- LOGIN
- MemberShip
- 2025-12-20 14:40:00
- New K-drugs for metabolic diseases make international debut
- by Son, Hyung Min | translator Alice Kang | 2025-06-30 06:07:28
Major Korean pharmaceutical and biotechnology companies have signaled their full-scale entry into global clinical trials, presenting new drug development results at overseas conferences.
The companies presented their achievements in developing new drugs for various metabolic diseases, including obesity, type 2 diabetes, and metabolic dysfunction-associated steatohepatitis (MASH).
Although most of the data disclosed is focused on early clinical or preclinical trials, the companies are attempting to diversify their mechanisms of action with triple agonists and oral small molecule formulations.
Hanmi reveals triple agonist data...
Oral and long-acting injectable formulations are also under development

At the conference, various domestic companies, including Hanmi Pharmaceutical, Ildong Pharmaceutical, Dong-A ST, and Inventage Lab, revealed the clinical results of their novel drug candidates.
Obesity drugs are rapidly emerging as a global R&D trend.
With Novo Nordisk and Lilly's GLP-1-based obesity treatments becoming global blockbuster drugs, latecomers are also intent on developing their versions.
Major domestic companies are conducting clinical studies on GLP-1 agonists in various areas, including obesity and MASH with drugs that have different methods of administration or focus on the quality of weight loss effects.
Hanmi Pharmaceutical announced the results of its Phase I clinical trials and preclinical data for HM15275, a GLP-1 class triple agonist, and HM17321, a UCN2-based candidate substance, at the conference.
First, Hanmi Pharmaceutical announced the results of the Phase I clinical trial for HM15275.
HM15275 is a new obesity drug candidate that acts on glucagon-like peptide (GLP-1), gastric inhibitory polypeptide (GIP), and glucagon (GCG).
No new drug with this mechanism targeting all three has been commercialized to date.
The Phase I clinical trial was conducted on 74 healthy and obese adults.
HM15275 was administered subcutaneously once a week for four weeks, followed by an evaluation of the candidate drug’s safety, tolerability, pharmacokinetics, and pharmacodynamics.
The results confirmed HM15275’s tolerability and safety.
Specifically, the average weight loss rate at day 29 in the highest dose group was 4.8%.
At day 43, the maximum weight loss rate was 10.6%.
Preclinical data for HM15275 demonstrated greater weight loss efficacy compared to semaglutide (brand name Wegovy) and tirzepatide (Zepbound), which are currently marketed as obesity treatments.
In animal models, switching from tirzepatide to HM15275 resulted in additional weight loss effects.
Hanmi Pharmaceutical also announced the results of preclinical studies on HM17321, a new drug candidate that simultaneously targets weight loss and muscle gain.
HM17321 is a UCN-2 analogue that selectively targets the CRF2 (corticotropin-releasing factor 2) receptor rather than GLP-1 or other incretin receptors.
It is being developed as an innovative first-in-class drug that selectively reduces fat while increasing muscle mass.
HM17321 demonstrated weight loss effects and improved body composition in both mouse models and non-human primate models.
Yunovia, a subsidiary of Ildong Pharmaceutical Group specializing in new drug research and development, is conducting Phase I clinical trials for ID110521156, a GLP-1 receptor agonist class new drug candidate targeting metabolic disorders such as diabetes and obesity.
ID110521156 is a low-molecular-weight compound-based drug, and the company aims to develop it as an oral synthetic new drug for diabetes and obesity with distinct advantages such as superior productivity and excellent ease of use over existing representative treatments like peptide injections.
Previously, Yunovia confirmed the efficacy of insulin secretion and blood glucose control through preclinical efficacy and toxicity evaluations.
It also demonstrated superior safety compared to competing drugs in the same class and confirmed the drug's characteristics in a recently completed Phase 1 single-ascending dose (SAD) trial.
According to the study poster presented at the conference, in the single-dose escalation trial, ID110521156 demonstrated good tolerability with fewer gastrointestinal side effects across the entire effective dose range, unlike existing GLP-1 class drugs.
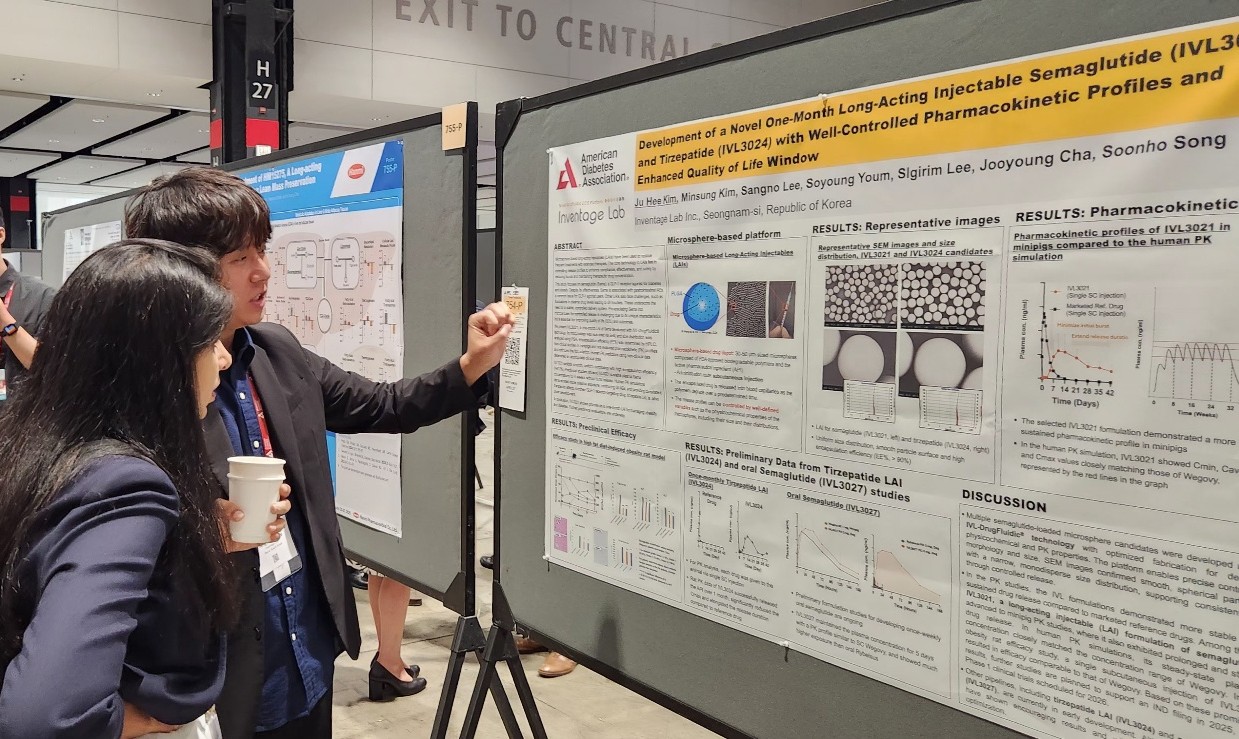
Inventage Lab also introduced preclinical data for its 1-month long-acting injectable formulations ‘IVL3021’ and ‘IVL3024’ based on semaglutide and tirzepatide, as well as its oral semaglutide formulation ‘IVL3027.’ After GLP-1 class obesity treatments such as Saxenda, Wegovy, and Zepbound emerged as global blockbuster drugs, the pharmaceutical industry has been actively pursuing formulation changes.
The existing drug Saxenda requires a once-daily injection, while Wegovy and Zepbound require weekly injections.
Oral formulations or long-acting injectables are expected to gain a competitive edge in terms of convenience of administration if commercialized.
According to the company's preliminary preclinical results, IVL3021 showed stable drug release in the blood over a one-month period.
Also, the long-acting injectable suppressed initial over-release and maintained stable drug release.
IVL3027 demonstrated high bioavailability compared to existing oral formulations and sustained drug release over a one-week period.
Metabolic dysfunction-related fatty liver disease was previously referred to as non-alcoholic steatohepatitis (NASH), but overseas academic societies such as the American Association for the Study of Liver Diseases have decided to change the name to metabolic dysfunction-associated steatohepatitis (MASH).
To date, Madrigal's rezdiffra is the only new drug for MASH that has cleared regulatory hurdles.
The U.S.
Food and Drug Administration (FDA) approved rezdiffra in March last year for the treatment of adult patients with non-cirrhotic MASH in combination with diet and exercise.
Rezdiffra is a selective thyroid hormone receptor (THR)-β agonist designed to target the core pathophysiological mechanisms of MASH within the liver.
The pharmaceutical industry is also developing new drugs for MASH that target not only THR-β but also GLP-1 and FGF21, which influence lipid metabolism.
DA-1241 is a synthetic new drug that activates GPR119.
Preclinical results have confirmed that DA-1241 improves blood sugar and lipid levels and directly acts on the liver to improve inflammation and fibrosis, making it a promising candidate for MASH treatment.
A Phase 2a clinical trial targeting patients with estimated MASH was completed in December last year.
Efruxifermin is a recombinant protein designed based on FGF21 (Fibroblast Growth Factor 21), a hormone secreted by the liver.
FGF21 is involved in energy consumption and the regulation of glucose and lipid metabolism in the body and is used as a target for the development of treatments for MASH, obesity, diabetes, and other conditions.
According to the study results presented at the conference, in the DA-1241+efruxifermin combination therapy group, approximately 94% of subjects showed an improvement of 2 points or more in NAS (Non-Alcoholic Steatohepatitis Activity Score) compared to baseline.
Additionally, the DA-1241+efruxifermin group showed a significant reduction in liver fibrosis area compared to the MASH control group that did not receive combination therapy, and in some individuals, a decrease in fibrosis stage was observed compared to pre-treatment levels.
Dong-A ST is currently conducting clinical studies on the combination therapy of DA-1241 with semaglutide, a GLP-1 agonist, in addition to a Phase II clinical trial on DA-1241 as monotherapy.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










