in 2023. CEO Jun-ho Hyun emphasized, “As the pharmaceutical distribution environment evolves and capital requirements grow, scaling up is no longer an option – it’s a necessity. We aim to establish a Korean-style large-scale distribution model and guide the future direction of the market.” The alliance anticipates increased distribution-related costs and volatility in the coming years. To address this, it aims to build a robust infrastructure and reduce labor dependency. Plans include exploring hospital market strategies, logistics outsourcing services, private-label (PB) healthcare products, and the potential introduction of Suzuken’s current Japanese business operations into the Korean market. CEO Seung-Uk Eom noted, “In the short term, we’ll focus on collaboration between logistics centers within the alliance, which is expected to reduce stockouts and delivery lead times through optimal inventory and shipping operations.” He added, “In the long term, we plan to build systems such as enterprise resource planning (ERP), web order systems (WOS), and customer relationship management (CRM). Given the significant time and cost required for IT system development, combining the long-standing expertise and ideas of Suzuken, Boksan Nice, and Dongwon Pharmaceutical will not only facilitate joint development but also greatly contribute to future logistics innovations such as the modernization of logistics and improvements in operational efficiency involving robots and AI. CEO Seung-Uk Eom added, “Beyond transportation management systems (TMS) and quality control standards, we will also build a foundation system for environmental, social, and governance (ESG) and seek ways to advance them.” CEO Jun-ho Hyun said, “Profit margins for pharmaceutical distributors have been steadily shrinking. Survival through sales promotion activities alone is becoming difficult. We must scale up and differentiate through cost reduction and pharmaceutical partnerships.” ”Will seek to implement Japanese-style wholesale structure in Korea" The alliance is eyeing the Japanese model, where pharmaceutical distribution is treated as a core part of national infrastructure, with government, pharma companies, wholesalers, and hospitals working in unison. Even logistics center placements are coordinated with government authorities, and disaster response systems are embedded into the distribution network. Japan’s market is dominated by major distributors like Medipal, Alfresa, and Suzuken, which fulfill roles as social infrastructure through close cooperation across the pharmaceutical supply chain. CEO Jun-jae Hyeon noted, “In Japan, systems are in place to ensure medicine continues to flow even during national disasters like earthquakes and tsunamis. We aspire to build such a socially integrated distribution system here in Korea.” Country Manager Seongwook Cho said, “, “Japan has established a virtuous cycle model that contributes to the national health insurance budget by minimizing the deterioration of medicine quality and the amount of expired medicines through infrastructure development. Although this may not be immediately achievable in South Korea, we will do our best to prepare for it." In addition, as more and more pharmaceutical companies are expected to develop new drugs such as biological agents, anticancer drugs, and orphan drugs, it is necessary to establish a system to manage and deliver these drugs. The association aims to provide one-stop services tailored to their needs. Country Manager Seongwook Cho said, “Suzuken already communicates and conducts business with many multinational pharmaceutical companies in Japan. We are aware that the companies have high standards for quality control and other global requirements. Our association’s goal is to meet the standards set by such global companies in various areas, including logistics and cold chain.” CEO Jun-jae Hyeon said, “In Japan, there are various pharmaceutical platforms, with distribution companies at the center of each. All transactions between healthcare institutions and related organizations are conducted through distribution companies. We will strive to establish a similar structure in Korea, where distribution companies play a central role in facilitating various activities.”
- Policy
- Growing role of the Korea Orphan & Essential Drug Center
- by Lee, Hye-Kyung Jun 19, 2025 06:03am
- "The emergency import of essential medicines through the Korea Orphan & Essential Drug Center (KOEDC) will be expanded, and support for pharmaceutical companies producing domestic products will be planned." President Lee Jae-myung made this pledge on his Facebook page on May 28, during his campaign for the presidency. President Lee emphasized the importance of the KOEDC, stating that he would strengthen national guarantees for the treatment of rare and intractable diseases. However, the KOEDC is a Ministry of Food and Drug Safety (MFDS)-affiliated organization with a fixed quota of only 30 personnel. KOEDC is responsible for the supply of rare and national essential medicines, establishing a stable supply base for national essential medicines, and supporting R&D. The center is currently understaffed. Kim Young-rim, CEO of Korea Orphan & Essential Drug Center (KOEDC)Kim Young-rim, CEO of KOEDC, stated at a briefing for journalists covering the MFDS on June 17, "The global supply chain has become increasingly important after COVID-19, and the role of the center is also growing." Kim added, "As a small organization with only 30 personnel, where individual roles and responsibilities are significant, it is necessary to devise ways to perform duties efficiently." In particular, the emergency import of essential medicines mentioned by President Lee is part of the national essential drug supply management plans implemented by the KOEDC. The KOEDC plays a crucial role in managing emergency imports of medicines that could be subject to potential supply shortages from overseas, domestically produced products under contract manufacturing, and emergency use authorizations for items deemed necessary to address public health crises. Kim stated, "The KOEDC is a specialized organization responsible for the stable supply of rare and essential medicines in Korea. This year, we plan to actively identify and promote tasks in line with the new government's initiatives to strengthen our role in ensuring a stable supply of rare and essential medicines." Regarding national essential medicines, strengthening on-site supply and demand monitoring, as well as emergency importation of drugs, is one of the main tasks for this year. The goal is to enhance management analysis of discontinued drugs, thereby shortening the designation period for emergency imported drugs and their subsequent domestic entry. Additionally, it aims to prioritize the classification of emergency imported drugs to facilitate appropriate inventory management. The KOEDC's role also includes planning the details for stable supply, such as identifying annual supply and demand plans for drugs requested by government ministries and expanding procurement sources. In addition to national essential medicines, the KOEDC also announced plans for rare diseases this year. Kim said, "For items that have been labeled as essential in areas where no existing treatments were available and have been designated as MFDS GIFT (Global Innovative products Fast Track) and approved, we will temporarily supply them during the domestic supply gap period before drug price negotiations, contributing to increased patient access." They are also planning expedited reimbursement applications for pediatric drugs such as 'Glucagen hypo kit,' 'Baqsimi Nasal Spray,' and 'Rapamune Syrup.' KOEDC is exploring ways to reduce patient burden by converting candidates to tax-exempt status after reviewing relevant provisions. The KOEDC has been conducting the "Research on Establishing a Domestic Stable Supply Base for National Essential Medicines" since 2022 to establish a domestic self-sufficiency base for national essential medicines in response to supply chain crises. Through Phase 1 of the project, conducted from 2022 to 2023, a domestic production base for two finished pharmaceutical products and three active pharmaceutical ingredients (APIs) has been established. Ahn Myung-soo, a division head at the KOEDC, stated, "Myung In Pharm used to import benserazide raw material from China, but we have developed it domestically and completed its DMF registration with MFDS." Ahn added, "While commercialization is a decision for the pharmaceutical company due to drug pricing issues, its significance lies in being registered in the DMF so that it can be used at any time." Furthermore, Korus Pharm's amiodarone injection received approval for export. With additional processing, a push for technology transfer, and further R&D investment, it could become commercialization-ready. Since last year, Phase 2 of the project has been underway, which includes the domestic development of both the API and finished drug for acetaminophen. Ahn added, "We have succeeded in achieving self-sufficiency through the domestic development of the API and finished pharmaceutical product for acetaminophen. A product approval application will soon be submitted to MFDS," and added, "This means that domestic self-sufficiency is possible if there are shortages, regardless of the global supply chain." In this regard, Kim stated, "There are limitations to securing a stable supply network through projects funded by the KOEDC." Kim added, "Budget and personnel support are necessary for these projects to be sustainable within the KOEDC's scope. To achieve this, we plan to work towards this continuously."
- Opinion
- [Reporter's View] Telemedicine in a 5-sided tug-of-war
- by Lee, Jeong-Hwan Jun 19, 2025 06:02am
- With the election and inauguration of President Lee Jae-Myung, the Democratic Party of Korea, which successfully changed the administration, submitted a bill to revise the Medical Service Act that narrows the scope of telemedicine’s initial consultation in the current pilot program to the National Assembly. As a result, the eyes of the ruling and opposition parties, public opinion, the health and medical community, and the platform industry are all focused on the bill. Specifically, six key stakeholders — the ruling and opposition parties, the government (Ministry of Health and Welfare), telemedicine users (patients), doctors, pharmacists, and platform companies — are all closely monitoring the Democratic Party’s proposed telemedicine legislation. The biggest issue is the scope and eligibility for initial non-face-to-face medical care stipulated in the bill proposed by Representative Jeon Jin-sook of the Democratic Party of Korea. Rep. Jeon Jin-sook's bill allows minors under 18 and seniors over 65 to apply for telemedicine from the initial consultation, while adults over 18 can only apply for follow-up consultations with telemedicine. This has led to differing arguments among legislative stakeholders. This is why the legislative battle over telemedicine, which erupted once in the 21st National Assembly, is likely to be repeated in the 22nd National Assembly. Two major differences from the 21st National Assembly are that the Democratic Party of Korea, which was the opposition party at the time, has moved onto the ruling party position, and that the number of patients using telemedicine has increased rapidly due to the unrestricted pilot project. In this context, the key stakeholders in the legislation surrounding telemedicine can largely be grouped into five categories: the government, patients, doctors, pharmacists, and platform companies. The National Assembly, which is responsible for reviewing the revision of the Medical Service Act, must gather all the different opinions of these five stakeholders, find common ground, and then persuade and consult with each other on points of disagreement before reaching a consensus between the ruling and opposition parties. The problem is that even before the bill was submitted to the National Assembly for review, the five stakeholders had such different positions that conflict constantly arose. First, the MOHW had to maintain the pilot program for unrestricted telemedicine while holding party-government consultations with the ruling Democratic Party of Korea on the new administration’s plan. During the Yoon Suk-yeol administration, the MOHW took the position that it would utilize the institutionalization of telemedicine as a means to resolve essential and regional medical shortages and promote the health and medical industry. However, the Lee Jae-myung administration is likely to pursue a different policy. The original bill proposed by Representative Jeon Jin-sook aims to conservatively legislate telemedicine as a supplement to face-to-face medical treatment, rather than as a means to promote the health industry. Doctors and pharmacists are both in a symbiotic and adversarial relationship concerning this legislation. While doctors and pharmacists share the same interests in minimizing the scope of telemedicine, they are busy attacking each other over the delivery of prescription drugs, with doctors in favor and pharmacists opposed. For now, doctors and pharmacists are likely to maintain a cooperative stance, opposing telemedicine and prescription drug delivery under the pretext of in-person medical care and in-person dispensing, while also agreeing that intermediary platforms must be prevented from disrupting the healthcare delivery system and pharmacy ecosystem and causing medical institutions and pharmacies to become dependent on platforms. Public opinion is divided regarding the legislation, but there is growing support for allowing telemedicine without restrictions, provided that safety is guaranteed. Patients who have experienced the gradual expansion of telemedicine since February 2020 have grown accustomed to the convenience of telemedicine. If legislation suddenly restricts access to telemedicine services they have been using, it is inevitable that there will be backlash. In particular, public opinion is likely to maintain its stance that the current scope of telemedicine pilot programs should be maintained or minimally reduced, citing reasons such as improving medical access for children and adolescents during late-night hours and enhancing the right to medical care for disabled individuals and the elderly with mobility difficulties. The platform industry is strongly advocating for the institutionalization of telemedicine through a negative list approach and the allowance of prescription drug delivery by courier in the 22nd National Assembly, as it had been during the 21st National Assembly. The argument is that telemedicine should be prohibited only in cases where specific risks have been identified and that telemedicine should be available without age restrictions in all other cases, in order to maintain the platform business that has been in business for 6 years. Ultimately, legislation on telemedicine will be reviewed by the National Assembly amid a five-way conflict of interests between the government, patients, doctors, pharmacists, and platforms. Currently, there are 3 bills (proposed by Rep. Choi Bo-yoon, Rep. Woo Jae-Joon, and Rep. Jeon Jin-sook, in order of proposal) to institutionalize telemedicine, but there is ample room for additional bills reflecting the positions of stakeholders to be proposed in the future. The Democratic Party of Korea, medical associations, and the platform industry are already at odds over the scope of telemedicine for initial consultations. In the 21st National Assembly, telemedicine bills failed to reach a consensus due to differing interests and were not passed. After that, doctors and medical students opposed the increase of 2,000 medical school enrollment quotas and took collective action, and the MOHW has been implementing unrestricted telemedicine under the pretext of alleviating the medical shortage. Some analysts say that the unconditional opposition of doctors to telemedicine and the opposition of pharmacists’ prescription drug delivery created a synergy effect, which led to the blocking of relevant bills. The 22nd National Assembly must not repeat the legislative turmoil experienced by the 21st National Assembly. The institutionalization of telemedicine is a common presidential campaign pledge of both ruling and opposition parties and an unstoppable trend. Unlimited telemedicine without legal grounds cannot be maintained. If legal loopholes are left unaddressed as they are now, it will inevitably create opportunities for illegal and irregular practices to proliferate. This is why the ruling and opposition parties must work together to minimize disagreements among stakeholders and establish legislation that reduces public inconvenience and prevents confusion. It is now time for the sharply divided stakeholders to gather in the National Assembly and engage in intense yet reasonable legislative discussions to achieve the stable domestic implementation of telemedicine.
- Company
- Takeda launches new drug 'Fruzaqla' in Korea
- by Whang, byung-woo Jun 18, 2025 10:28am
- Product photo of Takeda Pharmaceutical's Fruzaqla Takeda Pharmaceutical Korea (CEO Kwang-kyu Park) announced on June 16 that the company has officially launched its 'Fruzaqla (fruquintinib)' in South Korea. Fruzaqla is the first new treatment for metastatic colorectal cancer. This drug selectively inhibits Vascular Endothelial Growth Factor Receptor (VEGFR)-1,2, and 3. It is expected to provide a new treatment option for patients subjecting to fourth-line or later treatment who had limited treatment options previously. According to the 2024 statistics, colorectal cancer is one of the cancer types with prevalence ranking No.2 in South Korea. Approximately 20% of the patients are found to be metastatic at diagnosis. It has been reported that 50-60% of the patients who do not have metastasis during the initial diagnosis experience metastasis to other organs. In such cases, the survival rate is only 20.6%. However, treatment options for third-line treatment and above in metastatic patients are limited. Thus, many patients and doctors have voiced high demands for new treatment options that are effective and less burdening. Fruzaqla is a new treatment option for metastatic colorectal cancer that has emerged after over a decade and can be used regardless of specific biomarker status. Fruzaqla was designed to be effective by selectively inhibiting VEGFR-1, 2, and 3. It also minimizes off-targeted toxicity, thereby avoiding unnecessary targets. It has the mechanistic advantage of high-level drug exposure and continuous target inhibition. The efficacy·effectiveness of Fruzaqla has been demonstrated for adult patients with metastatic colorectal cancer who have previously been treated with a chemotherapy containing fluoropyrimidines, oxaliplatin, and irinotecan plus an anti-VEGF or anti-EGFR agent (for patients with wild-type RAS), and whose disease has progressed or who does not show tolerability following treatment with trifluridine/tipiracil and/or regorafenib. The basis of approval was the Phase 3 FRESCO-2 clinical study. The study results showed that the Fruzaqla group had a median overall survival (mOS) of 7.4 months, which was higher than the 4.8 months mOS of the placebo group, and also had a 34% lower mortality risk. Additionally, the Fruzaqla group had a median progression-free survival (mPFS) of 3.7 months, more than double the 1.8 months in the placebo group, corresponding to a 68% reduction in disease progression or death risk. Furthermore, Fruzaqla is an oral treatment that can be taken once daily with convenience without complicated mean conditions. It is expected to yield a positive impact on improving quality of life in addition to the treatment effects. Dr. Sang Cheul Oh, Korea University Guro Hospital (Korean Cancer Study Group's Colorectal Cancer Division Head), said, "Metastatic colorectal cancer, despite its high prevalence and aggressiveness, had been the key cancer type with unmet needs due to limited treatment options for fourth-line or later treatments." Dr. Oh added. "Fruzaqla works by selectively inhibiting VEGFR-1, 2, and 3, and is highly effective but reduced toxicity. It will be a significantly meaningful option for patients at later cancer stages who are undergoing fourth-line or later treatments." Kim Mi-seung, Takeda Pharmaceutical's oncology business unit, said, "Fruzaqla is an innovative new drug that can be used regardless of the specific biomarker status, emerged to the metastatic colorectal cancer treatment setting after 10 years, based on the FDA record. It is expected to solve unmet needs for a wide range of patients," and added," Takeda Pharmaceutical will continue to put efforts in providing improved treatment options for Korean patients, including those for metastatic colorectal cancer."
- Company
- Daiichi Sankyo exceeds ₩300B in sales…new drug drives
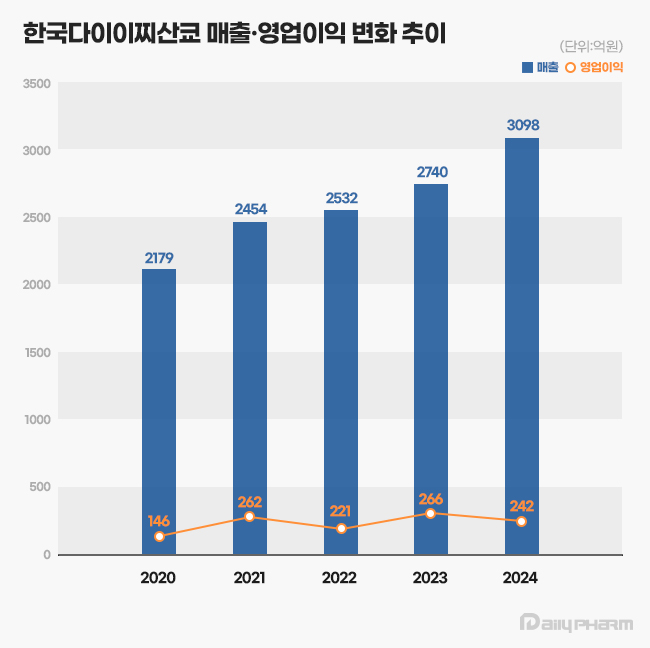
- by Son, Hyung Min Jun 18, 2025 06:01am
- Daiichi Sankyo Korea has exceeded KRW 300 billion in sales for the first time, led by its cardiovascular products, Antibody-Drug Conjugate (ADC), and new anticancer drugs. The company is successfully transitioning its portfolio towards new ADC drugs while maintaining robust growth from established cardiovascular products like Sevikar, Lixiana, and Olmetec. According to the Korea Financial Supervisory Service (FSS)'s electronic disclosure system on June 16, Daiichi Sankyo Korea's sales last year reached KRW 309.8 billion, a 13% increase from the previous year. During the same period, operating profit decreased by 9%, from KRW 26.6 billion to KRW 24.2 billion. Daiichi Sankyo Korea considers its sales for 2024 based on the Japanese fiscal year, covering April of last year to March of this year. Daiichi Sankyo Korea Daiichi Sankyo Korea's sales have been steadily increasing since 2020. The company first surpassed KRW 200 billion in revenue in 2020 with KRW 217.9 billion, followed by a continuous upward trend, reaching KRW 245.4 billion in 2021, KRW 253.2 billion in 2022, and KRW 274.0 billion in 2023. Notably, an analysis suggests that collaboration with the domestic pharmaceutical company Daewoong Pharmaceutical on some cardiovascular products, such as Lixiana and Sevikar, has created a synergistic effect. Daiichi Sankyo Korea signed co-promotion agreements for Sevikar in 2013 and Lixiana in 2015 with Daewoong Pharmaceutical, and this partnership continues to date. Among them, the highest revenue generator is the Direct Oral Anticoagulant (DOAC), Lixiana. According to market research firm UBIST, Lixiana's prescription sales last year was KRW 117.5 billion, a 12% increase compared to KRW 105.3 billion in 2023. DOACs are anticoagulants that prevent blood clots by directly acting on blood coagulation factors. They are increasingly being used in clinical settings as they replace warfarin, which inhibits Vitamin K metabolism. In Korea, Xarelto was approved in 2009, followed by Pradaxa and Eliquis in 2011, and Lixiana in 2015. Despite being the last to be launched among DOACs, Lixiana has rapidly increased its prescription performance, backed by demonstrated clinical data, and has maintained its market dominance since 2019. With annual growth of around 10%, its prescription performance nearly doubled in five years, from KRW 60.4 billion in 2019. Its market share in the overall DOAC market also expanded from 33% in 2019 to 45% last year. Sevikar, an olmesartan-based combination therapy for hypertension, continues to maintain its strong performance in prescription revenue. Sevikar's prescription revenue last year was KRW 68.8 billion, a 4% increase from the previous year. Despite numerous global and domestic pharmaceutical companies entering this market, Sevikar's prescription sales continue to grow. Sevikar's prescription revenue, which was KRW 53.4 billion in 2019, surpassed KRW 60 billion in 2022. In 2023, it recorded KRW 65.9 billion, demonstrating five consecutive years of increased prescription sales. The triple combination hypertension drug Sevikar HCT also maintained its growth trajectory. Sevikar HCT's prescription sales for the last year totaled KRW 42.1 billion, representing a 4% increase from the previous year. Daiichi Sankyo Korea generated approximately KRW 140 billion in prescription sales solely from olmesartan-based hypertension treatments, including Sevikar HCT (KRW 42.1 billion), Olmetec (KRW 30.6 billion), and Sevikar (KRW 68.8 billion). The 5 ADC Strategy...Will it achieve R&D success after Enhertu? Daiichi Sankyo Korea is working towards transitioning from a cardiovascular-focused company to a leader in oncology. The company is particularly concentrating its R&D capabilities on the ADC field, focusing on new growth engines. Following the already approved Enhertu, it is pursuing a '5 ADC strategy' and preparing for the launch of various other therapeutic agents, including Datroway, patritumab deruxtecan, DS-7300, DS-700, and DS-6000. An ADC is a new anticancer drug designed by linking an antibody that binds to a specific target antigen on the surface of cancer cells with a drug that has cell-killing capabilities using a linker. The advantage of ADCs is their ability to selectively target cancer cells by utilizing the antibody's target specificity and the drug's cytotoxic activity, thereby maximizing therapeutic efficacy while minimizing side effects. ADC anticancer agentWhile first-generation ADCs, such as Roche's Kadcyla, were initially limited to breast cancer indications, second-generation ADCs are successfully securing various other indications. Among these, Enhertu is a second-generation new ADC drug introduced by Daiichi Sankyo Korea. Enhertu is a next-generation ADC that links a monoclonal antibody with the same structure as trastuzumab (which binds to specific target receptors overexpressed on the surfaces of cancer cells) and a highly potent, novel topoisomerase I inhibitor payload via a tumor-selective, cleavable linker. Currently, Enhertu won domestic approval for HER2-positive breast cancer, gastric cancer, and non-small cell lung cancer, and is primarily used as a second-line treatment. Its potential as a first-line treatment for breast cancer is also currently being investigated. Daiichi Sankyo is also preparing to launch its second new ADC drug, Datroway. This ADC targets the Trop-2 protein and has been approved in the U.S. for the treatment of breast cancer. The Trop-2 protein is a cell membrane antigen overexpressed in breast cancer, particularly in over 90% of triple-negative breast cancer cases. Datroway binds to the Trop-2 protein and delivers cytotoxic substances into the cancer cells. It has the advantage of maximizing the benefits of targeted therapy and cytotoxic chemotherapy while minimizing damage to healthy cells. Currently, Daiichi Sankyo is co-developing and co-marketing Enhertu and Datroway with AstraZeneca. Daiichi Sankyo is also developing an ADC with Merck. patritumab deruxtecan, which targets HER3, showed efficacy in EGFR-mutated patients compared to platinum-based chemotherapy in the Phase 2 HERTHENA-Lung01 study. Daiichi Sankyo continues to conduct research for subsequent ADC candidates after Enhertu, which targets the HER2 biomarker. The company is also jointly conducting clinical studies with Merck on DS-7300, which targets B7-H3 (an emerging new biomarker in solid tumors), and DS-6000, a CDH6-targeting ADC.
- Opinion
- [Desk View] Disclosing drug reimb info must be done properly
- by Lee, Tak-Sun Jun 18, 2025 06:00am
- Due to concerns about the non-transparency of the selection process for medicinal product reimbursement, the Health Insurance Review & Assessment Service (HIRA) and the National Health Insurance Service (NHIS) are disclosing partial processes and candidates. HIRA discloses, through press releases, the review results from the Cancer Drug Reimbursement Committee, which discusses reimbursement criteria for anticancer agents, as well as review results from the Drug Reimbursement Evaluation Committee (DREC) regarding new drugs and the expanded usage scope of drugs under a risk-sharing agreement (RSA). NHIS updates the initiation of the negotiation process for drugs that have passed the HIRA review and the agreement status on its website. However, the problem is user-friendliness. Information disclosure is intended for public knowledge in the best interest of patients, yet it does not take the user's perspective into account. For instance, in February, HIRA released press reports of the results from the DREC review on the conditional appropriateness of expanded reimbursement criteria for 'Cabometyx.' According to the detailed report, "The efficacy·effectiveness of Cabometyx 20, 40, 60mg (cabozantinib, Ipsen Korea) are for clear cell renal cell carcinoma (ccRCC). Cabometyx is deemed appropriate for expanded reimbursement scope if Cabometyx's company were to accept a price below the evaluated amount." Cabometyx was initially listed for reimbursement in February 2019 as a 'monotherapy for patients with advanced renal cell carcinoma who had previously received VEGF-targeted therapy.' DREC's press release indicates that this drug, reimbursable for treating advanced renal cell carcinoma, can also be appropriate for reimbursement for treating ccRCC. However, ccRCC is the most common type of advanced renal cell carcinoma. Patients with ccRCC are already eligible for reimbursement, so expanding the reimbursement scope does not make sense. The expanded usage scope for this drug was recently reported following the NHIS's update of its website in May, which included news of ongoing negotiations. The negotiation outcome was 'failed.' However, even here, it's impossible to discern for which indication the negotiations were conducted and subsequently fell through. The NHIS only discloses the success or failure of drug negotiations in an Excel file. According to the HIRA press release, the negotiation for expanded reimbursement coverage could have been for 'ccRCC.' However, as previously mentioned, the scope for 'ccRCC' is broad, and it is already reimbursed. Ultimately, the information disclosed by both agencies makes it impossible to know for what specific indication the reimbursement scope of this drug is being expanded. The pharmaceutical company later confirmed that the drug was undergoing reimbursement expansion for 'treatment not only after VEGF-TKI-based first-line therapy but also after immunotherapy-based first-line therapy (ipilimumab+nivolumab or IO+TKI) in ccRCC.' Before the final indication was confirmed, some public even suspected that the HIRA might have mistakenly used 'clear cell renal cell carcinoma (ccRCC)' instead of 'non-clear cell renal cell carcinoma (nccRCC)' in their press release. This suspicion arose because there's a strong demand within the medical community to extend reimbursement coverage for Cabometyx to patients with nccRCC. The two agencies' unhelpful disclosure of information regarding drug reimbursement isn't limited to this instance. Recently, the NHIS announced the initiation of negotiations for Darzalex solution via a website update. However, simply looking at the website doesn't reveal the nature of these negotiations. Confusion arises because the NHIS lists all drugs, whether they are new drugs, drugs with waived price negotiations, or drugs with expanded usage scope, regardless as subjects for drug price negotiation. One can infer that Darzalex solution is undergoing negotiations for an expanded usage scope, given that this drug was mentioned in the HIRA deliberation results from a press release distributed in May, under the section for review results regarding the appropriateness of expanding the usage scope for RSA drugs. But even this doesn't clarify which indication the usage scope is being expanded for. The press release merely states its efficacy and effect as 'multiple myeloma.' Darzalex solution is a well-known drug used for multiple myeloma. Since several reimbursement criteria for multiple myeloma are already established, it remains unclear for which indication reimbursement is being expanded this time unless one directly asks a HIRA or NHIS official. The consumers of drug reimbursement information are generally patients with relevant diseases who are often in desperate situations. This type of information does not gain widespread public interest. Maybe because of this reason, HIRA's and NHIS's information disclosure is unilateral, unhelpful, and, at times, even irresponsible. It's as if they're implying, "You can figure it out with just this much information?" If information disclosure is initiated due to patient demands, then an extra degree of helpfulness should be added. If HIRA and the NHIS intend to continue providing drug reimbursement information in the future, they must do it properly. HIRA and the NHIS should accurately specify the scope of reimbursement coverage for the target diseases of the respective drugs. We are not asking them to disclose the background of their review outcomes or agreement results. We want them to at least, inform us why a particular drug is undergoing review, evaluation, or negotiation. In the future, we hope that those responsible for disclosing drug reimbursement information will adopt a more responsible approach.
- Company
- Zejula, a new standard ovarian cancer maintenance therapy
- by Whang, byung-woo Jun 18, 2025 06:00am
- Ovarian cancer is often diagnosed at an advanced stage due to the difficulty of early detection, and it is known for its high recurrence rate even after initial treatment. First-line maintenance therapy aimed at delaying recurrence as much as possible after surgery and chemotherapy became a key strategy that determines treatment outcomes for ovarian cancer. Recently introduced PARP inhibitors have emerged as a standard option for maintenance therapy, and the use of biomarkers to guide patient selection has been a major advancement, enabling better prediction of which patient subgroups are likely to benefit the most. In an interview with Dailypharm, Professor Jae Kwan Lee of the Department of Obstetrics and Gynecology at Korea University Guro Hospital, and Dr. Bradley Monk of the Florida Cancer Specialists & Research Institute stressed the need for institutional support for personalized treatment of ovarian cancer. Long-term efficacy of Zejula as first-line maintenance therapy for ovarian cancer proven Ovarian cancer is difficult to diagnose at an early stage and often recurs after initial treatment, raising the importance of maintenance therapy. This is why first-line maintenance therapy to delay recurrence as much as possible after surgery and chemotherapy has become a key strategy in ovarian cancer treatment. Professor Lee said, "First-line maintenance therapy is becoming a critical turning point in ovarian cancer treatment. HRd (homologous recombination deficiency)-positive patients showed an average progression-free survival period extension of approximately 2 years when receiving first-line maintenance therapy after surgery. Given the high recurrence rate of ovarian cancer, maintaining remission for as long as possible is key to successful outcomes, and first-line maintenance therapy serves as a highly effective strategy in this regard." Jae Kwan Lee, Professor of Obstetrics and Gynecology, Korea University Guro Hospital (President, Korean Society of Gynecologic Oncology)One of the changes in the domestic treatment environment for ovarian cancer came with the expansion of reimbursement criteria for the PARP inhibitor Zejula (niraparib) to HRd-positive ovarian cancer in October last year. The reimbursement extension of the PARP inhibitor Zejula was significant because of its biomarker. Approximately 50% of all ovarian cancer patients are HRd-positive, and about half of them, or 25%, have BRCA gene mutations. In addition, studies continue to demonstrate the efficacy of PARP inhibitors in HRd-positive patients. Professor Lee said, “In the past, reimbursement was limited to patients with BRCA mutations, so HRd-positive patients who were BRCA-negative could not choose to use Zejula due to the financial burden. However, since the reimbursement criteria were extended to include HRd-positive patients, many patients are actively starting Zejula treatment.” Zejula is currently one of the most promising PARP inhibitors for first-line maintenance therapy for ovarian cancer. In particular, the long-term follow-up data from the PRIMA study published last year has enhanced the reliability of Zejula. In the PRIMA trial, Zejula increased progression-free survival (PFS) by more than twofold in HRd-positive ovarian cancer patients compared to placebo. Additionally, at the time of clinical confirmation, the median PFS in the Zejula treatment group was 24.5 months, compared to 11.2 months in the placebo group, showing a significant difference. The 5-year PFS rate was also 35%, approximately twice as high as that of the placebo group. Dr. Monk stated, “Previously, there were concerns that long-term use of PARP inhibitors could lead to drug resistance, but this data confirms that such a possibility is low. These long-term follow-up results will serve as a strong source of reliability for doctors who have been hesitant about prescribing Zejula in the long term." He further explained, “Zejula can be used as a first-line maintenance therapy for all patients who respond to platinum-based chemotherapy (all-comer), but it is known to show the most effective results in HRd-positive patients. Since approximately half of all ovarian cancer cases are classified as HRd-positive, Zejula is increasingly being considered as a key option when setting treatment strategies.” “Diagnostic hurdles remain despite Zejula’s extended reimbursement for HRd-positive ovarian cancer” One of the main reasons for the popularity of Zejula is its ease of administration. While other PARP inhibitors require twice-daily dosing, Zejula can be taken once daily, improving medication adherence. Professor Lee said, “For patients to adhere to long-term maintenance therapy without becoming fatigued, treatment convenience is crucial. Zejula’s once-daily dosing regimen has had a positive impact on patients' ability to remain on therapy over the long term without discontinuation." Bradley Monk, MD, Medical Director of Late-Phase Clinical Research Program, Florida Cancer Specialists & Research Institute Dr. Monk added, "Zejula has the advantage of having relatively low drug-drug interactions, which makes it a safer option when used in combination with other drugs. This is a significant advantage for elderly patients with comorbidities or those receiving complex medication regimens.” Meanwhile, with the expansion of reimbursement criteria for HRd-positive ovarian cancer, it has become essential to determine whether a patient is HRd-positive before establishing a treatment strategy, but access to such HRd diagnostic tests remains a barrier. Currently, BRCA1/2 mutation testing for ovarian cancer patients is relatively affordable through national support programs and partial health insurance coverage. However, genomic panel testing required to confirm HRd status is not covered by insurance, leaving patients to bear the full cost of approximately KRW 2.5 million. Professor Lee pointed out, “HRd testing is essential for HRd-positive patients to receive Zejula treatment, but the fact that the test is not covered by insurance and must be paid out of pocket is a major institutional contradiction. Policy improvements should be made so that HRd diagnostic tests can settle as a diagnostic tool accessible under the same criteria as BRCA tests.” In contrast, access to HRd tests has been rapidly improving overseas. Dr. Monk stated, “Currently, more than 10 companies in the United States offer HRd tests, and some provide the service at very low costs. HRd diagnostic tests can serve as an important basis for predicting treatment response to PARP inhibitors such as Zejula.” For this reason, the Korean Society of Gynecologic Oncology is also known to be actively collecting supporting data to officially propose reimbursement for HRd tests to Korean health authorities. If HRd tests are promptly covered by health insurance, patients will be able to receive the necessary testing without financial burden and fully enjoy the benefits of targeted maintenance therapy such as Zejula. In addition, Professor Lee, who has been appointed as the President of the Korean Society of Gynecologic Oncology, emphasized his commitment to advancing precision medicine based on the genetic profiling of ovarian cancer. Professor Lee stated, “The society plans to focus on how to diagnose and manage the genetic characteristics of ovarian cancer. In other countries, there are already detailed clinical guidelines in place for individuals with BRCA mutations, and I believe similar protocols are needed in Korea as well." He concluded, "Since ovarian cancer often occurs alongside other cancers such as breast or endometrial cancer, collaboration with other specialties, including surgical departments, is essential. Establishing a multidisciplinary, patient-centered integrated care system through close coordination with various medical fields is another key priority for the society."