- LOGIN
- MemberShip
- 2025-12-21 04:26:54
- Product
- KPDS ‘Gov’t action needed to counter price hike pressure’
- by Jul 08, 2025 06:35am
- The Korean Pharmacists for Democratic Society (President: Kyung-Lim Jeon, KPDS) urged the government to take decisive action against Trump and multinational pharmaceutical companies' pressure to raise drug prices. In a statement released on the 4th, the KPDS stated: “Trump signed an executive order on May 12 demanding a reduction in drug prices within the United States, claiming that ‘the United States accounts for less than 5% of the world's population but generates three-quarters of the global pharmaceutical industry's profits.” This is intended to support U.S. pharmaceutical companies by expanding their access to foreign markets, enabling them to lower drug prices within the United States while increasing profits in other countries.” However, ironically, the U.S. pharmaceutical industry has expressed opposition to President Trump's “most-favored-nation (MFN)” policy for the U.S., citing concerns that its double standard could lead to reduced investment and lower new drug development. The KPDS also pointed out that the U.S. pharmaceutical industry has long opposed policies that view health as a basic social security for citizens and seek to establish a foundation for the health of individuals and society as a whole through a national health insurance system and drug price control policies. Rather, the companies demanded a society where pharmaceutical companies can sell drugs at monopolistic prices that guarantee maximum profits, packaging the anti-market system of patent monopolies as “fair trade.” They have also sought to extend patent periods or operate various monopolistic systems, such as through the new drug product exclusivity system, and have exploited loopholes in the patent system. The KPDS stated, “US President Trump's executive order to lower drug prices acted as a catalyst, turning into a storm of drug price hikes for South Korea and many other countries. The Korean government must recognize this change in the trend and respond proactively.” The KPDS proposed 3 countermeasures. The first is to improve the drug pricing system being promoted by the government to reflect the innovative value of treatments. The currently promoted system is not a system for patients, but rather a capitulation to the demands of multinational pharmaceutical companies to raise drug prices. Efforts to increase bargaining power to counter the rapidly rising prices of new drugs should be prioritized. They also urged the government to seek international cooperation to counter the pharmaceutical companies' unreasonable demands for price hikes. KPDS stated, "The Korean pharmaceutical market accounts for only about 1% of the global market. The Korean government's bargaining power is so weak that multinational pharmaceutical companies can easily abandon the Korean market if they so desire. Recently, Europe has been countering the high prices of new drugs by forming alliances with neighboring countries to negotiate drug prices with companies. Korea must also build alliances with neighboring countries in various ways to strengthen its bargaining power with multinational pharmaceutical companies in drug price negotiations." In addition, they proposed the need to establish policies that would substantially reduce drug costs. The fundamental reason for the high price of drugs in the United States is that the government has excessively overestimated the value of drugs under the pretext of fostering domestic pharmaceutical companies and has made no effort to control drug prices. KPDS added, “The reason why rebates by Korean pharmaceutical companies continue is that the drug pricing policies that are lenient to domestic companies provide incentives for companies to sell drugs even if they have to pay extra to doctors. Amid rapid aging, South Korea's drug pricing policy needs to be readjusted, and it is urgent to establish new policies to resolve drug pricing issues, such as the 2006 ‘Drug Price Rationalization Plan.’” "From the perspective of reasonable corporate profits, drug prices in South Korea are not low. There is a growing trend of high-priced new drugs receiving economic evaluation waivers during review, and generic drug prices are among the highest in the world. From the U.S. perspective, demanding that all countries pay high drug prices on the grounds that South Korea's drug prices are low is not justifiable. Instead, the U.S. should review and reform its overly protective pharmaceutical patent monopoly system.
- Company
- PCV21 emerges…evidence-based vaccination policies discussed
- by Whang, byung-woo Jul 07, 2025 06:10am
- "To improve pneumococcal disease prevention in Korea, an evidence-based pneumococcal vaccination policy is essential. It's crucial to evaluate the efficacy of existing vaccines and conduct cost-effectiveness assessments for new vaccines based on domestic data." Despite the implementation of the National Immunization Program (NIP), pneumococcal disease remains a significant public health issue in South Korea. Therefore, experts emphasize the need to strengthen vaccine strategies, particularly targeting high-risk populations. Dr. Jung Yeon Heo, a professor at Ajou University HospitalDr. Jung Yeon Heo, a professor at Ajou University Hospital's Infectious Diseases Department, an expert in the field, emphasized the need for a 'dual protection strategy,' which involves the direct vaccination of high-risk adults in addition to pediatric pneumococcal vaccination. Pneumococcal infection is known to be fatal for elderly people, causing not only pneumonia but also various invasive diseases such as bacteremia and meningitis. Specifically, adults aged 65 and older face a greater risk of pneumococcal pneumonia and invasive infection. The risk of infection further increases for adults with chronic diseases compared to healthy adults of the same age. Dr. Heo explained, "Invasive Pneumococcal Disease (IPD) primarily occurs in high-risk groups, including adults aged 65 and older, immunocompromised individuals, and patients with chronic kidney disease or heart disease," and added, "Generally, the prevalence of these chronic or immunocompromised conditions increases with age, leading to a higher risk of pneumococcal infection in elderly people." Since 2013, South Korea has provided protein conjugate vaccines (PCV) for children and 23-valent polysaccharide vaccines (PPSV23) for adults (aged 65 and older) through the NIP. However, while the pediatric PCV vaccination rate is high at approximately 97%, the PPSV23 vaccination rate for adults aged 65 and older is only about 54.5%. Currently, there are concerns about the intergenerational transmission of pneumococcal bacteria, as the number of grandparents caring for grandchildren increases. Regarding this, Dr. Heo stated that indirect effects of reduced adult pneumococcal infection can be expected from pediatric vaccination, based on domestic and international cases. However, he also emphasized the importance of direct vaccination for a sufficient preventive effect. Dr. Heo said, "While indirect effects of reducing pneumococcal disease in adults can be expected from pediatric vaccination, indirect effects alone are not sufficient for adequate prevention in adults," and stressed, "In addition to pediatric vaccination, adult pneumococcal vaccination is also crucial." The distribution of serotypes also highlights the importance of prevention in elderly people. According to Dr. Heo, the most common pneumococcal serotypes in Korean adults are 3 and 19A. Despite these two serotypes being included in the currently used 13-valent pneumococcal conjugate vaccine (PCV13), they still cause infections. Dr. Heo pointed out, "This shows that even though the domestic pediatric vaccination rate is high, nearing 95%, for some serotypes, pediatric vaccination alone is not sufficient for full prevention." He added, "For certain serotypes, indirect effects alone are insufficient for adequate prevention, providing evidence that adults also need pneumococcal vaccination." Discussion of sequential·single-dose vaccination strategies..."Vaccine characteristics must be considered" However, with the emergence of newly approved pneumococcal vaccines, there is also anticipation for expanding the scope of pneumococcal disease prevention. Recently, the Korean Society of Infectious Diseases issued revised recommendations, recommending sequential vaccination with PCV15 + PPSV23 or single-dose vaccination with PCV20 for all individuals aged 6 months and older, as well as for high-risk individuals aged 19-64. Regarding sequential vaccination, Dr. Heo explained, "Vaccination is needed to enhance the immunogenicity in high-risk groups for pneumococcal disease while including as many serotypes as possible. This strategy was proposed because combining PCV's strong immune induction effect with PPSV23's broad serotype coverage can lead to more comprehensive and potent preventive effects." He also stated, "If patient convenience is prioritized, a single injection of PCV20 might be a simpler approach." However, he added, "The main reason why the sequential vaccination strategy is recommended is due to considerations of PPSV23's efficacy and cost-effectiveness." Currently, PPSV23, provided free through the domestic NIP, is considered highly cost-effective as it can prevent a wide range of serotypes at a relatively low cost. Conversely, individuals who can afford to cover the cost may opt for non-reimbursed vaccination with a single dose of PCV20, which is not covered by insurance benefits. Dr. Heo advised, "For those who can bear the cost, a single-dose PCV20 strategy can be considered. However, for those who wish to benefit from the NIP, sequential vaccination with PCV15 and PPSV23 is a good choice." He further stated, "Since each vaccine has its pros and cons and overall effects are similar, it's difficult to conclude that one vaccine is superior to another." He added, "Physicians should thoroughly explain the characteristics and differences of each vaccine and then decide on the appropriate vaccination method together with the patient." "PCV21 vaccine is expected to be introduced...Expectation for adult prevention effectiveness" In this context, the 21-valent pneumococcal conjugate vaccine (PCV21) is expected to be approved this year. Regarding this, Dr. Heo explained, "Theoretically, the PCV21 vaccine can prevent the broadest range of serotypes in adults," and added, "At the Infectious Diseases Society conference, attendees showed a preference for PCV21 among the 15-valent, 20-valent, and 21-valent options." PCV21 is distinguished from existing vaccines by excluding serotypes included in the original PCV7 and incorporating the most non-vaccine type (NVT) serotypes whose adult incidence has increased due to serotype replacement phenomena following vaccine use. Dr. Heo stated, "Considering even the indirect effects of pediatric vaccination, the 21-valent vaccine could be the ideal vaccine." He added, "However, what strategy will be most effective for adults will need to be determined through real-world data from future field use." Dr. Heo also emphasized the establishment of an evidence-based vaccine policy to improve the pneumococcal prevention environment in South Korea. In South Korea, PPSV23 is currently provided free of charge to adults aged 65 and older. However, with the emergence of new vaccines, a multi-faceted review is necessary. Dr. Heo pointed out, "As new pneumococcal vaccines continue to be introduced, we need to closely analyze the efficacy of existing vaccines and domestic usage data. When introducing new vaccines, cost-effectiveness must also be reviewed." He added, "However, to respond to diverse serotype distributions and serotype changes resulting from vaccine use, a pneumococcal vaccine covering a broader range of serotypes is needed." Finally, Dr. Heo suggested, "While expanding the adult NIP is not easy at the moment due to cost issues, we have no choice but to follow the trend as vaccine technology advances. Systematic policy preparation considering complex factors is necessary."
- Company
- PKU drug Sephience receives orphan drug designation in KOR
- by Eo, Yun-Ho Jul 07, 2025 06:10am
- Sephience, a new drug for phenylketonuria (PKU), a rare metabolic disorder, has been designated as an orphan drug in Korea. The Ministry of Food and Drug Safety recently announced so through a public notice. Specifically, the drug is indicated for the treatment of hyperphenylalaninaemia (HPA) in adult and pediatric patients with phenylketonuria (PKU). Sephience (sepiapterin), which was developed by U.S. biopharmaceutical company PTC Therapeutics, recently received marketing authorization from the European Commission and is currently undergoing approval procedures with the U.S. FDA. More specifically, the drug is an oral formulation of tetrahydrobiopterin, a critical enzyme cofactor involved in the metabolism of various biological substances. Tetrahydrobiopterin is known to reduce phenylalanine levels in the blood of patients with phenylketonuria. The efficacy of Sephience was confirmed in the Phase III APHENITY trial. In the trial, the phenylalanine levels in the sepiapterin arm decreased by an average of 63%. In detail, 84% of patients achieved phenylalanine levels below 360 µmol/L, which is the target level according to treatment guidelines, and the majority of participants successfully controlled their levels. Meanwhile, phenylketonuria is an autosomal recessive metabolic disorder caused by a deficiency of the enzyme that breaks down phenylalanine, which is present in proteins at levels of 2% to 5%. This deficiency leads to seizures and developmental disorders. Patients born with this congenital deficiency of the enzyme are known to have congenital impairments compared to the general population, resulting in intellectual disabilities, light brown skin and hair, and seizures. Early diagnosis and treatment during infancy are essential, and patients are required to follow a lifelong diet restricted in phenylalanine. If left untreated, elevated phenylalanine levels in the blood can lead to hyperphenylalaninemia, causing severe damage to the brain, liver, heart, and kidneys over time.
- Policy
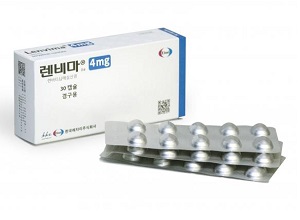
- Boryung's follow-on Lenvima, 'Lenvanib,' becomes reimbursed
- by Lee, Tak-Sun Jul 07, 2025 06:09am
- Product photo of LenvimaBoryung has successfully obtained reimbursement listing for all dosages of 'Lenvanib Cap (lenvatinib mesylate dimethyl sulfoxide),' its follow-on drug to the anti-cancer medication Lenvima (lenvatinib mesylate, Eisai), which is used to treat conditions such as liver cancer. With the patent dispute with the original manufacturer, Eisai, still ongoing, Boryung is expected to commence sales following the listing of reimbursement. According to industry sources on July 4, Boryung's Lenvanib Cap 10 mg and Lenvanib Cap 12 mg were listed for reimbursement this month. Consequently, all three approved products, including Lenvanib Cap 4mg, which was listed for reimbursement in May, can be reimbursed. Lenvanib Cap is the first follow-on drug to Eisai's Lenvima in Korea. Unlike Lenvima, it has a solvate (dimethyl sulfoxide) attached. Boryung proved its equivalence to Lenvima through bioequivalence testing. Through this, Boryung filed patent invalidation or circumvention, and most of its claims were accepted by the Intellectual Property Trial and Appeal Board. However, Eisai has appealed the rulings concerning the invalidation of the use patent and the circumvention of the composition patent, and these disputes are currently ongoing at the Intellectual Property Court of Korea. The drug price for Lenvanib Cap 4mg and Lenvanib Cap 10mg is the same at KRW 26,765, while Lenvanib Cap 12mg is KRW 29,442, making them slightly cheaper than the original drug. The original product, Lenvima Capsule 4mg and 10mg, costs KRW 29,739. There is no 12mg product for the original drug. The approved indications for both products are identical, covering a total of four efficacies·effects, ▲Locally recurrent or metastatic progressive differentiated thyroid cancer refractory to radioactive iodine ▲First-line treatment of unresectable hepatocellular carcinoma (HCC) ▲Combination therapy with pembrolizumab for the treatment of advanced endometrial carcinoma that is not MSI-H (microsatellite instability high) or dMMR (mismatch repair deficient), in patients who have received prior systemic therapy and whose disease has progressed, and for whom surgical or radiation therapy is not suitable ▲First-line treatment of advanced renal cell carcinoma in combination with pembrolizumab. Among these, the two companies are disputing over the use patent related to thyroid cancer. The substance patent expired in April. As Boryung has successfully obtained reimbursement listing for all three dosages of its follow-on drug to Lenvima, it is expected to proceed with sales based on the Intellectual Property Trial and Appeal Board's ruling. However, an analysis suggests that Boryung is likely to proceed cautiously with sales activities until the court results are finalized, as a loss in the ongoing patent litigation at the Intellectual Property Court of Korea could lead to market withdrawal and the risk of patent infringement compensation. The original Lenvima recorded sales of KRW 10.3 billion in 2023, based on IQVIA data.
- Policy
- MFDS reviews GMP standards for sterile preparations abroad
- by Lee, Hye-Kyung Jul 07, 2025 06:09am
- With the Ministry of Food and Drug Safety planning to enforce revised GMP standards for sterile drug products reflecting PIC/S international standards in December, it plans to identify research tasks on items that require international harmonization by analyzing the regulatory status of reference countries. Ahead of its re-entry into PIC/S in 2023, the MFDS announced the “Regulations on Drug Manufacturing and Quality Control (MFDS Notice)” that contained a risk assessment-based systematic contamination management strategy and operational plan to enhance the quality assurance level of sterile drug products. Instead of implementing PIC/S-level strengthened GMP for sterile preparations in December as planned, the MFDS has decided to establish guidelines for large-volume parenteral solutions, contamination control strategies (CCS), and PUPSIT (Pre-use Post-sterilization Integrity Testing), referring to the results of the “Study on the Implementation of Sterile GMP Regulatory Harmonization” currently being conducted by the Manufacturing Quality Innovation Committee of the Korea Pharmaceutical and Bio-Pharma Manufacturers Association. Additionally, the MFDS recently announced a bid for the “Study on Implementation Strategies for Regulatory Harmonization of Manufacturing and Quality Control Based on Global GMP Trend,” aiming to establish implementation strategies for manufacturing and quality control based on an analysis of international regulatory trends, including those of advanced pharmaceutical countries and PIC/S regulations, to support the stable adoption of regulatory systems within the domestic pharmaceutical industry. The objective of the study is to establish a strategic foundation for regulatory harmonization in the pharmaceutical GMP field and expand domestic pharmaceutical exports by conducting a gap analysis based on research into international GMP regulatory trends. To this end, the regulatory status and implementation cases of advanced pharmaceutical countries and major PIC/S member countries (such as the United States, Europe, and Japan) will be studied, and gap analysis will be conducted between domestic GMP regulations and PIC/S GMP regulations to identify research tasks requiring international regulatory harmonization. In particular, the gap analysis will include the period before and after the revision of domestic GMP standards for sterile drug products, and future research tasks will include consideration of aseptic testing for large-volume parenteral solutions, the establishment of a CCS, and the application of PUPSIT. In addition, the formation of a pool of domestic and international experts in related fields, and consulting with relevant experts when necessary or holding seminars or presentations for the domestic pharmaceutical industry with invited external GMP experts as needed will also be studied. Based on the results of this study, the Ministry of Food and Drug Safety (MFDS) plans to develop draft guidelines for research topics requiring international regulatory harmonization.
- Company
- ‘Need to institutionalize reinvestment of funds into R&D'
- by Kim, Jin-Gu Jul 07, 2025 06:09am
- A recommendation was made for the implementation of a system that reinvests the savings from drug price reductions by pharmaceutical and biotech companies into R&D for new drugs and ensures a fixed price during the early stages of a new drug's market launch. Professor Jeonghoon Ahn of the Department of Convergence Health Sciences at Ewha Womans University stated in an issue report published by the Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA) on the 3rd, “Post-marketing price management and R&D support must be designed to achieve policy coherence,” and proposed, “A predictable environment must be established to build a virtuous cycle in the pharmaceutical and biotech industry.” “Reinvesting savings from drug price cuts into R&D…differentiated price cut rates should be applied based on investment scale” Professor Ahn emphasized the need to move away from the dichotomy of viewing drug price cuts as a simple cost-cutting measure. He explained that a system should be established to encourage companies to reinvest the funds saved from drug price cuts into R&D. Professor Ahn proposed the institutionalization of a “drug price cut reinvestment system” as an alternative. He predicted that this system would enable efficient fiscal management without stifling industrial growth. Specifically, he proposed ▲differentiating drug price reduction rates based on the scale of a company's R&D investment, ▲institutionalizing the reinvestment of funds saved through drug price reductions into R&D, and ▲establishing a new program where the government and private sector jointly support high-risk stages of research such as Phase III clinical trials and allow recover of a certain portion of their support through sales upon success. Ahn also said that a system to guarantee the initial drug price of new drugs for a certain period of time is necessary. Similar to Japan's “price maintenance premium” system, this system guarantees drug prices for a certain period of time after the launch of a new drug and limits excessive price reductions during the patent period. Ahn also suggested that government-funded R&D should be reflected in drug pricing by allowing cost-based pricing for new drugs, and that a risk-sharing agreement should be established to enable companies to recover their investment. “Failed research should also be granted tax credits... Overall improvement of tax incentives is necessary” Along with improving the drug pricing system, Professor Ahn diagnosed that overall improvement of tax policies is necessary. In particular, he emphasized that failed research should also be included in tax credits, considering the possibility of failure in new drug development. In addition, he argued that tax support should be expanded for costs incurred during Phase III clinical trials, such as patient recruitment, CRO contracts, and data analysis. Furthermore, he noted the need for flexibility across the board in the system, including: ▲recognition of costs for purchasing clinical trial drugs and animals as material expenses; ▲clarification of criteria for verifying overseas clinical trial costs; and ▲relaxation of registration requirements for research institutes engaged in commissioned or joint R&D. Additionally, they suggested that the following issues should be reviewed: ▲reflecting the payment structure for each clinical stage in tax laws; ▲applying an exception to the minimum corporate tax rate based on the scale of R&D investment; ▲expanding the scope of tax credit carryover; ▲applying preferential tax rates on patent revenues earned through the introduction of a patent box system; and ▲shortening the depreciation period for research equipment. Professor Ahn said, “Given the high level of uncertainty in the pharmaceutical and bio industry, there must be substantial tax incentives for companies to decide on bold investments from a long-term perspective,” adding, “Tax and drug price systems must be organically linked for a virtuous cycle of R&D reinvestment to function.” “Risks of new drug development must be mitigated through predictable systems…Post-marketing management system needs reform” Professor Ahn pointed out that the current post-marketing drug price management system causes uncertainty in companies' R&D decision-making. Ahn criticized that the price-volume price linkage system and the actual transaction price reduction system are operated in a fragmented manner, resulting in repeated price reductions for certain items. They also pointed out that the drug price calculation and reduction criteria are unclear, making it difficult for companies to predict. To address these issues, he emphasized the need for structural improvements, including: ▲integrating the implementation timeline of the post-marketing drug price management system; ▲introducing an “R-zone” in the actual transaction price-based price reduction system; and ▲mitigating the concentration of price reductions on specific drug formulations. Professor Ahn stated, “The drug price management system and tax support measures must be designed in a predictable form so that companies can confidently invest in new drug development. This could lead to the enhancement of technological capabilities and global competitiveness in the domestic pharmaceutical and biotechnology industry.”
- Policy
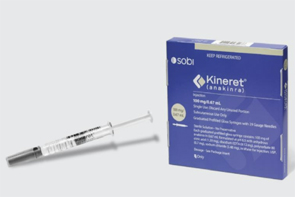
- MFDS reviews emergency import of 'anakinra'
- by Lee, Hye-Kyung Jul 07, 2025 06:08am
- Product photo of Kineret (anakinra) The Ministry of Food and Drug Safety (MFDS) is reportedly conducting a review of whether to urgently import anakinra (brand name Kineret) for the treatment of severe adverse reactions associated with CAR-T cell therapy. On June 2nd, in response to an official inquiry from specialized journalists, MFDS stated, "MFDS is currently reviewing the necessity of emergency import based on the safety, efficacy, and overseas usage status of this product and its indication." The current MFDS review is a follow-up action to the emergency import request for anakinra submitted by the Korean Society of Hematology. The Pharmaceutical Safety Bureau, which received the request, transferred the item to the MFDS Biopharmaceuticals Policy Division for an official review process. CAR-T cell therapy is an advanced, precision treatment that genetically modifies a patient's immune cells to target and attack cancer cells. It has garnered significant attention as a treatment offering different effects compared to conventional anti-cancer drugs, particularly for relapsed and refractory acute lymphoblastic leukemia, diffuse large B-cell lymphoma, and multiple myeloma. In South Korea, the treatment costs KRW 400-500 million. With national health insurance reimbursement applied, the patient's out-of-pocket burden has significantly decreased. Yet, while this high-cost therapy is supported with reimbursement, some adjuvant medications used to manage severe immune adverse reactions that can occur after treatment remain unreimbursed. 'Tocilizumab' or 'high-dose steroids' are primarily used as first-line agents. However, for patients who are refractory to these, anakinra, an Interleukin-1 (IL-1) inhibitor, serves as a crucial therapeutic alternative. However, anakinra's general use in Korea has been blocked since its withdrawal from the domestic market based on the manufacturer's decision. Anakinra is an interleukin-1 receptor antagonist manufactured by Sobi of Sweden. It's approved in countries such as the United States (FDA), Europe (EMA), and Japan (PMDA) for treating conditions including rheumatoid arthritis, CAPS (Cryopyrin-Associated Periodic Syndromes), FMF (Familial Mediterranean Fever), and Still's Disease. The 2025 European CAR T Handbook by the GoCART Coalition recommends high-dose anakinra administration for Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), and its guidelines include co-administration with corticosteroids for patients with IEC-HS (Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome). Specifically, the guidelines suggest anakinra as an essential treatment option for high-risk patients who do not respond to steroid and tocilizumab therapies. Furthermore, a study by Gazeau N et al. (Transplant Cell Ther. 2023) showed that among 43 patients who developed Grade 2 or higher complications after CAR-T therapy and were administered anakinra, the treatment-related mortality rate within 28 days was 0%, and the overall response rate was 77%. Meanwhile, in South Korea, anakinra is currently supplied only in a limited manner through the Korea Orphan & Essential Drug Center (KOEDC) as an urgently imported drug. Yet, it is not permitted for treating adverse reactions of CAR-T. Consequently, some hospitals are attempting to offer limited prescriptions through their own Institutional Review Board (IRB) approvals. However, the treatment is neither reimbursed nor guaranteed legal protection, making nationwide application impossible. The Korean Society of Hematology stated, "Currently, medical professionals are forced to off-label use anakinra, even risking illegal practice, to save patients' lives," and added, "This does not align with the fundamental principles of national public health systems and the right to life, and urgent institutional reform is desperately needed." The Korean Society of Hematology, which has submitted requests to the Ministry of Health and Welfare (MOHW) and the HIRA for several years following the reimbursement of CAR-T cell therapy, submitted a request via the 'Anti-Corruption & Civil Rights Commission (e-People)' portal in April regarding the allowance of off-label use of anakinra and institutional improvement. In response, MFDS replied that "upon request from a central administrative agency or professional organization, we can review the expansion of the reimbursement scope based on the necessity for patient treatment, availability of alternatives, and overseas approval status." In response, the Korean Society of Hematology formally submitted an emergency import request for anakinra to the MFDS Pharmaceutical Safety Bureau on May 19. The request included information such as product details, approval status in major overseas countries, usage cases in the U.S. and Europe, the necessity of its use in life-threatening situations, domestic demand prediction (less than 100 cases annually), and the lack of drug alternatives. The Korean Society of Hematology stated, "Rapid immune modulation is essential in life-threatening severe complications, and currently, there are no drugs with a similar mechanism to anakinra available in South Korea." They emphasized, "This emergency import request is an essential measure to protect patient lives and ensure access to treatment, and prompt institutional reform is required."
- Company
- 'Tecentriq' reattempts at lung cancer adjuvant therapy reimb
- by Eo, Yun-Ho Jul 04, 2025 06:06am
- Product photo of Tecentriq 'Tecentriq,' immunotherapy for cancer, will be submitted again for expanded insurance reimbursement of adjuvant therapy for lung cancer. According to our press coverage, Roche Korea applied for expanded reimbursement of its PD-L1 inhibitor Tecentriq (atezolizumab) and is awaiting review by the Cancer Disease Review Committee (CDRC) of the Health Insurance Review & Assessment Service (HIRA). It is their third attempt. The detailed indication that the company is applying for expanded reimbursement is 'adjuvant therapy after resection and platinum-based chemotherapy for Stage II-IIIA non-small cell lung cancer (NSCLC) where PD-L1 is expressed in 50% or more of tumor cells (TC).' Tecentriq was first submitted to the CDRC in May 2023; however, at that time, reimbursement criteria for the drug had not been established. Then, the company made a second attempt, but it did not pass the CDRC in July of last year. At that time, Roche presented overall survival (OS) improvement results at the American Society of Clinical Oncology (ASCO) meeting, but the company did not receive the outcome. Consequently, it is to be watched whether Tecentriq receives a different outcome at the third attempt. Meanwhile, Tecentriq is indicated for various types of lung cancer and was the first immunotherapy to be approved for first-line treatment of extensive-stage small cell lung cancer in combination with carboplatin and etoposide (chemotherapy). Furthermore, it continues to conduct various clinical studies to address unmet medical needs in advanced or metastatic NSCLC, either as a monotherapy or in combination with other targeted therapies, chemotherapy, or immunotherapies. NSCLC is a key lung cancer type that accounts for approximately 85-90% of lung cancer, which is the leading cause of cancer death in Korea. A significant number of patients are diagnosed at the locally advanced or metastatic stage, and about half of NSCLC patients who undergo complete resection still experience cancer recurrence after surgery. NSCLC poses a heavy burden on these patients.
- Policy
- National Office of Investigation announces rebate crackdown
- by Lee, Jeong-Hwan Jul 04, 2025 06:06am
- Amidst the National Police Agency's announcement of a special crackdown on illegal drug rebates following the launch of the new administration, the Ministry of Health and Welfare is also busy assessing the situation. The MOHW plans to actively consult with the National Police Agency if it receives a specific request for cooperation to crack down on illegal rebates. On the 2nd, an official from the MOHW explained, “The issue of illegal rebates is understood to be part of a national policy agenda related to the National Police Agency's special crackdown, aimed at eradicating corruption and promoting integrity among public officials.” However, the official said that no separate request for cooperation has been made by the National Police Agency to MOHW for the special crackdown on rebates so far. The Ministry of Health and Welfare is currently maintaining its routine of monitoring cases and referring them to investigative agencies such as the police, in accordance with the guidelines for handling illegal pharmaceutical rebates. The ministry also plans to respond to future requests for cooperation. The official said, “The MOHW requests investigations by the police or the prosecution when it receives external reports or complaints related to rebates. This is in accordance with our guidelines for handling rebates. The police or the prosecution launches an investigation, then reports the results to the MOHW.” He added, “There are cases where the police request an authoritative interpretation to the MOHW when legal interpretation is necessary, such as whether there has been a violation of the Pharmaceutical Affairs Act, and some investigative agencies request the submission of expenditure reports. In response to some requests earlier this year, we provided (submitted expenditure reports) within the scope that could be disclosed.” He added, “There are no immediate plans or policies regarding the media reports, but we are cooperating with the prosecution and the police and are requesting investigations if necessary.”
- Opinion
- [Reporter’s View] NHI finances and reimb expansions
- by Lee, Jeong-Hwan Jul 04, 2025 06:06am
- Striking a balance between improving the financial sustainability of the national health insurance system and enhancing patient access to drugs for rare and intractable diseases is a national issue. The two issues that repeatedly come into conflict have long occupied the attention of all political parties in their search for solutions, yet a clear and definitive answer has remained elusive. With ample finances, there would be no need to agonize over finding concrete solutions. The problem is that the surplus funds available for drug expenses within the health insurance budget are becoming increasingly scarce. The global paradigm for new drugs is rapidly shifting from chemical drugs to biopharmaceuticals. Global big pharmas are focusing on developing ultra-high-priced one-shot treatments for severe and intractable diseases for which there are no effective drugs, and the number of biopharmaceuticals approved in Korea is increasing accordingly. This in turn is increasing the health insurance authorities' concerns. There are an increasing number of cases where expensive drugs with therapeutic effects proven through clinical trials are being developed, but there are insufficient financial resources to ensure patient access to each and every drug that emerges with health insurance coverage. Another issue is that the entry of ultra-high-priced drugs for severe and intractable diseases developed by global big pharmas into the Korean market poses an unintended threat to domestic pharmaceutical companies. In order to increase reimbursement for ultra-high-priced drugs within the limited national health insurance budget, the easiest measure is to reduce the insurance ceiling price (drug price) of existing drugs to create financial leeway. This means that the prices of drugs manufactured by most domestic pharmaceutical companies that maintain their business with generic drugs are likely to fall. Drug price experts often describe the repeated administrative practice of lowering prices of already approved drugs to fund high-cost drugs as a zero-sum game. This expression metaphorically describes the reality of having to balance two conflicting priorities: expanding treatment opportunities for patients with severe and rare diseases while also supporting the domestic pharmaceutical industry, both of which are inextricably linked to the limited national budget. As such, the new administration is tasked with the challenge of reducing or eliminating the conflict zone between two zero-sum goals: “enhancing the sustainability of the national health insurance finances” and “improving access to new drugs.” Among the many ways to solve this problem, this reporter has two suggestions for the new administration based on the opinions of drug price experts. First, I ask the government to take the courage to pursue a new agreement on the allocation of health insurance drug expenses and consider the establishment of a separate fund for severe and intractable diseases, targeting high-cost drugs, as an additional funding source. This means reducing health insurance drug costs for mild diseases (by increasing patient copayments) and launching a process to gather opinions and reach a consensus across society and the public on expanding reimbursement for severe diseases, while at the same time securing additional financial resources with the mission of improving access to high-cost drugs. Both are expected to face significant challenges in implementation, but given the recurring zero-sum nature of the issue, it is an issue that cannot be ignored any longer. President Lee Jae-myung has repeatedly pledged to design a true Korea. We hope that the process of deriving a social consensus on a genuine health insurance policy aligned with the principles and philosophy of the National Health Insurance System will take its first steps in tandem with the design of a true Korea.