- LOGIN
- MemberShip
- 2025-12-20 14:39:55
- Daiichi Sankyo exceeds ₩300B in sales…new drug drives
- by Son, Hyung Min | translator Hong, Ji Yeon | 2025-06-18 06:01:42
Daiichi Sankyo Korea has exceeded KRW 300 billion in sales for the first time, led by its cardiovascular products, Antibody-Drug Conjugate (ADC), and new anticancer drugs.
The company is successfully transitioning its portfolio towards new ADC drugs while maintaining robust growth from established cardiovascular products like Sevikar, Lixiana, and Olmetec.
According to the Korea Financial Supervisory Service (FSS)'s electronic disclosure system on June 16, Daiichi Sankyo Korea's sales last year reached KRW 309.8 billion, a 13% increase from the previous year.
During the same period, operating profit decreased by 9%, from KRW 26.6 billion to KRW 24.2 billion.
Daiichi Sankyo Korea considers its sales for 2024 based on the Japanese fiscal year, covering April of last year to March of this year.
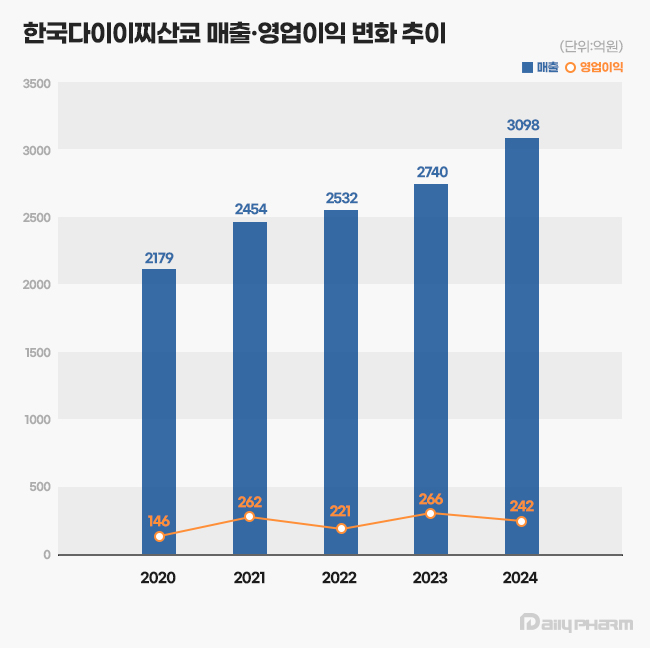
The company first surpassed KRW 200 billion in revenue in 2020 with KRW 217.9 billion, followed by a continuous upward trend, reaching KRW 245.4 billion in 2021, KRW 253.2 billion in 2022, and KRW 274.0 billion in 2023.
Notably, an analysis suggests that collaboration with the domestic pharmaceutical company Daewoong Pharmaceutical on some cardiovascular products, such as Lixiana and Sevikar, has created a synergistic effect.
Daiichi Sankyo Korea signed co-promotion agreements for Sevikar in 2013 and Lixiana in 2015 with Daewoong Pharmaceutical, and this partnership continues to date.
Among them, the highest revenue generator is the Direct Oral Anticoagulant (DOAC), Lixiana.
According to market research firm UBIST, Lixiana's prescription sales last year was KRW 117.5 billion, a 12% increase compared to KRW 105.3 billion in 2023.
DOACs are anticoagulants that prevent blood clots by directly acting on blood coagulation factors.
They are increasingly being used in clinical settings as they replace warfarin, which inhibits Vitamin K metabolism.
In Korea, Xarelto was approved in 2009, followed by Pradaxa and Eliquis in 2011, and Lixiana in 2015.
Despite being the last to be launched among DOACs, Lixiana has rapidly increased its prescription performance, backed by demonstrated clinical data, and has maintained its market dominance since 2019.
With annual growth of around 10%, its prescription performance nearly doubled in five years, from KRW 60.4 billion in 2019.
Its market share in the overall DOAC market also expanded from 33% in 2019 to 45% last year.
Sevikar, an olmesartan-based combination therapy for hypertension, continues to maintain its strong performance in prescription revenue.
Sevikar's prescription revenue last year was KRW 68.8 billion, a 4% increase from the previous year.
Despite numerous global and domestic pharmaceutical companies entering this market, Sevikar's prescription sales continue to grow.
Sevikar's prescription revenue, which was KRW 53.4 billion in 2019, surpassed KRW 60 billion in 2022.
In 2023, it recorded KRW 65.9 billion, demonstrating five consecutive years of increased prescription sales.
The triple combination hypertension drug Sevikar HCT also maintained its growth trajectory.
Sevikar HCT's prescription sales for the last year totaled KRW 42.1 billion, representing a 4% increase from the previous year.
Daiichi Sankyo Korea generated approximately KRW 140 billion in prescription sales solely from olmesartan-based hypertension treatments, including Sevikar HCT (KRW 42.1 billion), Olmetec (KRW 30.6 billion), and Sevikar (KRW 68.8 billion).
The 5 ADC Strategy...Will it achieve R&D success after Enhertu? Daiichi Sankyo Korea is working towards transitioning from a cardiovascular-focused company to a leader in oncology.
The company is particularly concentrating its R&D capabilities on the ADC field, focusing on new growth engines.
Following the already approved Enhertu, it is pursuing a '5 ADC strategy' and preparing for the launch of various other therapeutic agents, including Datroway, patritumab deruxtecan, DS-7300, DS-700, and DS-6000.
An ADC is a new anticancer drug designed by linking an antibody that binds to a specific target antigen on the surface of cancer cells with a drug that has cell-killing capabilities using a linker.
The advantage of ADCs is their ability to selectively target cancer cells by utilizing the antibody's target specificity and the drug's cytotoxic activity, thereby maximizing therapeutic efficacy while minimizing side effects.
Among these, Enhertu is a second-generation new ADC drug introduced by Daiichi Sankyo Korea.
Enhertu is a next-generation ADC that links a monoclonal antibody with the same structure as trastuzumab (which binds to specific target receptors overexpressed on the surfaces of cancer cells) and a highly potent, novel topoisomerase I inhibitor payload via a tumor-selective, cleavable linker.
Currently, Enhertu won domestic approval for HER2-positive breast cancer, gastric cancer, and non-small cell lung cancer, and is primarily used as a second-line treatment.
Its potential as a first-line treatment for breast cancer is also currently being investigated.
Daiichi Sankyo is also preparing to launch its second new ADC drug, Datroway.
This ADC targets the Trop-2 protein and has been approved in the U.S.
for the treatment of breast cancer.
The Trop-2 protein is a cell membrane antigen overexpressed in breast cancer, particularly in over 90% of triple-negative breast cancer cases.
Datroway binds to the Trop-2 protein and delivers cytotoxic substances into the cancer cells.
It has the advantage of maximizing the benefits of targeted therapy and cytotoxic chemotherapy while minimizing damage to healthy cells.
Currently, Daiichi Sankyo is co-developing and co-marketing Enhertu and Datroway with AstraZeneca.
Daiichi Sankyo is also developing an ADC with Merck.
patritumab deruxtecan, which targets HER3, showed efficacy in EGFR-mutated patients compared to platinum-based chemotherapy in the Phase 2 HERTHENA-Lung01 study.
Daiichi Sankyo continues to conduct research for subsequent ADC candidates after Enhertu, which targets the HER2 biomarker.
The company is also jointly conducting clinical studies with Merck on DS-7300, which targets B7-H3 (an emerging new biomarker in solid tumors), and DS-6000, a CDH6-targeting ADC.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










