- LOGIN
- MemberShip
- 2025-12-20 12:49:07
- 'Wegovy' dominating the South Korean obesity drug market
- by Chon, Seung-Hyun | translator Hong, Ji Yeon | 2025-05-27 06:17:53
Novo Nordisk's Wegovy has dominated the South Korean obesity treatment market, establishing a monopolistic competition with over 70% market share.
In just six months since its launch in Korea, Wegovy generated a sensation, surpassing KRW 100 billion in cumulative sales.
Wegovy's success has expanded the obesity drug market to its largest size ever, while sales of Saxenda, which previously led the market, sharply declined.
According to pharmaceutical market research firm IQVIA, on May 26, the obesity drug market reached KRW 108.6 billion in the first quarter of this year, a 162.3% increase compared to KRW 41.4 billion in the same period last year.
This is the first time the quarterly obesity drug market size has exceeded KRW 100 billion.
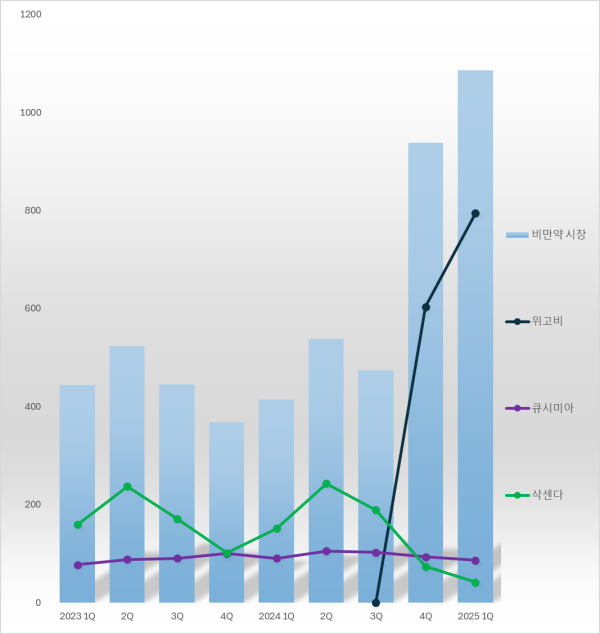
Legend: Bars-obesity market, Black-Wegovy, Purple-Qsymia, Green-Saxenda (Unit: KRW 100 million, Source: IQVIA) Novo Nordisk's Wegovy clearly stood out.
In the first quarter, Wegovy's sales recorded KRW 79.4 billion, accounting for a dominant 73.2% of the entire obesity drug market.
Wegovy, which was approved by the Ministry of Food and Drug Administration (MFDS) in April 2023, is a GLP-1 analogue containing semaglutide, known to reduce HbA1c.
Novo Nordisk developed Wegovy as a once-weekly obesity treatment using semaglutide after observing weight loss effects in patients during clinical trials of GLP-1 class diabetes drug candidates.
Wegovy gained immense popularity immediately after its domestic launch in October last year.
Despite its high price, prescription demand surged due to its significant weight loss efficacy.
Wegovy's sales reached KRW 60.3 billion in the fourth quarter of last year, propelling it to the top of the obesity drug market.
The obesity drug market size was KRW 47.4 billion in the third quarter of last year, but it soared by 97.9% to KRW 93.8 billion in just one quarter following Wegovy's launch.
Wegovy held a 63.4% market share in the obesity drug market in the fourth quarter of last year, and its share has continued to rise this year.
Under six months since its domestic release, Wegovy's cumulative sales reached KRW 139.8 billion, exceeding KRW 100 billion.
Wegovy is thriving globally due to its groundbreaking weight loss effects.
Wegovy's sales last year reached DKK 58.26 billion (approximately KRW 11.7 trillion), an 85.7% increase from DKK 31.343 billion in 2023.
Demand for Wegovy surged to the point of shortages after its launch in the U.S.
market.
Even before its domestic launch, Wegovy gained global fame for shortages, having been rumored as the weight loss secret of international celebrities like Tesla CEO Elon Musk.
Despite its high price of around KRW 500,000, Wegovy gained explosive interest immediately after its domestic release, leading to supply shortages.
Even with the suspension of non-face-to-face prescriptions, demand for Wegovy continued to rise.
Initially, Wegovy was actively prescribed through non-face-to-face medical consultations.
When concerns were raised that Wegovy was being indiscriminately prescribed via non-face-to-face consultations regardless of a person's weight or obesity status, health authorities suspended non-face-to-face prescriptions for obesity treatments from December 16 of last year.
The introduction of Wegovy has led to a reduction in sales of Saxenda and Qsymia, which previously dominated the obesity drug market.
The presence of Novo Nordisk's Saxenda has significantly declined in the obesity drug market.
Saxenda's first-quarter sales were KRW 4.2 billion, down 72.3% from KRW 15.1 billion in the same period last year.
Compared to its KRW 24.2 billion sales in the second quarter of last year, its sales have sharply declined to less than 20%.
Saxenda, launched in Korea in 2018, was the world's first obesity drug approved as a GLP-1 analog.
Saxenda's active ingredient, liraglutide, is identical to Victoza's, prescribed for type 2 diabetes patients, differing only in dosage and administration.
The analysis suggests that Wegovy, a GLP-1 class drug similar to Saxenda, has further taken Saxenda's market.
With reduced domestic supply since Wegovy's launch, there are rumors of Saxenda's production being discontinued.
After becoming the obesity treatment market leader with sales of KRW 42.6 billion immediately after its launch in 2019, Saxenda maintained its lead for five consecutive years until 2023.
Saxenda's sales reached KRW 66.8 billion in 2023, accounting for 37.5% of the obesity treatment market that year.
However, following the introduction of Wegovy, Saxenda's sales sharply declined, and its market share in the first quarter of this year was only 3.8%.
The first-quarter sales of Alvogen Korea's Qsymia decreased by 3.9% year-on-year to KRW 8.6 billion.
Launched in late 2019, Qsymia is a combination drug containing 'phentermine' and 'topiramate'.
Alvogen Korea secured domestic marketing rights from U.S.-based Vivus in 2017.
Alvogen Korea entered a co-promotion agreement with Chong Kun Dang in late 2019 and began full-scale sales in Korea.
Qsymia recorded KRW 10.2 billion in sales in the third quarter of last year but fell to 9.3 billion KRW in the fourth quarter, when Wegovy was launched, and has further decreased this year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










