- LOGIN
- MemberShip
- 2025-12-21 06:19:29
- Opinion
- [Reporter’s View] Doctors agree Korea’s reimb is too slow
- by Eo, Yun-Ho Jul 02, 2025 06:09am
- Even prescribing physicians believe that the pace of new drug reimbursement listing in Korea is too slow. This is an issue not only for doctors or patients, but obviously for pharmaceutical companies as well. Recently, the Korean Research-based Pharmaceutical Industry Association (KRPIA) released the results of a survey of 100 medical professionals in Korea. In January, a global polling agency Ipsos Research surveyed domestic clinical experts from various medical departments to ask their opinions on access to new drugs in Korea. According to the survey results, all medical professionals unanimously answered that the period required for MFDS approval to health insurance coverage is “long,” with 74% stating it is “too long.” Regarding the appropriate period from approval to health insurance coverage, 81% of medical professionals answered “up to 10 months,” with 41% deeming “within 6 months” as appropriate. As of 2022, it took an average of 608 days (approximately 20 months) for innovative new drugs to be approved by the MFDS and listed for health insurance reimbursement in Korea. This is twice the appropriate period (10 months) cited by most medical professionals and significantly longer than what is required for other major countries such as Germany (281 days), Japan (301 days), and France (311 days). Furthermore, experts who directly treat patients on-site responded that the widespread introduction of innovative new drugs would be of practical help in treating patients. Eighty-three percent of medical professionals expected that “if drugs already in common use overseas are covered by health insurance in Korea, patient treatment outcomes will improve significantly.” A large proportion of medical professionals (85%) also responded that “if reimbursement criteria are relaxed to enable early or wider scope of use, even for drugs already covered by health insurance, this will significantly improve patient treatment outcomes.” In addition, 95% of medical professionals urged the MOHW to introduce a “fast-track listing procedure or system” for health insurance coverage, similar to the MFDS's Global Innovate Products on Fast Track (GIFT) system, which shortens the drug approval review period for severe or life-threatening diseases by up to 75%. Medical professionals who participated in the survey also expressed concerns about Korea's low access to new drugs. Ninety-four percent pointed out that “access to new drugs in Korea is lower than overseas,” and 97% answered that “the government must set appropriate and reasonable drug prices to prevent the ‘Korea Passing’ phenomenon, where multinational pharmaceutical companies abandon the launch of innovative new drugs due to domestic regulations on pharmaceuticals.” Although this is a recurring issue, the criticism carries more weight as it comes from the front lines of medical care. Of course, the government is not without effort. Every year, health authorities introduce various systems to improve the speed of drug listing. The “parallel approval-evaluation-negotiation pilot project” is already in its second phase. In addition, the deadlines for regulations of drugs that go through the general listing process are being shortened in general, in both the evaluation and negotiation stages of the Health Insurance Review and Assessment Service and the National Health Insurance Service. However, whether the process is really speeding up remains in question. The problem lies in the process of enforcing regulations. No matter how well the system supports prompt reimbursement of drugs, there are always factors that can cause delays. Ultimately, patients are the ones left to suffer. They are left to wait anxiously, without a clue about what will happen next. Measures to shorten the process of listing drugs for insurance reimbursement must be accompanied by greater transparency and disclosure.
- Company
- Generics of Jardiance expected to compete for KRW 100B mkt
- by Kim, Jin-Gu Jul 02, 2025 06:08am
- Product photo of JardianceThe substance patent for Jardiance (empagliflozin), a diabetes treatment generating annual sales of approximately KRW 100 billion, is set to expire this October. Generic competition in the SGLT-2 inhibitor diabetes drug market is likely to heat up again. Approximately 50 companies have already secured approvals for related generic products. In November, the substance patent of Pfizer's oral autoimmune disease treatment, 'Xeljanz (tofacitinib),' will expire. Thiswill be followed by the first generic launch for a drug in the Janus Kinase (JAK) inhibitors category, with over 60 generics poised to enter a market valued at approximately KRW 15 billion annually. The priority sales period for 'Cetus (pranlukast)' generics, treatment for asthma and allergic rhinitis, will also end in October. Therefore, generic drugs without this priority sales marketing authorization are expected to enter the market. 'Generic Jardiance' expected to launch in October...variable is the 'unlisted patent' According to the Ministry of Food and Drug Safety (MFDS), on June 30, Boehringer Ingelheim's Jardiance substance patent is set to expire on October 23. The pharmaceutical industry anticipates a mass launch of generics around the time of the patent expiration. Generic companies have successfully circumvented the remaining listed patents in their patent disputes with Boehringer Ingelheim, excluding the substance patent. Currently, 49 companies have received product approvals for 333 empagliflozin-based single and fixed-dose combination products. Specifically, 48 generic companies have obtained approval for 100 Jardiance generic products, while 36 companies have approval for 213 Jardiance Duo (empagliflozin + metformin) generic products. For Esglito (empagliflozin + linagliptin) generics, five companies have obtained approval for 10 products. Products with active ingredients or combinations not found in the original drug are also ready for launch. Chong Kun Dang received approval for four follow-on products combining empagliflozin + sitagliptin, and and Daewon Pharm received approval for six follow-on products combining empagliflozin + sitagliptin + metformin. Generic competition is expected to heat up again in the diabetes drug market. Generic companies have signaled their entry into the empagliflozin market, which has grown to over KRW 100 billion annually. According to pharmaceutical market research firm UBIST, the combined prescription sales of Jardiance and Jardiance Duo last year reached KRW 108.2 billion, an 11% increase from KRW 97.5 billion in 2023. The withdrawal of Forxiga (dapagliflozin) from the market is analyzed to have significantly contributed to Jardiance's upward trend. AstraZeneca decided to withdraw Forxiga from the Korean market at the end of 2023. However, they retained Xigduo, a combination product containing metformin. Forxiga was supplied domestically until the middle of last year. Jardiance took over the KRW 50 billion market gap left by Forxiga. Jardiance's prescription sales increased by 22% over the year, from KRW 15 billion in Q4 2023 to KRW 18.2 billion in Q4 2024. In Q1 this year, it further increased to KRW 19 billion. Additionally, Jardiance Duo, the metformin combination product, shows a steady upward trend. In Q1 this year, it recorded KRW 10.8 billion in prescription sales, representing a 10% year-over-year (YoY) increase. Given that annual prescription sales of Jardiance and Jardiance Duo totaled KRW 100 billion, challenges from generics are expected to be intense. The variable lies in 'unlisted patents.' Boehringer Ingelheim Korea holds over nine patents related to Jardiance, Jardiance Duo, and Esglito that are registered with the Korean Intellectual Property Office (KIPO) but not listed in the MFDS patent registry. While their absence from the listed patents does not affect product approval, there is a risk of patent infringement upon launch. Boehringer Ingelheim Korea plans to enforce its patent rights vigorously. In the case of Trajenta's (linagliptin) substance patent expiration last year, Boehringer Ingelheim Korea proactively warned generic companies about patent infringement by sending certified mail. In response, generic companies are challenging the undisclosed Jardiance patents through avoidance or invalidation efforts. The industry anticipates that a significant number of generic companies will proceed with their generic launches after the substance patent expires. This is because many generic companies launched products with similar patent infringement risks when Trajenta's substance patent expired. On November 22, Pfizer's Xeljanz patent will expire. All eyes are on this because it is the patent expiration of one of the oral autoimmune disease treatments. Fifty-five companies have received product approvals for 63 generic items. These companies are set to challenge the KRW 14.4 billion Xeljanz market. Furthermore, competition with other original JAK inhibitor drugs, including Xeljanz, is also expected. JAK inhibitors, such as Xeljanz, Rinvoq (upadacitinib), Olumiant (baricitinib), and Jyseleca (filgotinib), have been launched in Korea. Prescription sales for these drugs last year totaled KRW 62.2 billion, a 56% increase YoY. Priority sales period for 'Cetus' with market worth KRW 46 billion annually, expires in October…more generics expected to enter Product photo of CetusThe priority sales period for generic Cetus, an asthma and allergic rhinitis treatment with annual sales of KRW 46 billion, will expire on October 1. This will allow the launch of generics that did not receive priority sales rights. Previously, generic companies filed a passive rights scope confirmation trial against Sam-A Pharm's Cetus formulation patent. Subsequently, in October last year, they received a favorable judgment from the Intellectual Trial and Appeal Board. This judgment was confirmed without an appeal from Sam-A Pharm. Based on their first-instance victory, the challenging companies have successively obtained generic approvals. Among these, Dasan Pharmaceutical's 'Prituss,' Dongkook Pharmaceutical's 'Pranpid,' Green Cross's 'Neofran,' and Daewoong Bio's 'Cituone' received priority sales rights. The priority sales period runs from November last year to October this year. Generic companies that did not receive priority sales rights during this period can launch their products after October. The launch of follow-on generics from Hanwha Pharmaceutical and Dongkoo Bio is expected. These companies have previously won patent disputes against Sam-A Pharm. Hanwha Pharmaceutical has even obtained generic product approval under the name 'Citurien'. Despite Cetus's annual prescription sales of KRW 46 billion, and the fact that earlier launched Cetus generics have not firmly established themselves in the asthma and allergic rhinitis treatment market, analysis suggests that follow-on drugs launching about a year later will still have significant competitiveness. In Q1 this year, Cetus generics recorded only KRW 600 million in prescription sales, representing about a 5% market share in the overall pranlukast market. In contrast, the original Cetus saw a slight increase to KRW 11.4 billion in Q1 this year compared to the same period last year.
- Company
- Asthma drug 'Trelegy 200 Ellipta Inhaler' becomes available
- by Eo, Yun-Ho Jul 01, 2025 06:03am
- Product photo of The asthma treatment 'Trelegy 200 Ellipta Inhaler' is now available for prescription at tertiary general hospitals. According to industry sources, Trelegy 200 Ellipta Inhaler (fluticasone furoate·umeclidinium·vilanterol) has passed the drug committees (DC) of Korea's 'Big 5' tertiary general hospitals, including Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary's Hospital, Asan Medical Center in Seoul, and Sinchon Severance Hospital. Trelegy 200 Ellipta Inhaler has been included in the insurance reimbursement list since March last year. It can be prescribed to patients with 'severe asthma that is not properly managed with combination therapy containing intermediate-dose or high-dose inhaler corticosteroid or long-acting beta2-agonists.' The efficacy of Trelegy 200 Ellipta was demonstrated through the Phase 3 CAPTAIN study, which compared Trelegy Ellipta and dual combination therapy FF/VI (fluticasone furoate/ vilanterol) in 2,436 adult asthma patients aged 18 and above whose asthma cannot be controlled despite treatment with dual combination therapy ICS/LABA. As the primary endpoint, each cohort’s Forced Expiratory Volume in 1 second (FEV1) changes at Week 24 were evaluated. The results demonstrated statistical significance as Trelegy Ellipta-treated cohort showed 110 mL more FEV1 compared with FF/VI-treated cohort. The safety profile of Trelegy Ellipta for asthma treatment in this study was consistent with the known profile of the individual drug components and their combinations. The most common side effects were nasopharyngitis v(13-15%), headache (5-9%), and upper respiratory tract infection (3-6%). Severe adverse responses occurred similar in all treatment groups. Dr. Jae-Won Jeong, a Professor at Inje University Ilsan Paik Hospital, explained, "Asthma is a chronic respiratory disease that requires lifetime management. It's important for patients who experience worsening of asthma to receive proper treatments." Additionally, Dr. Jeong said, "Major guidelines also recommend using triple combination therapy, with LAMA added to the ICS/LABA, as the optimal treatment option before the start of oral corticosteroid treatment for patients with severe asthma whose symptoms are not controlled with double therapy of ICS/LABA." Meanwhile, Trelegy 200 Ellipta is a once-daily single-inhaler triple combination therapy, approved in September 2022 as a maintenance therapy for Korean adult patients. It obtained approval in 10 countries, including the United States, Japan, Canada, and Taiwan, and is being prescribed globally. Additionally, Trelegy 100 Ellipta, approved for the first time in May 2018 in South Korea, is a once-daily single inhaler triple combination therapy for COPD and asthma. It was approved in June 2021 for national health insurance reimbursement as the COPD maintenance therapy. In March 2024, it expanded the scope of reimbursement for asthma indication.
- Company
- Dong-A ST-Ipsen to comarket prostate cancer drug Diphereline
- by Son, Hyung Min Jul 01, 2025 06:02am
- On the 30th, Dong-A ST signed a joint sales agreement with Ipsen Korea for the premature puberty and prostate cancer treatment ‘Diphereline.’ Dong-A ST (CEO Jae-Hoon Jeong) announced on the 30th that it has signed a joint sales agreement with Ipsen Korea (CEO Mi-sun Yang) for the premature puberty and prostate cancer treatment ‘Diphereline.’ Diphereline is a GnRH (gonadotropin-releasing hormone) agonist developed by the multinational biopharmaceutical company Ipsen, used to treat central precocious puberty and prostate cancer. The agreement ceremony was attended by Dong-A ST CEO Jae-hoon Jeong, Ipsen Korea CEO Mi-sun Yang, along with other officials from both companies, who reaffirmed their commitment to strengthening the partnership. Under the agreement, the two companies will jointly conduct domestic promotional and marketing activities for Diphereline starting July 1. Sales targeting general hospitals will be conducted jointly by both companies, while sales targeting clinics and private hospitals will be handled exclusively by Dong-A ST. Dong-A ST owns the growth hormone ‘Growtropin’ and urology drugs ‘Zydena’ and ‘Flivas,’ based on which the company accumulated extensive sales and marketing experience and expertise in the fields of pediatric endocrinology and urology. Ipsen Korea supplies various anticancer drugs in Korea, including ‘Diphereline,’ ‘Cabometyx’ for advanced renal cell carcinoma, hepatocellular carcinoma, and differentiated thyroid cancer, and ‘Somatuline’ for acromegaly and neuroendocrine tumors. Recently, the company has also entered the field of rare biliary diseases and plans to launch ‘Bylvay,’ a new drug for progressive familial biliary disease, in Korea. The two companies plan to actively expand Diphereline’s stance in the domestic market, forging synergy between the companies’ accumulated capabilities and expertise. Mi-sun Yang, CEO of Ipsen Korea, said, “Diphereline is the global standard therapy for children with precocious puberty and prostate cancer patients struggling with the boundaries between masculinity and cancer treatment. We plan to leverage Ipsen's scientific approach and Dong-A ST's expertise and network in the domestic market to bring Diphereline closer to patients in Korea.” Jae-hoon Jeong, CEO of Dong-A ST, remarked, “This collaboration with Ipsen Korea will serve as a crucial opportunity to expand the domestic supply of Diphereline and improve patient access. By combining the strengths and expertise of both companies, we aim to provide domestic patients with more effective treatment options and create synergies in the fields of precocious puberty and anticancer agents.”
- Company
- Metabolic disease drug development landscape evolves
- by Son, Hyung Min Jul 01, 2025 06:02am
- Innovative efforts in the development of new drugs for metabolic disorders such as obesity and diabetes are steadily gaining traction in Korea and abroad. The success of standalone GLP-1 therapies has driven the evolution toward multi-hormonal agents, and recently, major global pharmaceutical companies and biotech firms are accelerating new drug development by targeting not only GLP-1, GIP, and glucagon combinations but also new metabolic hormones such as FGF21 and IGF-1. In essence, a multi-target approach that goes beyond simple weight loss to regulate energy metabolism, insulin sensitivity, and liver fat improvement is becoming a reality. Development of multi-agonists starts with Mounjaro... Biomed announces Phase II trial According to industry sources on the 30th, the US pharmaceutical and biotech company Biomed Industries recently announced the results of its Phase II clinical trial for NA-931, an oral quadruple receptor agonist candidate for obesity and metabolic disorders. The results were presented at the American Diabetes Association's annual diabetic conference (ADA 2025) that was held in Chicago from the 21st to the 24th. NA-931 is a small-molecule drug that acts on four receptors: glucagon-like peptide (GLP-1), gastric inhibitory peptide (GIP), glucagon (GCG), and insulin-like growth hormone type 1 (IGF-1). Recently, research has also been conducted on quadruple receptor agonists that utilize additional metabolic-related hormones such as IGF-1, FGF21, and PYY, in addition to GLP-1, GIP, and GCG. FGF21 is a hormone produced in the liver that is involved in fat oxidation, insulin sensitivity, and temperature regulation. It is gaining attention as a next-generation target due to its ability to induce metabolic improvements similar to those observed with intermittent fasting. PYY is an appetite-suppressing hormone derived from the gut that can contribute to enhancing satiety by interacting with GLP-1. The Phase II clinical trial presented at the conference was a 13-week, randomized, double-blind, placebo-controlled, parallel-group study involving 125 adults with obesity (BMI ≥ 30 kg/m²) or overweight (BMI ≥ 27 kg/m²). The primary endpoints were the safety, tolerability, and weight loss efficacy of the investigational drug NA-931. Results showed that the group administered NA-931 at a dose of 150 mg once daily experienced an average weight reduction of 13.8% compared to baseline, with a statistically significant reduction of 12.4 percentage points compared to the placebo group. The incidence of treatment-emergent adverse events was also favorable. Gastrointestinal (GI) adverse events were mostly mild, with nausea and vomiting observed in 7.3% of participants and diarrhea in 6.3%. No muscle mass reduction was reported, and there was no clinically significant difference in the incidence of GI-related adverse events among subjects treated with NA-931 compared with placebo. The researchers explained, “NA-931 appears to induce weight loss while preserving muscle mass and has a lower incidence of side effects than existing drugs. It can be regarded as a viable alternative that can improve the shortcomings of existing GLP-1 class treatments.” Development of quadruple receptor agonists continue… to treat MASH in addition to obesity Clinical studies of quadruple receptor agonists targeting indications other than obesity are also actively underway. Among domestic companies, Wonjin Biotechnology has entered the clinical trial phase for a quadruple receptor agonist. Wonjin Biotechnology received approval in the US last year for the Investigational New Drug (IND) application for its candidate compound ‘OGB21502’ for the treatment of metabolic dysfunction-associated steatohepatitis (MASH) and fibrosis. OGB21502 is an innovative drug candidate that combines GLP-1, glucagon, FGF21, and an IL-1 receptor agonist (IL-1RA), an immune modulator, to simultaneously regulate metabolic disorders and chronic inflammation. OGB21502 induces appetite suppression and energy metabolism activation through GLP-1 and glucagon receptors while promoting lipid metabolism via FGF21 action. Additionally, by blocking IL-1 signaling involved in inflammation, the company expects it to demonstrate a multi-layered therapeutic effect by preventing inflammatory exacerbation of metabolic diseases. According to Wonjin Biotechnology, there is growing evidence that therapies blocking IL-1 signaling are effective in alleviating liver fibrosis. In fact, anakinra, a recombinant IL-1 receptor antagonist, has been shown in multiple studies to inhibit NLRP3 activation and reduce hepatic stellate cell (HSC) activity and blood-based fibrosis markers. In preclinical studies, OGB21502 demonstrated a reduction in lipid markers, including cholesterol and triglycerides, as well as the expression of fibrosis-related markers, compared to semaglutide and FGF21 analogs in obese mouse models. In a mouse model of non-alcoholic fatty liver disease (NAFLD), liver tissue staining analysis revealed that OGB21502 significantly reduced the non-alcoholic fatty liver disease activity score (NAS) and improved steatosis and lobular inflammation.
- Company
- AstraZeneca Korea launches ‘Lung Health Checkbus’ campaign
- by Whang, byung-woo Jul 01, 2025 06:00am
- Launch ceremony for AstraZeneca Korea AstraZeneca Korea announced on the 30th that it held a ceremony for the launch of its ‘Lung Health Checkbus’ campaign at the COEX Square in Seoul on the 27th. The campaign aims to help people detect lung nodules that they are not aware of at an early stage by operating buses equipped with AI-based chest X-ray imaging nationwide. AstraZeneca Korea, which has been making various efforts to create a world where lung cancer is no longer a cause of death, has partnered with the Korea National Tuberculosis Association and medical AI solution company Maihub to operate the ‘Lung Health Checkbus’ nationwide. Lung cancer is the leading cause of cancer-related deaths in Korea, according to 2023 data. Over the 5-year period from 2018 to 2022, the relative survival rate was 79.8% when the cancer was detected at an early stage, but the rate dropped sharply to 12.9% when the cancer had spread to distant parts of the body. However, it has been reported that more than 40% of patients are diagnosed with cancer at an advanced stage of metastasis, emphasizing the importance of regular screening. Low-dose chest CT is an effective screening method that can accurately detect lung cancer and reduce mortality, and AI-equipped chest X-rays are more effective than conventional X-rays in detecting lung nodules. According to a study comparing the lung nodule detection rates of AI-equipped chest X-rays and conventional X-rays at a single institution in Korea, the lung nodule detection rate in the AI group was more than twice that of the non-AI group. Se-Hwan Chon, General Manager of AstraZeneca Korea, said, “Lung cancer can affect anyone, so lung cancer screening is necessary even for non-smokers. In particular, lung cancer has a significantly higher survival rate when detected early, so it is important to detect it early through regular screening.” On the same day, AstraZeneca Korea signed a three-party memorandum of understanding (MOU) with the Korea National Tuberculosis Association and Maihub to ensure the successful operation of this campaign. Many citizens had chest X-rays taken at the lung health check bus set up on-site and received reports analyzed by artificial intelligence (AI) to check their lung health status for themselves. Min-Seok Shin, Chair of KNTA, said, “We find it meaningful that KNTA can expand its social responsibility beyond respiratory diseases to a broader range of diseases through the campaign. We plan to continue supporting customized health management programs to ensure that everyone, including medically vulnerable groups, can easily check their lung health. Hyuck Yang, CEO of Maihub, said, “This campaign is a meaningful example of public and private sectors coming together to create an AI-based chest X-ray examination environment that is easily accessible to everyone, going beyond simply providing technology. Maihub will contribute to the early detection of lung nodules through AI-based image interpretation solutions and strive to build a digital healthcare ecosystem.” Meanwhile, AstraZeneca Korea is a member of the Lung Ambition Alliance (LAA), a global non-profit collaboration organization, and is conducting various lung cancer awareness improvement activities to create a future where lung cancer is no longer a cause of death.
- Company
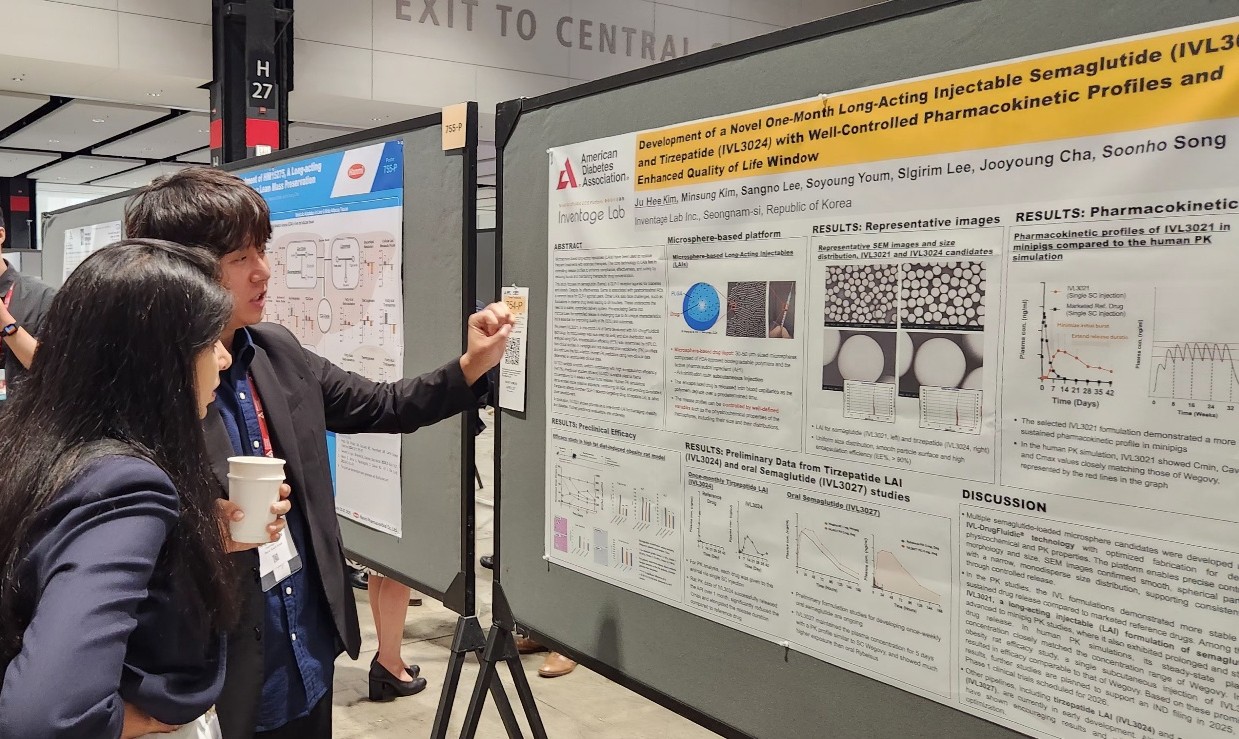
- New K-drugs for metabolic diseases make international debut
- by Son, Hyung Min Jun 30, 2025 06:07am
- Major Korean pharmaceutical and biotechnology companies have signaled their full-scale entry into global clinical trials, presenting new drug development results at overseas conferences. The companies presented their achievements in developing new drugs for various metabolic diseases, including obesity, type 2 diabetes, and metabolic dysfunction-associated steatohepatitis (MASH). Although most of the data disclosed is focused on early clinical or preclinical trials, the companies are attempting to diversify their mechanisms of action with triple agonists and oral small molecule formulations. Hanmi reveals triple agonist data... Oral and long-acting injectable formulations are also under development 30According to industry sources, the American Diabetes Association Diabetes Conference was held in Chicago, USA, from the 21st to the 24th. At the conference, various domestic companies, including Hanmi Pharmaceutical, Ildong Pharmaceutical, Dong-A ST, and Inventage Lab, revealed the clinical results of their novel drug candidates. Obesity drugs are rapidly emerging as a global R&D trend. With Novo Nordisk and Lilly's GLP-1-based obesity treatments becoming global blockbuster drugs, latecomers are also intent on developing their versions. Major domestic companies are conducting clinical studies on GLP-1 agonists in various areas, including obesity and MASH with drugs that have different methods of administration or focus on the quality of weight loss effects. Hanmi Pharmaceutical announced the results of its Phase I clinical trials and preclinical data for HM15275, a GLP-1 class triple agonist, and HM17321, a UCN2-based candidate substance, at the conference. First, Hanmi Pharmaceutical announced the results of the Phase I clinical trial for HM15275. HM15275 is a new obesity drug candidate that acts on glucagon-like peptide (GLP-1), gastric inhibitory polypeptide (GIP), and glucagon (GCG). No new drug with this mechanism targeting all three has been commercialized to date. The Phase I clinical trial was conducted on 74 healthy and obese adults. HM15275 was administered subcutaneously once a week for four weeks, followed by an evaluation of the candidate drug’s safety, tolerability, pharmacokinetics, and pharmacodynamics. The results confirmed HM15275’s tolerability and safety. Specifically, the average weight loss rate at day 29 in the highest dose group was 4.8%. At day 43, the maximum weight loss rate was 10.6%. Preclinical data for HM15275 demonstrated greater weight loss efficacy compared to semaglutide (brand name Wegovy) and tirzepatide (Zepbound), which are currently marketed as obesity treatments. In animal models, switching from tirzepatide to HM15275 resulted in additional weight loss effects. Hanmi Pharmaceutical also announced the results of preclinical studies on HM17321, a new drug candidate that simultaneously targets weight loss and muscle gain. HM17321 is a UCN-2 analogue that selectively targets the CRF2 (corticotropin-releasing factor 2) receptor rather than GLP-1 or other incretin receptors. It is being developed as an innovative first-in-class drug that selectively reduces fat while increasing muscle mass. HM17321 demonstrated weight loss effects and improved body composition in both mouse models and non-human primate models. Yunovia, a subsidiary of Ildong Pharmaceutical Group specializing in new drug research and development, is conducting Phase I clinical trials for ID110521156, a GLP-1 receptor agonist class new drug candidate targeting metabolic disorders such as diabetes and obesity. ID110521156 is a low-molecular-weight compound-based drug, and the company aims to develop it as an oral synthetic new drug for diabetes and obesity with distinct advantages such as superior productivity and excellent ease of use over existing representative treatments like peptide injections. Previously, Yunovia confirmed the efficacy of insulin secretion and blood glucose control through preclinical efficacy and toxicity evaluations. It also demonstrated superior safety compared to competing drugs in the same class and confirmed the drug's characteristics in a recently completed Phase 1 single-ascending dose (SAD) trial. According to the study poster presented at the conference, in the single-dose escalation trial, ID110521156 demonstrated good tolerability with fewer gastrointestinal side effects across the entire effective dose range, unlike existing GLP-1 class drugs. Inventage Lab also introduced preclinical data for its 1-month long-acting injectable formulations ‘IVL3021’ and ‘IVL3024’ based on semaglutide and tirzepatide, as well as its oral semaglutide formulation ‘IVL3027.’ After GLP-1 class obesity treatments such as Saxenda, Wegovy, and Zepbound emerged as global blockbuster drugs, the pharmaceutical industry has been actively pursuing formulation changes. The existing drug Saxenda requires a once-daily injection, while Wegovy and Zepbound require weekly injections. Oral formulations or long-acting injectables are expected to gain a competitive edge in terms of convenience of administration if commercialized. According to the company's preliminary preclinical results, IVL3021 showed stable drug release in the blood over a one-month period. Also, the long-acting injectable suppressed initial over-release and maintained stable drug release. IVL3027 demonstrated high bioavailability compared to existing oral formulations and sustained drug release over a one-week period. Poster presentation by Inventage Lab (Source=Inventage Lab). MASH clinical trial results also announced Dong-A ST and its subsidiary MetaVia announced the results of non-clinical studies on DA-1241, which is being developed as a treatment for MASH, and combination therapy that uses efruxifermin, a fibroblast growth factor (FGF21) analog. Metabolic dysfunction-related fatty liver disease was previously referred to as non-alcoholic steatohepatitis (NASH), but overseas academic societies such as the American Association for the Study of Liver Diseases have decided to change the name to metabolic dysfunction-associated steatohepatitis (MASH). To date, Madrigal's rezdiffra is the only new drug for MASH that has cleared regulatory hurdles. The U.S. Food and Drug Administration (FDA) approved rezdiffra in March last year for the treatment of adult patients with non-cirrhotic MASH in combination with diet and exercise. Rezdiffra is a selective thyroid hormone receptor (THR)-β agonist designed to target the core pathophysiological mechanisms of MASH within the liver. The pharmaceutical industry is also developing new drugs for MASH that target not only THR-β but also GLP-1 and FGF21, which influence lipid metabolism. DA-1241 is a synthetic new drug that activates GPR119. Preclinical results have confirmed that DA-1241 improves blood sugar and lipid levels and directly acts on the liver to improve inflammation and fibrosis, making it a promising candidate for MASH treatment. A Phase 2a clinical trial targeting patients with estimated MASH was completed in December last year. Efruxifermin is a recombinant protein designed based on FGF21 (Fibroblast Growth Factor 21), a hormone secreted by the liver. FGF21 is involved in energy consumption and the regulation of glucose and lipid metabolism in the body and is used as a target for the development of treatments for MASH, obesity, diabetes, and other conditions. According to the study results presented at the conference, in the DA-1241+efruxifermin combination therapy group, approximately 94% of subjects showed an improvement of 2 points or more in NAS (Non-Alcoholic Steatohepatitis Activity Score) compared to baseline. Additionally, the DA-1241+efruxifermin group showed a significant reduction in liver fibrosis area compared to the MASH control group that did not receive combination therapy, and in some individuals, a decrease in fibrosis stage was observed compared to pre-treatment levels. Dong-A ST is currently conducting clinical studies on the combination therapy of DA-1241 with semaglutide, a GLP-1 agonist, in addition to a Phase II clinical trial on DA-1241 as monotherapy.
- Company
- Adempas may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jun 30, 2025 06:06am
- Adempas, a new treatment for pulmonary arterial hypertension that has emerged after a long wait, is now available for prescription at general hospitals in Korea. According to industry sources, Bayer Korea's Adempas (riociguat) has been approved by the Drug Committee (DC) of tertiary hospitals in Korea, including Samsung Medical Center and Seoul National University Hospital. As it has been listed for reimbursement since this month (June), the number of medical institutions that can prescribe it is expected to continue to increase. Adempas was approved in Korea as an orphan drug in June 2014 and is available in 5 dosage forms. It is indicated for: ▲Improvement of exercise capacity in adult patients with chronic thromboembolic pulmonary hypertension (CTEPH, WHO Group 4) who are unable to undergo surgery or who have persistent or recurrent symptoms after surgery ▲Improvement of exercise capacity adult patients with pulmonary arterial hypertension (WHO Group 1) who are classified as having functional class II or III. In particular, it was known as the first new drug for CTEPH. CTEPH is caused by patients who develop chronic pulmonary embolism, which leads to fibrotic stenosis and occlusion, resulting in pathological vascular remodeling and increased resistance in the pulmonary artery. CTEPH is a chronic disease that causes progressive dyspnea and right heart dysfunction, which weakens the heart. Symptoms include dyspnea, fatigue, chest pain, dizziness, peripheral edema, cough, and hemoptysis, which reduces the patient’s quality of life. Ultimately, it can progress to heart, kidney, and liver failure, which can lead to death. Meanwhile, Adempas is a stimulator of soluble guanylate cyclase (sGC), an enzyme found in the heart and lungs, and its efficacy has been confirmed in two Phase III clinical trials in patients with chronic thromboembolic pulmonary hypertension. Results showed improvement in exercise capacity, which was the primary endpoint, and good tolerability. No unexpected adverse reactions were reported. In the CHEST-1 study, when comparing the 6-minute walking test (6MWT) results after 16 weeks with the baseline, results showed that the group of patients who received riociguat showed statistically significant improvement compared to the group of patients who received placebo. In the PATENT-1 study, the change in the 6MWT score after 12 weeks of treatment, showed statistically significant improvement in the riociguat arm compared to placebo, meeting the primary endpoint.
- Opinion
- [Reporter's View] Divisional restructuring needed
- by Lee, Hye-Kyung Jun 30, 2025 06:06am
- During his candidacy, President Lee Jae-myung pledged to designate the biotech industry as a cutting-edge sector and build it as the future growth engine, aiming to position South Korea as one of the world's five strongest biotech countries. Based on an analysis that the previous government was short on investment in the pharmaceutical and biotech industry, President Lee will likely plan to implement policies geared at establishing special funds and fostering talents with expertise to strengthen national investment and responsibility in the pharmaceutical and biotech industry. Firstly, there will be expanded support at the R&D center, focusing on venture technologies. To achieve this, regulatory support is crucial. To set a goal of becoming a strong biotech country, aggressive R&D must be pursued. Additionally, supporting measures for venture firms with less regulatory experience are also necessary. Celltrion, for instance, which successfully overtook the No. 1 place in exporting biopharmaceuticals in South Korea, was established in 2002 as a biotech venture company. Therefore, when aggressive R&D takes place, there will be more venture firms with fewer years of regulatory experience that will robustly grow. The problem lies in whether the Ministry of Food and Drug Safety (MFDS), which is responsible for providing regulatory support to make South Korea one of the top five global biotech powerhouses, can properly fulfill its role. In particular, the division responsible for pharmaceutical approvals is operating as a temporary task force (TF), with a fixed staff of only seven people managing all approval-related tasks for biopharmaceuticals, Korean traditional medicines (han-yak), and quasi-drugs. Although the current government has promised active investment in biotech, it remains uncertain to determine how much will be allocated for pre-registration or applications for new drug approval. Furthermore, the government implemented an innovative measure to shorten the new drug approval duration from 420 to 295 days this year, which also includes biopharmaceuticals. According to the number of approved new drugs by year, the number of chemical medicines decreased from 29 in 2023 to 11 in 2024. In contrast, the number of biopharmaceuticals increased during the same period, from 8 to 12 cases. For biopharmaceuticals, which are often high-priced, cutting-edge therapies for rare·intractable diseases or cancers with significant medical demand, the approval process is complex. If the approval division isn't functioning properly, it becomes challenging to ensure swift internal and external communication or make highly reliable decisions based on scientific·legal reviews. Related to this, it's puzzling why the Pharmaceutical Approval Management Division remains a task force (TF). This bio approval TF was established in May 2024 when the Vice Minister's direct reporting divisions for overall approval management and advanced product approval were reorganized and redistributed across three bureaus: Pharmaceutical Safety Bureau, Biopharmaceuticals and Herbal Medicines Bureau, and Medical Device Safety Bureau, to strengthen the linkage between medical product approvals and policy. While this reorganization converted two existing divisions into three, resulting in 'Approval Divisions' for pharmaceuticals and medical devices, only bio approvals were structured as a "team"-based TF. Reportedly, MFDS faced limitations in creating new official divisions under its organizational regulations, leading to the establishment of permanent divisions for pharmaceuticals and medical devices, which handle a relatively higher volume of public complaints and regulatory support). Yet, it's difficult to comprehend. At the same time, the bio-related function was set up as a temporary task force. This is particularly difficult to understand given that becoming a biotech powerhouse has been a national priority since the previous administration, and it's a commitment significant enough to be a presidential pledge in the current government. The Pharmaceutical Approval Management Division serves as the manager for the entire approval process and acts as a communication channel between external stakeholders and the MFDS. Operating as a temporary organization with only seven full-time staff means that even the absence of a single employee can jeopardize the swift processing of applications and approvals. If the approval department doesn't function properly, it becomes difficult to ensure quick internal and external communication or make reliable decisions based on scientific and legal reviews. While the current proportion of pharmaceuticals in the overall medical product industry in South Korea may still be low, there's significant potential for further growth, depending on the role of the President Lee government. To accelerate the commercialization of domestically developed biopharmaceuticals and secure a leading global position, the MFDS will likely need to reorganize its structure to provide proactive regulatory support.
- Policy
- More generic 'Vimovo' drugs with naproxen+PPI enters the mkt
- by Lee, Tak-Sun Jun 30, 2025 06:05am
- Product photo of the original drug Generic drugs containing the same active ingredients as the 'Vimovo' (naproxen+esomeprazole magnesium trihydrate), a combination of an anti-inflammatory drug and an anti-ulcer agent, are set to be released. A generic has not been approved since Chong Kun Dang's 'Naxen S Tab' was approved in 2024. Considering the characteristics of a generic containing two types of active ingredients, proving a pharmaceutical equivalence test on each active ingredient may have been challenging. Furthermore, analysis suggests new product entry to this market poses a challenge due to 'Naxozole,' a salt-changed product that was launched at a relatively lower price, with a strong presence. According to the industry on June 27, four generic Vimovo drugs, including KyungDong Pharm's 'Nasopra Tab 500/20 mg,' will be listed with reimbursement next month. KyungDong Pharm is the organizing company. The company's Nasopra Tab 500/20 mg met two types of requirements. Thus, the price was assessed at KRW 715, the same as the original Vimovo. In contrast, the drug price of the generics meeting only one requirement was set as KRW 608. These include Genuonesciences' 'Gemovo Tab 500/20 mg,' Mother's Pharmaceutical's 'Vimo M Tab. 500/20 mg,' and Dongkook Pharmaceutical's 'Exoraxen Tab 500/20 mg.' These drugs treat symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis in patients at risk of gastric or duodenal ulcers associated with NSAIDs, such as naproxen, or in those who are not adequately treated with low-dose naproxen or other NSAIDs. Joined later by four companies, products containing the same active ingredients as Vimovo have now increase from two to six. Previously, LG Chem's 'Vimovo Tab 500/20 mg' and Chong Kun Dang's 'Naxen S Tab' were the only generics available. Furthermore, Naxen S Tab has been on the market for over 10 years since its approval in 2014. Even without re-examination periods or patent barriers, generic versions have not been made available, likely due to the significant difficulty of conducting bioequivalence tests for this combination therapies. To prove bioequivalence for generic Vimovo drugs, it's necessary to compare the human absorption rates of each component, naproxen and esomeprazole. This process is complex, and achieving favorable results is challenging. Korean pharmaceutical companies have shifted their development efforts towards modified drugs that use a different salt of esomeprazole. Currently, there are five such salt-changed products available, including Hanmi Pharm's 'Naxozole Tab. 500/20 mg. Naxozole recorded KRW 25.8 billion in outpatient prescription sales last year, according to UBIST data, surpassing the original Vimovo (KRW 21.7 billion). The issue, however, is that Naxozole is relatively low-priced, at KRW 445 per tablet, which is the cheapest among competing products. Naxen S, a generic version of Vimovo, is relatively inexpensive at KRW 490 and recorded KRW 4.2 billion in outpatient prescription sales last year. In contrast, these newly reimbursed generic drugs are priced KRW 100-200 higher than Naxen S, based on the estimation criteria. Therefore, it's uncertain whether they will exhibit drug price competitiveness against Naxozole and Naxen S in the market. However, an analysis suggests that if they enter the market with high CSO (Contract Sales Organization) fees based on their higher drug prices, they could increase their market share. Amid other generic companies also preparing for market entry, attention is now focused on the market performance of the products from KyungDong Pharm and the other three companies that have recently obtained reimbursement.