- LOGIN
- MemberShip
- 2026-03-10 00:45:09
- Policy
- Pharma-MOHW disagree on drug pricing policy
- by Lee, Jeong-Hwan Jan 16, 2026 08:52am
- The Korean pharmaceutical industry has announced its stance that the government's drug price reform plan, which centers on generic price cuts and preferential pricing for "Innovative Pharmaceutical Companies," is too vague and requires both a delay in implementation and significant revisions.The industry requested a sufficient grace period, arguing that the plan excludes long-term impact assessments on the pharmaceutical-bio industry and public health, which could lead to industrial contraction.In contrast, the government stated it has no intention of delaying its plan to submit and finalize the reform at next month's (February) Health Insurance Policy Deliberation Committee for full implementation in July. This has left the gap between the two sides unbridged.As the pharmaceutical industry and the government have not bridged the divide in opinion, the conflict over drug pricing reform is expected to continue.On the 14th, the "National Assembly Forum on Drug Price Policies for Becoming a New Drug Powerhouse," hosted by Democratic Party Representatives Lee Un-ju, Seo Young-seok, and Kim Yoon, brought together the MOHW and industry representatives to exchange views.Pharmaceutical Industry, "Concerns over job losses, supply instability, and slowed growth"Hong Jeong-Ki, Managing Director of the Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA), pointed out that repeated drug price cuts, executed 10 times since 1999, have gradually shrunkne the industry's overall profitability and investment capacity.Hong also expressed concerns that large-scale cuts to generic prices, as foreshadowed by the MOHW, would inevitably deepen reliance on low-cost imported raw materials and finished imported drugs.While price cuts might yield short-term fiscal savings, Hong warned that, based on implementation of policies in the past, they could lead to long-term employment shrinkage, instability in the supply of essential medicines, and a slowdown in industrial growth.Hong argued that the implementation of price cuts should be reconsidered after a comprehensive assessment of the impact on the industry and public health. Hong argues the need for a sufficient grace period for a successful landing and the establishment of a government-industry governance system.Furthermore, he requested fair compensation for companies that contribute to innovation and supply stability.The compensation aims to provide uniform preferential pricing for all Innovative Pharmaceutical Companies and to grant price benefits to non-certified companies that demonstrate excellent R&D performance and facility investment."The current reform plan is structured such that damages from strengthened regulations increase while compensation for innovation and supply stability remains limited," Hong pointed out. "This could act as a barrier to becoming one of the world's top five pharmaceutical powerhouses."He emphasized, "The implementation of drug price cuts must be reconsidered after an assessment of both short- and long-term impacts. We request a sufficient grace period to ensure a soft landing for the system," adding, "It is also necessary to establish a government-industry governance structure for constructive discussions on drug price policy."He further stressed, "By specifying the innovation-based price addition period and ensuring fair value compensation, we must build a virtuous cycle that accelerates innovation, such as through R&D reinvestment, and leads to the creation of global new drugs," and maintained, "Cuts to the prices of existing listed generics should be minimized to secure investment capacity and ensure supply stability.""Preferential pricing plan fails to reflect company contribution and diversity"Kim Sang Jong, Managing Director of Hanmi Pharmaceutical, also criticized the reform, stating it could weaken the foundation for domestic companies to continue investing in new drugs.Kim argued that cutting existing generic prices without accounting for a company's contribution to new drug R&D creates a structure in which larger companies with higher investment volumes incur greater absolute losses. In his view, the policy is becoming an overall price cut premised on the notion that Korean generics are expensive compared to overseas markets, rather than a system that guarantees fair returns on investment.Notably, Kim highlighted that limiting preferential pricing to "Innovative Pharmaceutical Companies" and a few others fails to benefit the many firms that contribute to the industry through Phase 2/3 trial investments, manufacturing facility expansions, quality advancements, and employment of research personnel.Kim added that extending the price increase period for Innovative Pharmaceutical Companies by three years is only effective in the early stages of the patent-expired market. Once generic market share expands in the later stages, prices drop significantly, making the compensation effect felt by companies very limited."The resources needed for new drug R&D, maintaining production facilities for stable supply, and maintaining employment currently come from generic sales," Kim criticized. "It is questionable whether an overall price-cutting policy aligns with reward investment."Kim further criticized, "The financial resources required for new drug R&D, maintaining production facilities for stable supply, and sustaining employment come from the sales of existing listed generics. For now, this is unavoidable," adding, "While the MOHW stated it would focus on outcomes such as innovation, I question whether implementing a blanket price-cutting policy, based on the idea that high generic prices can now be lowered, actually aligns with the policy goal of rewarding investment."He added, "It is questionable whether it is truly necessary to further divide preferential drug pricing among Innovative Pharmaceutical Companies into upper and lower tiers. Once the three-year preferential period ends, generic expenditures drop significantly. We are not sure if this can be considered a reward for Innovative Pharmaceutical Companies," adding, "Pharmaceutical companies also need to analyze the reorganization of the drug price system. Since the announcement on November 28 last year, time has been needed to run simulations to determine how to manage operations in line with the policy goals. A grace period (for the drug price cuts) is necessary."MOHW "Current system has reached its limit... will reform to minimize industry concerns"Despite such requests from Hong and Kim to delay implementation and revise the preferential pricing policy, the MOHW rejected these requests, stating that the current system has reached its limit and cannot be further delayed.Kim Yeon-Sook, Head of the Division of Pharmaceutical Benefits at the MOHW, explained, "This reform is being implemented because the current system has reached its limit. It is a structural policy change designed to improve these limits, strengthen the supply stability of both new and essential drugs, and maintain the sustainability of national health insurance," and explained, "Other countries also periodically adjust generic prices while considering fiscal efficiency.""While our drug price system has previously lacked predictability and fallen short of achieving the goal of generic activation, this reform is different," Kim added. "Unlike past approaches focused solely on reducing drug expenditures, this reform includes mechanism to reward innovation based on investment, even for generics."Kim concluded, "The government views this reform as a golden time for structural improvement and a leap forward for the industry, centered on Innovative Pharmaceutical Companies. We will implement a drug price system that leads to rewards for the industry to minimize the concerns of domestic pharmaceutical firms."
- Company
- ‘Mounjaro’ enters pricing negotiations for diabetes
- by Eo, Yun-Ho Jan 16, 2026 08:52am
- Passed Drug Reimbursement Evaluation Committee review at year-end...Clinical data suggest potential for remissionMounjaro, the blockbuster obesity drug that has taken the market by storm, has entered the final stage toward reimbursement listing for diabetes treatment in Korea.According to industry sources, the Ministry of Health and Welfare has recently instructed the National Health Insurance Service (NHIS) to begin price negotiations for Mounjaro (tirzepatide), Eli Lilly Korea’s dual GIP/GLP-1 receptor agonist.Mounjaro passed the Drug Reimbursement Evaluation Committee under the Health Insurance Review and Assessment Service (HIRA) in December.Accordingly, once the NHIS negotiation team is formed, substantive discussions are expected to commence. It remains to be seen whether Mounjaro can swiftly conclude price negotiations and secure its place on the reimbursement list in Korea.Mounjaro is currently approved in Korea as an adjunct to diet and exercise for improving glycemic control in adults with type 2 diabetes, as well as for chronic weight management in adults with obesity or overweight accompanied by at least one weight-related comorbidity (hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease).The recommended starting dose is 2.5mg once weekly for both indications, intended solely for treatment initiation. After four weeks, the dose is increased to 5mg once weekly. If further dose escalation is required, the dose may be increased by 2.5mg at intervals of at least four weeks, up to a maximum of 15 mg once weekly.Mounjaro has drawn significant attention as the first drug to demonstrate the potential for remission in diabetes.In the Phase III SURPASS trial, which supported its regulatory approval, Mounjaro showed statistically superior reductions in HbA1c and body weight compared with all comparator treatments, including semaglutide (Ozempic), insulin degludec, and insulin glargine.Furthermore, at the European Association for the Study of Diabetes (EASD) conference held last September, Lilly presented results from the SURPASS-CVOT Phase III clinical trial, which directly compared Mounjaro with the GLP-1 receptor agonist Trulicity, adding data on cardiovascular prevention effects and overall survival improvement.
- Policy
- ‘Drug innovation is concentrated around specific diseases’
- by Jung, Heung-Jun Jan 16, 2026 08:52am
- The OECD has raised concerns that pharmaceutical innovation is increasingly concentrated in specific disease areas, while placing healthcare financial sustainability at the center of policy debate.It has stressed that the price increases of new drugs must be reviewed to determine if they actually lead to meaningful health gains, and that a comprehensive evaluation is necessary when assessing access to medicines.On the 15th, the Health Insurance Review and Assessment Service (HIRA) announced the results of its research on the Revised OECD Health System Performance Assessment framework indicators.The OECD HSPA framework is designed to compare how effectively national healthcare systems are functioning across countries.According to HIRA’s analysis, pharmaceuticals and medical technologies were newly positioned as core foundational elements determining system performance in the 2024 revised OECD HSPA framework.The revision reflects lessons from the COVID-19 pandemic, during which vulnerabilities in global supply chains for essential medicines and medical devices, as well as shortages in intensive care capacity and healthcare infrastructure, were exposed.Sustainability perspectives were particularly strengthened. The HIRA research team explained, “(The revised framework) critically questions whether rising prices of new drugs translate into actual improvements in health value and addresses the sustainability of health finances as a key policy challenge.”They added, “The OECD also points out the imbalance created by innovation being concentrated in specific disease areas, and calls for a fundamental review of the pharmaceutical incentive structure and the rationality of current pricing models.”Changes were also made to concretize the concept of drug accessibility. This signifies that accessibility should be evaluated as a multidimensional, people-centered concept, not merely based on a product's availability.The research team stated, " The revised framework defines access to medicines as a multidimensional concept encompassing availability, affordability, geographic accessibility, acceptability, and quality. This implies the need for a comprehensive assessment of barriers across the entire medicine lifecycle, including regulatory approval, health technology assessment, reimbursement listing, and prescribing and dispensing practices.”They further noted, ”The OECD has published a report that serves as a foundation for developing indicators to monitor such complex dimensions of access."
- Policy
- MOHW·KHIDI to focus on fostering innovative pharma companies
- by Lee, Jeong-Hwan Jan 16, 2026 08:52am
- The Ministry of Health and Welfare (MOHW) and the Korea Health Industry Development Institute (KHIDI) have set fostering of 'Innovative Pharmaceutical Companies' as a primary focus for the New Year. As the executive government bodies overseeing Innovative Pharmaceutical Companies, the MOHW and KHIDI plan to fully utilize the certification system for Innovative Pharmaceutical Companies as a core engine for the global expansion of the domestic pharmaceutical and bio-industry.These are the details from the New Year's policy directions announced by the MOHW Pharmaceutical and Bio-Pharma Industry Division on the 15th, as well as the major business report from KHIDI.Currently, the Innovative Pharmaceutical Company certification system is facing a shift. The core of the government's drug price system reform plan, which it announced will be implemented this year, involves significantly differentiating drug price preference rates based on whether a company holds the Innovative Pharmaceutical Company certification. The MOHW is preparing to improve the certification system to address controversies over its practical effectiveness.In this context, the MOHW and KHIDI have expressed their determination to leverage innovative pharmaceutical companies to advance the domestic pharmaceutical industry.First, Yim Kang-seop, Head of the Division of Health Industry Policy at the MOHW, plans to lead the structural improvement of the domestic industry through the reorganization of the certification system.Since the certification status is linked to the drug price reform plan, which determines the preferential price ratios for pharmaceutical companies, the reorganization aims to provide companies with specific signals regarding innovation.Yim explained, "Over the past 10 years, innovative pharmaceutical companies have contributed to some extent in shifting the domestic industry ecosystem toward a focus on new drugs. However, even among the 48 certified companies, a significant number still rely on generics, accounting for more than 40% of their sales. We must objectively acknowledge that some companies are becoming complacent with generics."Yim further emphasized, "We will strengthen systems so that innovative pharmaceutical companies can drive changes across the entire industrial ecosystem. We are engaged in sophisticated, strategic deliberation to ensure the drug pricing system functions as a mechanism that drives innovation, new drug development, and global expansion. We will enhance performance through the certification system reform."KHIDI also stated its goal of actively fostering the pharmaceutical industry to realize the Lee Jae Myung administration's national task of becoming one of the global top 5 pharmaceutical and bio-health powerhouses.First, KHIDI will support the National Bio-Innovation Committee, a governmental governance body, to provide more professional and systematic support for bio-health policies, and will establish statistics-based policies to respond to AI and new technology environments.Additionally, KHIDI will take full responsibility for managing the expanded healthcare R&D budget of KRW 1.2 trillion.KHIDI plans to focus on strengthening industry competitiveness by identifying policies that systematically cultivate innovative pharmaceutical companies.Hong Heon-Woo, KHIDI's Director of Planning, announced, "We will systematically foster innovative pharmaceutical companies and pursue global expansion through linked support from AI-based advanced medical device R&D to commercialization," adding, "KHIDI's plan includes striving to achieve KRW 2 trillion in healthcare R&D by 2030 and promoting strategic R&D investment centered on promising technologies by discovering representative technologies that citizens can experience."
- Policy
- Minister Jeong "Regional·essential healthcare doctor system"
- by Lee, Jeong-Hwan Jan 16, 2026 08:52am
- Minister of Health and Welfare Jeong Eun KyeongMinister of Health and Welfare Jeong Eun Kyeong stated, "The results of the Medical Personnel Supply and Demand Estimation Committee, while subject to realistic constraints, are the best possible outcome based on currently predictable data and a consensus-driven process."The Ministry of Health and Welfare (MOHW) reaffirmed its position that the newly increased medical workforce will be dedicated exclusively to personnel working in regional and essential healthcare sectors.Minister Jeong made these remarks during the 3rd meeting of the Health and Medical Policy Deliberation Committee held at the International Electronics Center in Seocho-gu, Seoul, at 4 p.m. on the 13th.The meeting discussed detailed plans for the deliberation criteria for the scale of medical personnel training from 2027, following the 1st meeting held on December 29 last year.First, the committee reconfirmed its agreement for the estimation results of the Medical Personnel Supply and Demand Estimation Committee (the Estimation Committee). The Estimation Committee, composed of a majority of members recommended by provider organizations, held 12 rounds of discussions.The meeting discussed the 1st deliberation criterion from the 1st meeting, focusing on resolving regional healthcare disparities and shortages of essential and public medical personnel. Specifically, the committee discussed a plan to apply the increase in medical personnel quota after 2027 to the quota for the regional doctor system.Furthermore, it was decided to consider the scale and timing of graduates resulting from the establishment of a "Public Medical Training School" (tentative name) and the creation of new medical schools in regions currently without them.The committee will also consider the second and third deliberation criteria, detailing future changes in the medical environment and healthcare policy shifts, by including all combinations of the three demand models and two supply models adopted by the Estimation Committee.Regarding the fourth criterion, related to ensuring the quality of medical education, the committee explored ways to keep the fluctuation rate of the 2027 admission quota within a certain level compared to the 2026 recruitment (3,058 students total) and to ensure that small-scale medical schools can secure an appropriate number of students for education.The committee also decided to consider the reality that students from the 2024 and 2025 classes are currently attending lectures together.For the final criterion, related to ensuring predictability and stability, the committee discussed applying the quota based on the 2025 estimate for five years from 2027 to 2031, in line with the mandatory five-year cycle for supply and demand estimation.Given that students entering during this period will graduate over five years from 2033 to 2037, 2037 was set as the base year for supply and demand management. The plan for the next estimation, to be conducted in 2029, was reviewed, taking into account the timing of the next quota application (the 2032 academic year) and the advance notice system for university admissions.The Health and Medical Policy Deliberation Committee plans to incorporate the results of these discussions on the application of deliberation criteria and submit training scale proposals for multiple scenarios at the next meeting.Minister Jeong stated, "We plan to review the scale of the medical workforce after 2027 based on the estimation results. In today's meeting," adding, "we will discuss agendas to detail the deliberation criteria applied to the various estimation results derived from the demand and supply models presented by the Estimation Committee."Minister Jeong added, "The most important principle is that the ultimate goal of discussing the scale of the medical workforce is to strengthen regional, essential, and public healthcare, which is currently in crisis," and stressed that, "We must also consider upcoming policy changes, such as the legislation for training and supporting regional doctors and the special law for strengthening essential healthcare and resolving regional medical gaps, which is currently awaiting passage in the National Assembly."Minister Jeong concluded, "We have previously failed to apply crucial deliberation criteria such as the qualitative level of medical education, the situation at educational sites, and sufficient predictability for those sites to train high-quality medical personnel," adding, "Through thorough discussion in today's meeting, we plan to refine further the review criteria presented in the first meeting."
- Company
- GLP-1’s expansion has only just begun
- by Son, Hyung Min Jan 16, 2026 08:52am
- SGLT-2 inhibitors, which began as treatments for type 2 diabetes, have established themselves as standard-of-care (SOC) after demonstrating clinical efficacy in major metabolic diseases such as heart failure and chronic kidney disease.Beyond blood glucose control, they have demonstrated cardiovascular and renal protective effects and the potential to improve long-term prognosis. As a result, they are regarded as having reshaped the treatment paradigm itself, beyond being drugs for specific diseases.Now, GLP-1–based therapies are taking over that baton.GLP-1 drugs have expanded their indications from type 2 diabetes to obesity, cardiovascular disease, kidney disease, and metabolic dysfunction–associated steatohepatitis (MASH), evolving into comprehensive treatment options covering the full spectrum of metabolic disease. Recently, their potential has even been discussed in Alzheimer’s disease, addiction disorders, and cancer prevention, raising the possibility that GLP-1 drugs could evolve into a systemic therapeutic platform rather than a single-disease therapy.This shift is most clearly evident in Novo Nordisk's ‘Ozempic/Wegovy (semaglutide)’ and Eli Lilly's ‘Mounjaro (tirzepatide)’, the leading GLP-1 drugs in the global market. While both drugs originated in diabetes, they have since differentiated themselves through distinct strategies for expanding indications and selecting mechanisms, broadening their respective domains.Building on the clinical and commercial success of GLP-1 drugs, domestic and multinational pharmaceutical companies are accelerating efforts to simultaneously pursue indication expansion and next-generation mechanism development, significantly broadening the scope of the GLP-1 market. The industry is now watching closely whether GLP-1 drugs can move beyond their single category as obesity treatments to become the core pillar for metabolic diseases overall.Beyond obesity and diabetes to cardiovascular, renal, and liver diseases... the current state of GLP-1 expansionThe expansion of indications for GLP-1 receptor agonists is already translating into actual approvals and late-stage clinical results.Originally developed for obesity and type 2 diabetes, GLP-1 drugs are now extending into cardiovascular disease, kidney disease, and MASH, positioning themselves as treatment options covering the entire spectrum of metabolic disorders.Notably, semaglutide expanded its therapeutic scope beyond obesity and diabetes treatments by securing evidence for reducing major adverse cardiovascular events (MACE).By accumulating clinical evidence encompassing not only diabetes patients but also high-risk cardiovascular populations, GLP-1 therapies have clearly demonstrated their potential to contribute to long-term prognosis improvement beyond weight loss.In addition, it has expanded into chronic kidney disease (CKD), strengthening its presence in a field once pioneered by SGLT-2 inhibitors.More recently, accelerated approval for MASH has pushed GLP-1 drugs into liver disease territory.Their mechanistic strengths in terms of weight loss, improved insulin resistance, and anti-inflammatory effects have translated into reduced hepatic fat accumulation and improved fibrosis, making them a promising new option in an area with limited therapeutic choices.Tirzepatide is also rapidly expanding its footprint. Beyond obesity and diabetes, it has secured approval for obstructive sleep apnea (OSA) and continues clinical development in cardiovascular and renal disease.Its strategy centers on managing obesity-related complications simultaneously, leveraging its strong weight-loss efficacy.This demonstrates how GLP-1 agonists are evolving beyond merely treating obesity-associated conditions to become therapies capable of modulating the pathophysiology of the disease itself.From single-mechanism drugs to multi-agonists … evolves to a ‘platform technology’If indication expansion represents the competition over what can be treated, diversification of mechanisms and delivery methods represents competition over how to treat. The GLP-1 market has already moved beyond single-mechanism competition and entered the development phase for multi-agonist combination therapies that target multiple receptors simultaneously.Early GLP-1 drugs focused on appetite suppression and satiety enhancement. However, dual and triple receptor agonists that simultaneously modulate various hormone receptors involved in metabolic regulation, such as glucagon-like peptide-1 (GLP-1), glucagon (GCG), and amylin receptor agonists, have recently emerged in succession.This multi-agonist approach differentiates itself by maximizing weight loss effects while also targeting lipid metabolism improvement, increased energy expenditure, and long-term metabolic stability.Dual GLP-1/GIP agonists, represented by Mounjaro, simultaneously promote insulin secretion and improve insulin resistance, while triple GLP-1/GIP/GCG agonists are evaluated as a strategy to further enhance weight loss and metabolic improvement effects.With the addition of GLP-1/amylin combination strategies, the GLP-1 class itself is effectively being redefined not as a single drug class but as a therapeutic platform. Recently, Novo Nordisk completed clinical trials for its platform therapy ‘CagriSema (semaglutide/caglinotide)’ and submitted a marketing application to the U.S. Food and Drug Administration (FDA). The mechanism aims for additional effects, such as delayed glucose absorption in the intestine, by combining semaglutide with caglinotide.Competition over delivery methods is also underway. While most currently commercialized obesity treatments are injectables, new delivery options like long-acting injections, oral formulations, and patches have entered the clinical stage.Oral GLP-1 agents, in particular, are seen as a key variable that could significantly expand treatment accessibility by reducing the psychological burden associated with injections. This holds the potential to broaden the target population for obesity treatment from specialized care settings to routine chronic disease management.Novo Nordisk and Lilly are leading in this area. Novo Nordisk has already secured approval for its oral Wegovy formulation, while Lilly has completed clinical trials for ‘Oroforglifron’ and submitted its FDA application. Unlike Mounjaro, Oroforglifron utilizes a single GLP-1 receptor agonist.Long-acting injectables are also rapidly entering late-stage clinical trials. Amgen's ‘MariTide’ uses an amino acid linker to conjugate two GLP-1 molecules with a fully human monoclonal anti-human GIPR antibody. This is known to produce greater weight loss effects than GLP-1 monotherapy.Since existing GLP-1 obesity treatments like Saxenda, Wegovy, and Zepbound became global blockbuster drugs, the pharmaceutical industry has been racing to develop new formulations. The existing drug Saxenda requires once-daily administration, while Wegovy and Zepbound require weekly injections. This is why a long-acting injectable formulation is expected to gain a competitive edge in terms of treatment convenience if commercialized.In Phase II clinical trials, MariTide demonstrated a maximum 20% weight loss rate at week 52 in non-diabetic obese patients. Based on this data, Amgen has fully launched the global Phase III clinical study, MARITIME. Amgen also plans to initiate Phase III studies this year for atherosclerotic cardiovascular disease (ASCVD), heart failure (HF), and obstructive sleep apnea (OSA).Domestic and multinational pharmaceutical companies are actively joining this trend. Multinational pharmaceutical firms are pursuing strategies to preempt next-generation standard treatments through multi-mechanism pipelines, while domestic pharmaceutical and biotech companies are also joining the global competition by leveraging dual/triple receptor agonists and novel delivery technologies.Ultimately, the competition in GLP-1 mechanisms and delivery methods is converging toward simultaneously fulfilling efficacy, convenience, and long-term manageability. This suggests that GLP-1 therapies are establishing themselves not as a temporary trend, but as a core platform for chronic metabolic disease treatment that will endure for decades to come.Latecomers are conducting clinical trials focused on differentiating themselves from existing drugs in areas like administration convenience, quality of weight loss, and preventing rebound effects. Next-generation obesity drugs that enable greater weight loss with a single dose are analyzed to potentially surpass existing drugs in administration convenience. With indications poised to expand, the value of obesity drugs is also being set against the benchmark of an average weight loss rate of 20%.
- Company
- GSK 'Omjjara' expecting to get reimbursement greenlight
- by Eo, Yun-Ho Jan 15, 2026 08:47am
- Product photo of Ojjaara/OmjjaraPreviously failed at its first attempt, the new drug 'Omjjara' for treating myelofibrosis is eying another chance.According to sources, GSK Korea's myelofibrosis treatment, Omjjara (momelotinib), is expected to be reviewed by this year's first Drug Benefit Evaluation Committee (DBEC) of the Health Insurance Review and Assessment Service (HIRA) today (January 15).Omjjara passed the Cancer Drug Review Committee (CDRC) in March of last year. However, during the mediating process for the DEBC consideration, there has been a difference of opinion between GSK and HIRA regarding the designation of a substitute medicine for drug pricing, which has suspended the listing process. After that, GSK supplemented its documents last year, discussed with HIRA, and reached a positive conclusion for DBEC consideration. It will be watched to see whether Omjjara will succeed in finalizing the reimbursement listing process.Omjjara has a triple mechanism of action that blocks JAK1, JAK2, and ACVR1 (activin A receptor type 1). In the treatment of myelofibrosis, inhibiting JAK1 and JAK2 can improve a patient's systemic symptoms and reduce enlarged spleen, while inhibiting ACVR1 can help alleviate anemia by decreasing hepcidin expression.Anemia management is a key unmet need in the treatment of patients with existing myelofibrosis. Anemia, which increases transfusion dependency, is a problem beyond simple dizziness and can lead to severe, life-threatening conditions depending on its severity.Based on the Phase 3 SIMPLIFY-1 and MOMENTUM clinical studies, Omjjara was shown to significantly reduce transfusion dependency and improve major symptoms, such as splenomegaly, in myelofibrosis patients with anemia, regardless of prior JAK inhibitor therapy.In the SIMPLIFY-1 study, which confirmed the clinical efficacy and safety of Omjjara compared to Jakavi (ruxolitinib) in a first-line treatment setting for myelofibrosis patients who had no prior experience with JAK inhibitors, Omjjara demonstrated non-inferiority to ruxolitinib in spleen volume response at week 24, the primary endpoint.The transfusion independence (TI) rate for each patient group was 66.5% in the Omjjara group and 49.3% in the ruxolitinib group, indicating that transfusion dependency was significantly lower in the Omjjara group.Professor Seo-Yeon Ahn of the Department of Hematology-Oncology at Chonnam National University Hwasun Hospital stated, "JAK inhibitors previously used for drug treatment of myelofibrosis showed effects in alleviating splenomegaly and systemic symptoms. However, there were unmet needs such as worsening anemia or increasing transfusion dependency. Omjjara has confirmed significant clinical value in the prognosis of myelofibrosis patients and managing anemia."
- Opinion
- [Reporter’s View] Need rationale to establish a new fund
- by Lee, Jeong-Hwan Jan 15, 2026 08:47am
- The Ministry of Health and Welfare has made clear its intention to secure approximately KRW 1 trillion in savings for the National Health Insurance (NHI) system by implementing a drug pricing reform centered on a dramatic reduction in generic drug reimbursement rates (from 53.55% to the low 40% range).At the same time, the ministry has announced plans to expand reimbursement coverage for innovative new medicines in order to strengthen patient access.Domestic pharmaceutical companies, whose operations rely heavily on generics as a primary revenue source, are criticizing this move, calling it “health insurance administration that cuts domestic generic drug prices to pay for multinational pharma’s innovative new drugs.”Their opposition stems from the fact that the announced pricing reform includes virtually no price-reduction mechanism for new drugs, while the government continues to send positive signals toward expanding reimbursement for increasingly expensive high-priced innovative drugs.Critics argue the policy has failed to strike a balance within the limited health insurance budget between domestically produced generics and innovative drugs largely supplied by multinational pharmaceutical companies.As a result, it is becoming increasingly clear that expanding reimbursement for new drugs, fostering the domestic generics industry, and maintaining the sustainability of the NHI system are difficult goals to achieve simultaneously under a single insurance budget.Amid this situation, the proposal by Jung-gu Kang, President of the Health Insurance Review and Assessment Service (HIRA), is drawing renewed attention.At a policy briefing held on January 12 under Minister of Health and Welfare Eun-Kyoung Jeong, Kang strongly advocated for a new approach to expanding reimbursement for ultra-high-priced drugs such as anticancer drugs and treatments for rare and intractable diseases.He reiterated the need to establish a separate fund dedicated exclusively to reimbursing ultra-high-priced “one-shot” curative therapies that demonstrate near-complete disease remission after a single administration.Kang had already raised this idea in his New Year’s address in 2025, proposing a separate fund for ultra-high-priced drugs with limited cost-effectiveness in order to improve patient access.The intent is to create a separate funding source outside the national health insurance budget to increase the coverage rate for high-cost treatments used for severe diseases.Discussions on establishing a separate fund for ultra-high-priced new drugs have been raised by multiple lawmakers in the 20th, 21st, and current 22nd National Assemblies, with several lawmakers introducing bills on the matter.A prime example is the bills to amend the National Health Insurance Act and the Lottery Act to allow the National Health Insurance Service to receive lottery revenue allocations as stipulated by the Lottery Fund Act.Minister Jeong believes it is necessary to consolidate the Ministry of Health and Welfare's position and rationale based on Kang's proposal and the pending legislative bills in the National Assembly. This would involve creating a separate fund dedicated to covering the reimbursement of ultra-high-priced treatments costing hundreds of millions to billions of won, thereby alleviating the burden on the NHIS budget.Prior to her appointment as Minister, during her confirmation hearing, Jeong had expressed some skepticism regarding the establishment of a separate fund for rare diseases.At the time, Jeong stated, “If the required finances exceed the fund's size due to factors like the application of high-cost treatments, flexible management could become difficult. Rather than establishing a separate fund, the priority should be given to continuously expanding reimbursement coverage.”Now, with domestic pharmaceutical companies strongly opposing the generic price cuts for KRW 1 trillion in NHIS savings, Jeong’s more forward-looking review of a separate fund may be necessary to avoid criticism that the government is simply ‘wringing a dry towel’ through drug price controls.Just as the ministry is aggressively considering the establishment of a Regional Healthcare Development Fund to strengthen local, essential, and public healthcare, a similar approach should be applied to the dual mission of expanding access to ultra-high-priced medicines and fostering Korea’s pharmaceutical and biotech industry.
- Policy
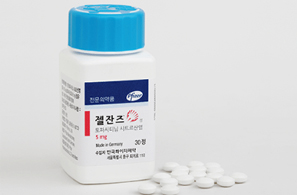
- Companies do not renew Xeljanz generic licenses
- by Lee, Tak-Sun Jan 15, 2026 08:47am
- Pfizer’s rheumatoid arthritis drug Xeljanz TabDespite the five year await for Xeljanz’s patent expiry, an increasing number of companies are abandoning sales of the generic versions of their rheumatoid arthritis treatment ‘Xeljanz.’This is largely attributed to drastic changes in the competitive landscape compared to the time of development. Some in the industry point to the negative consequences of so-called “blind approvals,” where companies follow competitors without question.According to the Ministry of Food and Drug Safety (MFDS) on the 14th, the license for Youngil Pharm’s Youngil Tofacitinib Tab 5 mg (tofacitinib aspartate) expired on the 14th and was removed from the product registry. This marks the 29th tofacitinib product to expire without renewal.By March, an additional six products are scheduled to expire, suggesting that more companies may forgo renewal.Of the 45 products that remain approved, only 14 have been listed for reimbursement.This situation reflects how the market environment changed dramatically during the 5-year wait for the original product’s substance patent expiry.Generic manufacturers attempted to bypass the extended substance patent by developing salt variants. Had this strategy succeeded, launch would have been possible after November 23, 2020, instead of waiting until the original substance patent expired on November 23, 2025.However, after legal disputes, the Supreme Court ruled in favor of the originator, Pfizer. The latecomers who received approval in 2020 had to wait until the substance patent expiry date of November 23, 2025.As a result, companies spent five years unable to launch, merely waiting for patent expiry. When the patent finally expired in November last year, several companies launched their products.However, the market had changed substantially compared to five years earlier. While Xeljanz became a blockbuster product generating sales in the KRW 10 billion range due to expanded reimbursement, its growth faced limitations as strong competitors emerged.New JAK inhibitors like Lilly's Olumiant and AbbVie's Rinvoq have entered the market since then. According to 2024 UBIST outpatient prescription data, Xeljanz recorded KRW 14.3 billion, Olumiant KRW 17.1 billion, and Rinvoq KRW 26.1 billion, showing Olumiant and Rinvoq surpassed Xeljanz in terms of sales.Furthermore, to counter latecomers, Pfizer released a more convenient extended-release formulation for Xeljanz, making it even harder for competitors to challenge it. The extended-release formulation, Xeljanz XR Tab 11mg, also holds a patent valid until March 12, 2035.As a result, more generic manufacturers are choosing not to renew their approvals despite the original drug’s substance patent expiry.Some point out that this incident highlights one of the drawbacks of the ‘blind approval’ system for latecomers. Many domestic pharmaceutical companies operating primarily in generics tend to develop products simply in line with the original drug’s patent expirations.Moreover, under Korea’s patent-linkage system, the first generic applicants that successfully bypass patents are granted nine months of market exclusivity. This has triggered a rush of follow-the-leader development, where competitors simply follow suit once a rival starts developing a generic.An industry executive commented, “In the case of some Xeljanz generics, the market has certainly changed, but fundamentally, the problem lies in companies blindly following competitors instead of pursuing independent business strategies, which has now backfired.”
- Company
- The evolution of next-gen cancer drugs continues
- by Son, Hyung Min Jan 15, 2026 08:47am
- If cytotoxic chemotherapy, targeted therapies, and immuno-oncology drugs once reshaped the cancer treatment paradigm, the latest trends in anticancer drug development are rapidly shifting toward next-generation approaches.The focus has moved toward more precise targeting strategies that improve efficacy while minimizing damage to normal tissues. In this process, antibody–drug conjugates (ADCs), bispecific and multispecific antibodies, and radiopharmaceuticals have emerged as core modalities in oncology R&D.Furthermore, the development axis is expanding to include targeted protein degraders (TPDs) and cell and gene therapies (CGTs). Consequently, the competition for new oncology drugs is no longer just about individual drugs; it is being restructured into a competition of modalities, encompassing platforms and delivery technologies.This shift is not merely a trend; it also represents the direction of R&D capital allocation, on how global pharmaceutical companies are simultaneously concentrating their M&A, licensing, and partnerships.This shift extends beyond technological trends, as confirmed by where global capital and transactions are actually concentrated. According to the market research institution Evaluate, the global prescription drug market is projected to reach USD 1.7 trillion by 2030, with growth driven by emerging modalities such as ADCs, multispecific antibodies, RNA-based therapies, gene and cell therapies, and radiopharmaceuticals.Ultimately, from the perspective of big pharma, the growth axis in oncology over the next decade must be designed not by filling pipelines one indication at a time, but by preemptively securing platforms that can be expanded across multiple cancer types upon commercialization.DailPharm examined how the oncology development landscape is being reshaped around ADCs, bispecific antibodies, and radiopharmaceuticals, modalities that have already proven their market impact.Precision drug delivery becomes standard therapy... ADC market competition intensifiesThe first modality to drive market momentum has been ADCs. ADCs are therapeutics designed to deliver cytotoxic drugs (payloads) more selectively to tumors. They do this by linking the payload to an antibody that binds to specific antigens on the surface of cancer cells via a linker. This precision delivery strategy combines the selectivity of targeted therapies with the killing power of chemotherapy while minimizing damage to normal cells.The drug symbolizing this field is Daiichi Sankyo and AstraZeneca's ‘Enhertu (trastuzumab deruxtecan)’. Enhertu's sales surged from KRW 3.72 trillion in 2023 to approximately KRW 5.6 trillion in 2024, proving that ADCs can evolve into blockbuster therapies rather than remain as late-line treatment options.Enhertu’s expansion across breast, gastric, and non-small cell lung cancer demonstrates the core strength of ADC platforms. Once the delivery system gains clinical trust, pipeline value can grow exponentially through indication expansion. Enhertu has already achieved standard-of-care status in each of its approved indications.Following HER2, the market's focus has shifted to another target, with TROP-2 taking center stage. The emergence of Daiichi Sankyo and AstraZeneca's Datroway (datopotamab deruxtecan) and Gilead’s Trodelvy (sacituzumab govitecan) has reinforced market confidence through strong sales growth.Trodelvy grew 24% year-over-year from USD 1.063 billion in 2023 to USD 1.315 billion in 2024. This signifies that TROP-2-targeted ADCs have moved beyond potential and are now being validated through actual prescriptions and sales. Notably, they have demonstrated consecutive successes in HR+/HER2- and triple-negative breast cancer.The reason ADCs are no longer just hit products for specific companies but have become a competitive axis across the entire industry lies in their high technological barriers and scalability. Given the complex combination of antibody, platform, linker, and payload required, it is difficult to solve all the puzzles through in-house development alone.Consequently, global pharmaceutical companies are adopting a strategy of concurrently pursuing acquisitions, partnerships, and licensing to rapidly internalize proven components. Payload competition has also intensified. While microtubule inhibitors (Microtubule-disrupting agent Monomethyl Auristatin E, MMAE) dominated early ADCs, topoisomerase-1 (TOP1) inhibitor payloads are now expanding. Meanwhile, drugs like Astellas’ Padcev (enfortumab vedotin) continue to leverage MMAE payloads in combination with immunotherapy to seek synergies.Ultimately, the decisive factor for ADCs is shifting from the fundamental skill of precise delivery to how rapidly they can expand indications and combination therapies.Bispecific antibodies expand from blood cancers to solid tumors … multispecific antibodies enter the arenaBispecific and multispecific antibodies represent the next evolutionary stage of antibody-based cancer therapy.Early development focused primarily on hematologic malignancies, where redirecting T cells toward tumor cells is easier to implement, and clinical efficacy can be demonstrated more rapidly.Starting with Amgen’s Blincyto (blinatumomab), followed by Roche’s Lunsumio (mosunetuzumab) and Columvi (glofitamab), Johnson & Johnson’s Tecvayli (teclistamab) and Talvey (talquetamab), and AbbVie’s Epkinly (epcoritamab), most bispecific antibodies initially gained approval in blood cancers.However, the landscape is changing. Leveraging accumulated clinical experience and manufacturing know-how, bispecific antibodies are now expanding into solid tumors.Solid tumors have long been considered a challenging domain due to their complex tumor microenvironment (TME), limited immune cell infiltration, and the difficulty of managing toxicity in normal tissues. Nevertheless, some bispecific antibodies gained approval or entered late-stage clinical trials for solid tumors, signaling that bispecific platforms are no longer confined to hematologic malignancies.Some experts interpret this as a process where bispecific antibodies are establishing themselves not as a technology for a single indication, but as an anti-cancer platform premised on indication expansion.The fundamental concept of bispecific antibodies lies in simultaneously targeting two different targets. They adopt a structure where a single antibody binds to two different antigens, or simultaneously binds to two different epitopes on the same antigen.This enables the simultaneous pursuit of increased binding affinity, enhanced signal blockade, and immune cell induction effects, which are outcomes difficult to achieve with monoclonal antibodies alone. Particularly in oncology, the core strategy involves simultaneously capturing tumor cell antigens and immune cell antigens to direct the immune response to the tumor site.Recently, pharmaceutical companies globally have begun actively developing multispecific antibodies that target three antigens simultaneously, beyond bispecific antibodies. This is not merely about adding one more target; it represents an attempt to combine elements like tumor targeting, immune activation, and immune checkpoint inhibition within a single molecule to achieve more sophisticated immune modulation.This approach aims to embed the combination strategy using immuno-oncology drugs within a single molecule. If successful, it is expected to simultaneously enhance treatment convenience and efficacy.Another reason multispecific antibodies are gaining attention is their potential to cross the blood-brain barrier (BBB). The BBB is considered a major challenge in anticancer drug development. While drug delivery to the brain is critical for treating CNS metastases or primary brain tumors, most antibodies and small-molecule drugs face limitations in crossing the BBB.Multispecific antibodies offer the potential to cross the BBB via receptor-mediated transcytosis by incorporating structures that bind to specific receptors present on the BBB surface. Consequently, they are also gaining attention as a next-generation anticancer strategy targeting CNS tumors or brain metastases.Furthermore, recent antibody designs increasingly combine antibodies that bind to antigens regulating immune cell activity with those that bind tumor-specific antigens. This aims to enhance immune activation while reducing non-specific toxicity.For example, one approach targets both antigens regulating T-cell activation signals and tumor-specific antigens simultaneously. This design amplifies immune responses exclusively within the tumor microenvironment. This represents an attempt to structurally mitigate the systemic toxicity issues of existing immune checkpoint inhibitors and is considered a particularly meaningful approach in the field of solid tumors.In summary, bispecific and multispecific antibodies are no longer technologies confined to hematologic malignancies. Building on validated mechanisms and clinical experience in blood cancers, the field has entered a stage of actively pursuing indication expansion into solid tumors.Simultaneously, the evolution of antibody-based anticancer platforms is evident through the addition of multi-target strategies, including triple antibodies, BBB-permeable designs, and precision-linking structures between immune cells and tumor cells. This symbolically demonstrates that anticancer therapy is moving beyond the era of targeting single targets, advancing to a sophisticated stage where tumors, immunity, and delivery pathways are designed simultaneously.Radiopharmaceuticals: from diagnosis to therapy… big pharma M&A fuels growthThe third major oncology R&D axis is radiopharmaceuticals. This approach involves administering compounds conjugated with radioactive isotopes, which reach the target and then emit radiation to damage the tumor. The shift of a market historically dominated by diagnostics toward therapeutic applications represents one of the most dramatic changes in recent years.While estimates from market research institutions vary, the consensus is that the radiopharmaceutical market will expand over the medium to long term. Some reports even suggest it could grow to around USD 10 billion this year. More significant than the numbers is that this growth expectation is actually triggering M&A and partnerships among multinational pharmaceutical companies.With beta-particle-based ligand therapies now on the track to commercialization, the global industry is shifting its focus to alpha-particle-based therapies, which offer higher energy, shorter range, and more precise killing power.Leading candidates include Actinium-225 and Astatine-211. These are gaining attention as next-generation targeted therapies because they possess a shorter range and higher LET (Linear Energy Transfer) compared to the existing beta-particle-based lutetium-177. This allows for precise targeting of cancer cells while minimizing damage to surrounding healthy tissue.Novartis, developer of Lutathera and Pluvicto, acquired radiopharma developer Mariana. AstraZeneca partnered with Fusion Pharmaceuticals. The US, Canada, and Europe are already treating the securing of actinium production and purification infrastructure as a national-level strategy.BMS acquired RayzeBio for USD 4.1 billion in late 2023 and formalized the completion of the acquisition in February 2024, integrating the radiopharmaceutical platform into the group.Notably, Lilly signed a joint development agreement with Aktis Oncology even after acquiring Point Biopharma. Recently, Lilly participated as an anchor investor in Aktis’ IPO process, reaffirming its commitment to positioning radiopharmaceuticals as a long-term growth pillar. Through the PointBio acquisition, Lilly secured the prostate cancer treatment candidate PNT2002 and the neuroendocrine tumor treatment candidate PNT2003.Radiopharmaceuticals are attractive because they enable indication expansion through combinations of targets (ligands/antibodies) and isotopes. Furthermore, if a value chain extending from diagnosis to therapy (theranostics) is established, platform lock-in effects can be anticipated.Simultaneously, the demanding industrial infrastructure requirements, including isotope supply chains, manufacturing (CMC), logistics, and administration infrastructure, are interpreted as driving a stronger tendency among global pharmaceutical companies to internalize capabilities through acquisitions rather than simple partnerships.Ultimately, the rapid reshaping of the oncology development landscape is not merely a succession of individual companies launching new drugs. While ADCs offer precision delivery, bispecific antibodies provide multi-target and immune engagement, and radiopharmaceuticals deliver targeted radiation. Each presents distinct solutions, but all believe that those possessing platforms are required to design the next pipeline.If cytotoxic, targeted, and immuno-oncology therapies formed the major pillars of treatment, the decisive factor now is securing which modality on that foundation and expanding it quickly. The success or failure of R&D hinges not on individual compounds but on modality (platform) competitiveness. Big pharma's acquisitions, investments, and technology deals signal that this competition is already fully underway.