- LOGIN
- MemberShip
- 2025-12-20 11:46:28
- Company
- GSK Korea appoints Gunnar Riediger as new General Manager
- by Whang, byung-woo Jul 31, 2025 06:14am
- Gunnar Riediger, new Country President & General Manager of GSK Korea GSK Korea announced on the 30th that it has appointed Gunnar Riediger as its new General Manager, effective August 1. The new GM joined GSK in 2004 through the company's global talent development program, the “Future Leaders Program,” and has led healthcare operations across Latin America for over 20 years. He has held key roles including Vaccines Business Unit Head, BioTech Business Unit Head, and Global Vaccines Market Lead at GSK Brazil. Based on these experiences, as a seasoned leader, Riediger worked to maximize the potential of GSK's pipeline, pioneered innovative market entry strategies, and consistently invested in leadership development programs focusing on challenging thinking and accountability, achieving outstanding results. Ridiger then served as Country President & General Manager of GSK Colombia since 2023, successfully launching key products across the company’s vaccine, specialty medicine, and oncology portfolios. Based on these achievements, GSK Colombia has been recognized as one of the fastest-growing multinational pharmaceutical companies in Colombia in 2024, among other accolades. “I am honored to join GSK Korea, a company that has achieved remarkable results to date,” said Riediger. “I look forward to driving the next chapter of GSK Korea's growth by continuously supplying innovative vaccines and medicines to improve the quality of life for Korean patients and strengthening collaboration to advance Korea’s biopharmaceutical industry.”
- Policy
- MFDS says, abortion drug approval cannot be reviewed
- by Lee, Hye-Kyung Jul 30, 2025 06:16am
- Product photo of Hyundai Pharm filed a marketing authorization application for oral abortion drug 'Mifegymiso Tab (mifepristone·misoprostol)' last year. However, it has been confirmed that, under the current state of legislative void, the review cannot even begin. On the 30th, the Ministry of Food and Drug Safety's (MFDS) Pharmaceutical Approval Management Division responded to an inquiry from specialized journalists, stating, "The review of Mifegymiso Tab can only commence once a legal basis is established, and currently, review is not possible." They added, "Hyundai Pharm also understands that the efficacy, indications, dosage, and administration, etc., can only be set once the allowance and permissible gestational age for medication-induced abortion are clearly defined by law." Mifegymiso is a drug that induces early pregnancy termination. It is already used in over 90 countries, including the United States and France, since the World Health Organization (WHO) designated it as an 'essential medicine' in 2005. In South Korea, Hyundai Pharm signed an exclusive supply agreement with LinePharma UK and submitted its third marketing authorization application in December 2024, following previous submissions in 2021 and 2023. The two previous applications were voluntarily withdrawn due to requests for supplementary data. An MFDS official explained, "The establishment and review of key assessment items, such as efficacy, effect, dosage, administration, and Risk Management Plan, are only possible once the allowance and permissible gestational age for medication-induced abortion are legally defined." They added, "Hyundai Pharm is also aware of this and has agreed through internal discussions that it is difficult to proceed with approval at this time." Under the current legal framework, only surgical abortion methods are permitted, and medication-induced termination lacks a legal basis. Although the Constitutional Court of Korea ruled the abortion ban unconstitutional in 2019, amendments to the Criminal Act and the Mother and Child Health Act have not yet been made, leading to an ongoing legislative void. Consequently, MFDS has not yet taken official action, such as refusing approval or suspending review, for Mifegymiso Tab. It is being formally managed as 'under review.' The official emphasized, "Internal discussions regarding alternative methods, such as conditional approval or restricted use, have not reached a feasible level." The MFDS's position is that it cannot make a judgment alone, as this is an issue that requires prior social consensus, possibly in the form of legal amendments. Hyundai Pharm announced the submission of the Mifegymiso marketing authorization application through a public disclosure on December 31 last year. The disclosure contained results from three clinical trials conducted in the U.S. and Mexico, where Mifegymiso showed abortion success rates of 94.9%, 96.2%, and 97.3%, respectively. Hyundai Pharm explained that these study results demonstrate the effectiveness of the regimen, which involves taking 200mg of oral mifepristone followed by 800mcg of misoprostol, for terminating pregnancies up to 63 days (9 weeks) of gestation. Mifegymiso is a combipack product consisting of one 200mg mifepristone tablet and four 200mcg misoprostol tablets. The method involves first taking mifepristone, which inhibits the action of progesterone (essential for maintaining pregnancy), and then, 1-2 days later, taking misoprostol, which promotes uterine contractions. This is the method for medical abortion up to 63 days (9 weeks) of gestation recommended by the WHO. An official from Hyundai Pharm stated, "There have been no new updates since the marketing authorization application in December 2024." They added, "We are striving to introduce a safe and effective drug based on the results of three clinical trials conducted in the U.S. and Mexico." Meanwhile, in April 2019, the Constitutional Court of Korea ruled the criminal code's abortion ban unconstitutional, citing respect for women's right to self-determination over their bodies. Consequently, abortion procedures became de facto legalized as of January 1, 2021. In the 21st National Assembly, a total of seven bills related to the abolition of the abortion ban, including amendments to the Criminal Act and the Mother and Child Health Act, were proposed but ultimately expired without proper discussion. In the 22nd National Assembly, Democratic Party of Korea lawmakers Nam In-soon and Lee Su-jin recently proposed amendments to the Mother and Child Health Act.
- Company
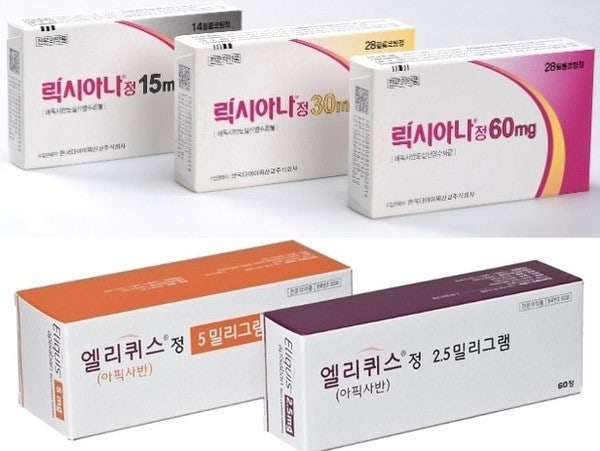
- Lixiana solely leads DOAC mkt… Xarelto, Eliquis↓
- by Kim, Jin-Gu Jul 30, 2025 06:16am
- (From top left clockwise) Lixiana, Eliquis, Pradaxa, and Xarelto Original direct-acting oral anticoagulant (DOAC) drugs have faced mixed fortunes in the Korean market. Prescription sales of Lixiana (edoxaban) increased by 8% year-on-year, solidifying its lead in Korea’s market. Its market share in the DOAC market expanded to 49%, and the analysis is that Lixiana will continue its lead in the market until the expiration of its substance patent next year. Eliquis (apixaban) and Xarelto (rivaroxaban) showed sluggish performance. Lixiana saw a 30% decline in prescriptions due to price reductions following the launch of generics in the fourth quarter of last year. Xarelto, whose patent expired earlier, has also shown a clear downward trend in sales in recent years. DOAC prescriptions in the first half of the year reach KRW 122.4 billion...Lixiana solidifies sole lead in the market According to the pharmaceutical market research institute UBIST on the 28th, the domestic DOAC market prescription volume in the first half of this year was KRW 122.4 billion. This is a 4% decrease from the KRW 127.1 billion it had rendered in the first half of last year. DOACs are anticoagulants that prevent blood clots by directly acting on blood coagulation factors. They have replaced warfarin, which inhibits vitamin K metabolism, and are increasingly being used in clinical practice. In Korea, Xarelto was approved in 2009, followed by Pradaxa and Eliquis in 2011, and Lixiana in 2015. When the product first appeared, it was commonly referred to as NOAC (New Oral Anticoagulant), but as it has been more than 10 years since its initial approval, it is now called DOAC (Direct Oral Anticoagulant), referring to its mechanism of action that directly acts on coagulation factors. Quarterly major DOAC prescriptions (Lixiana, Eliquis, Eliquis generic, Xarelto, Xarelto generics, Pradaxa) Lixiana has further strengthened its lead in the market. Lixiana’s prescription sales in the first half of this year reached KRW 59.9 billion, an 8% increase from the KRW 55.7 billion in the same period last year. Lixiana was the last DOAC to be released, but the drug quickly increased its prescription sales and has maintained its market leadership since 2019. With an annual growth of around 10%, prescriptions rose from KRW 60.4 billion in 2019 to KRW 117.5 billion last year, nearly doubling in five years. Its share in the total DOAC market expanded to 49% in the first half of this year. This means that Lixiana accounts for nearly half of the KRW 260 billion in the DOAC market. The industry expects the market dominance to continue until the end of next year. The substance patent for Lixiana will expire in November next year. About 20 domestic pharmaceutical companies are expected to release generic versions at that time. Currently, 13 companies have been approved to sell generic versions of Lixiana, including Nexpharm Korea, Dong-A ST, Samsung Pharm, Shinil Pharmaceutical, Shinpoong Pharm, Anguk Pharmaceutical, Ildong Pharmaceutical, Genuone Science, Union Korea Pharm, Korea Prime Pharm, Hutecs Korea Pharmaceutical, and Handok Pharm. In addition, Samjin Pharmaceutical, HLB Pharmaceutical, Theragen Etex, and DongKwang Pharm have filed for an invalidation trial (passive scope confirmation trial) regarding the Lixiana formulation patent. If they win the first trial, they will be able to release generic versions in line with the expiration of the substance patent, like other companies. Furthermore, Alico Pharmaceutical and Korean Drug have been approved to conduct bioequivalence tests for the release of their generic versions of Lixiana. Eliquis·Xarelto sales in clear decline…due to the release of generic versions and drug price cuts On the other hand, Eliquis and Xarelto are in a clear downward trend. The sales decline of both products is analyzed to be affected by patent expirations and the subsequent entry of generics. Eliquis sales fell 30% from KRW 38.8 billion in the first half of last year to KRW 27.1 billion in the first half of this year. This is due to the impact of price reductions caused by the entry of generics. Eliquis’ price was reduced by 30% in September last year. Quarterly prescriptions of Eliquis and Eliquis generics Eliquis generics re-entered the DOAC market in the fourth quarter of last year. Eliquis generics were originally released in June 2019. At that time, generic companies released their products based on the first and second instance rulings in favor of them in patent litigation. However, the situation reversed in April 2021 when the Supreme Court overturned the first and second court rulings. The generic drugs were immediately withdrawn from the market. Following the expiration of Eliquis' substance patent in September last year, the generics returned to the market after three and a half years. Eliquis generics have been expanding prescriptions since their return to the market, reaching KRW 200 million in the fourth quarter of last year, KRW 600 million in the first quarter of this year, and then KRW 1.1 billion in the second quarter. By product, the situation is similar to that before the generics withdrew from the market three and a half years ago. At the time, the top two generic products in terms of prescription sales, Chong Kun Dang’s ‘Liquisia’ and Samjin Pharmaceutical's ‘Elxaban,’ have maintained their positions as the top two generics even after re-entering the market. Xarelto recorded sales of KRW 15.3 billion in the first half of this year, maintaining its prescription sales as in the same period last year. However, the downward trend has been evident since 2021. Compared to KRW 28.9 billion in the first half of 2021, sales have decreased by half in four years. This is due to the entry of generic drugs. Xarelto generics first appeared in the second quarter of 2021. About 40 products were released simultaneously in line with the expiration of Xarelto’s substance patent. Subsequently, prescriptions steadily increased, reaching KRW 4 billion in the first half of 2022, KRW 8 billion in the first half of 2023, KRW 12.4 billion in the first half of 2024, and KRW 13.7 billion in the first half of this year. Quarterly prescriptions of Xarelto and Xarelto generics By product, Hanmi Pharmaceutical's Riroxban recorded KRW 4.3 billion, Samjin Pharm’s Rivoxaban KRW 2.5 billion, and Chong Kun Dang’s Riroxia KRW 2.2 billion. The remaining products had prescription sales of less than KRW 1 billion in the first half. As sales of the original product slowed down, generics expanded their influence, and the market share of generics in the rivaroxaban-containing DOAC market reached 47% as of the first half of this year. Another original product, Pradaxa (dabigatran), is struggling to gain traction in the DOAC market. Pradaxa's prescription sales in the first half of the year were KRW 4.7 billion, a slight decrease from the KRW 4.8 billion in the same period last year.
- Product
- Platforms promote Wegovy's lowest price is ₩390,000
- by Kang, Hye-Kyung Jul 30, 2025 06:10am
- Non-face-to-face treatment platforms are facing backlash from pharmacies after launching activities promoting the lowest prices for non-reimbursable drugs. Doctornow, a leading non-face-to-face medical consultation platform, is being criticized for encouraging price competition for non-reimbursable drugs and causing confusion among consumers. A local pharmacist stated, “Recently, Doctornw has posted promotional content on its social media accounts, such as ‘How to buy diet injections for KRW 200,000 less,’ 'The real way to buy semaglutide diet injections for KRW 390,000,‘ and 'Hair loss medication consultation fee + medication cost: KRW 9,060.’ I believe this is likely to cause distrust and confusion among consumers toward pharmacies.” For example, in a video titled ‘How to buy diet injections for KRW 200,000 less,’ it says, 'Wow! I used to buy weight-loss injections for KRW 600,000, but I was paying KRW 200,000 more. If you don't know the latest way to buy diet injections cheaply, you're losing out 100%. If you know this method, you can get a prescription for KRW 200,000 less, at an unbelievable price!“ The video states that all you need is to access Doctornow. The video explains that you can find the lowest price at a pharmacy near you by clicking ”Find a Pharmacy,“ then clicking ”Get a Prescription" to select a nearby and affordable doctor, going to the hospital for a consultation, and then getting the injection at the lowest-priced pharmacy. However, the video has been criticized for causing consumer distrust and confusion about pharmacies. This pharmacist said, "Doctornow claims that the real price for semaglutide diet injections is KRW 390,000. It suggests that pharmacies are taking a middleman margin. However, the purchase price of the semaglutide diet injection, Wegovy, far exceeds the 390,000 won suggested by Doctornow.” When credit card transaction fees are added to the purchase price, it is practically impossible to meet the criteria for ”buying cheaply and well" suggested by Doctornow. When Dailpharm searched for the lowest price of Wegovy on Doctornow, the price varied depending on the region, but was usually over KRW 430,000. It was found that there are pharmacies in the Gyeonggi area that sell the drug for KRW 394,000 and KRW 395,000, but in the Gyeongsangbuk-do, Chungcheongbuk-do, and Gyeongsangnam-do areas, the price of the non-reimbursable injectable was set around KRW 430,000 to KRW 450,000. Doctornow also presented KRW 9,060 as the lowest hair loss medication consultation fees available. Another pharmacist said, “There are differences between pharmacies when it comes to the price of non-reimbursable drugs. However, I think it is problematic under the Pharmaceutical Affairs Act for non-face-to-face consultation platforms to arbitrarily set the lowest price and misrepresent it as the ‘standard’. Shouldn't sanctions be imposed on the platform for such excessive promotional activities?” Pharmacists also voiced concerns about the institutionalization of telemedicine, as the majority of non-face-to-face prescriptions made are for non-reimbursable drugs for cosmetic purposes. According to a survey conducted by the Guro District Pharmaceutical Association and the Jungnang, Gwangjin, and Gangdong District Pharmaceutical Associations among their members, 46% of pharmacists stated that they had accepted non-face-to-face prescriptions in the past three months. When asked about the proportion of non-face-to-face prescriptions for cosmetic purposes, such as hair loss, weight loss, and eye drops, among the prescriptions received, over 80% of respondents answered that they accounted for the majority of non-face-to-face prescriptions. The associations stated that the majority of pharmacists who accepted non-face-to-face prescriptions in the last three months responded that they were for cosmetic purposes. We are concerned about the reality that non-face-to-face medical consultations are being used as a means to purchase non-reimbursable drugs for cosmetic purposes, such as hair loss, diet, and eye drops, which deviates from the original purpose. We believe that non-face-to-face prescriptions for cosmetic purposes should also be excluded or restricted by the system, similar to psychotropic drugs.” Meanwhile, during her confirmation hearing as nominee for Minister of Health and Welfare, Eun-kyung Jeong stated, “The institutionalization of telemedicine should be aimed at ensuring the stability of medical care and improving primary care, rather than expanding platform profits. During discussions with the National Assembly, appropriate regulatory measures should be discussed to address concerns that platforms will effectively take over clinics and pharmacies or promote profit expansion.”
- Company
- Expanded reimb for Jardiance…management of CRM disease
- by Whang, byung-woo Jul 30, 2025 06:10am
- Product photo of Jardiance Boehringer Ingelheim announced on the 29th that its SGLT2 inhibitor, Jardiance (empagliflozin), will be reimbursed by the National Health Insurance for the treatment of chronic kidney disease (CKD), starting August 1, according to the Ministry of Health and Welfare notification. According to this notification, Jardiance will be reimbursed for the treatment of chronic kidney disease patients who meet all of the following conditions: ▲Being stably treated with an ACE (Angiotensin-converting-enzyme) inhibitor or Angiotensin II receptor blocker at the maximum tolerated dose for at least 4 weeks ▲Having an estimated glomerular filtration rate (eGFR) between 20-75 ml/min/1.73m2 ▲Having a positive urine dipstick test (1+ or more) or a urine albumin/creatinine ratio (uACR) of 200mg/g or more. With this expanded reimbursement, Jardiance will be reimbursed by the National Health Insurance for all three indications: Type 2 diabetes, chronic heart failure, and chronic kidney disease. The expanded reimbursement is expected to strengthen its position as a crucial therapeutic option in the integrated management strategy encompassing cardio-renal-metabolic (CRM) diseases. The latest reimbursement approval is based on the results of the EMPA-KIDNEY Phase 3 clinical study, a large-scale and broad-patient population SGLT2 inhibitor study in the area of chronic kidney disease treatment. The study involved 6,609 chronic kidney disease patients with various underlying causes and comorbidities across the spectrum of CKD severity, regardless of Type 2 diabetes status or the use of renin-angiotensin system inhibitors. The study results showed that Jardiance significantly reduced the relative risk of kidney disease progression and cardiovascular death by 28% compared to placebo. This effect was consistently observed regardless of the presence of diabetes or albuminuria. Notably, unlike previous SGLT2 inhibitor studies, which primarily focused on patients with high urine albumin/creatinine ratio, the EMPA-KIDNEY study included patients with low urine albumin/creatinine ratio, which is significant. Ju-Young Moon, Professor of Nephrology at Kyung Hee University Hospital at Gangdong (Director of Insurance and Legislation at Korean Society of Nephrology), stated, "While it is crucial to detect and initiate treatment for chronic kidney disease at the earliest possible stage, fundamental treatment options available to patients have long been limited," and added, "With the expanded reimbursement of Jardiance, which can slow the progression to end-stage renal disease, more active and efficient treatment becomes possible, and a positive change is expected across the entire treatment environment." Jiyoung Park, Head of CRM Franchise at Boehringer Ingelheim Korea, stated, "It is significant that Jardiance, which has presented the possibility of an integrated approach to cardio-renal-metabolic diseases beyond blood sugar control, can now offer treatment opportunities to more patients by expanding its reimbursement coverage to chronic kidney disease," and added, "We will continue to do our best to expand treatment benefits for patients and contribute to improving the treatment environment based on various clinical evidence and treatment backgrounds."
- Company
- Neurophet partners with Roche for AI tech verification
- by Whang, byung-woo Jul 30, 2025 06:10am
- Neurophet (Co-CEO Jun-gil Bin, Dong-hyun Kim), a specialized AI company in brain disease diagnosis and treatment, announced on the 29th that it has officially signed a joint research agreement with Roche and has begun full-scale collaboration Neurophet has already been sharing data with Roche, and with the official announcement of the collaboration, technical verification and follow-up discussions are expected to proceed rapidly. Through the agreement, Neurophet has secured large-scale clinical data that is difficult to obtain in medical settings, laying the foundation for obtaining medical device certification and reliability verification in each country. Furthermore, starting with this research collaboration, Neurophet aims to pursue additional technologies and business collaboration opportunities with Roche. Neurophet has been expanding its brain imaging analysis business pipeline through collaboration with global big pharma. The company provides quantified analysis results that enable objective evaluation of the efficacy of clinical subjects by analyzing brain MRI (magnetic resonance imaging) and PET (positron emission tomography) image data required in the drug development stage. Such collaboration with big pharma is expected to serve as a key factor in expanding into the global market, going beyond the dissemination of solutions, and will serve as the backbone for Neurophet’s overseas business expansions. Jun-gil Bin, Co-CEO of Neurophet, stated, “Securing a research contract in a situation where it is difficult to obtain large-scale medical data is encouraging. We are pleased to be able to partner with Roche, one of the world’s largest pharmaceutical companies. We will do our best to develop this into a business partnership and utilize it for new technology development and clinical trials in the future.”
- Company
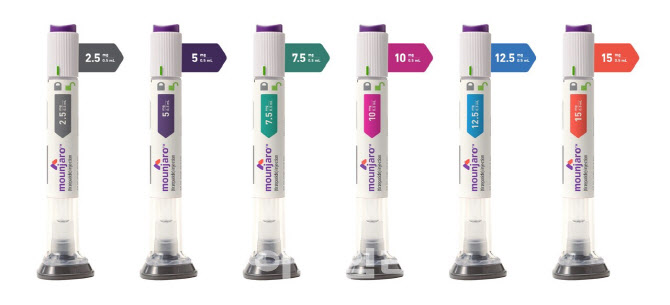
- Attention drawn to reimb status of 'Mounjaro' for diabetes
- by Moon, sung-ho Jul 30, 2025 06:09am
- As the official launch of Mounjaro (tirzepatide, Eli Lilly Korea), approved in Korea as an adjunct therapy for chronic weight management following its indication for adult type 2 diabetes, is imminent, the reimbursement status of the drug is garnering attention. The focus is on whether it will be recognized and reimbursed as the first 'innovative new drug' for a chronic disease. Product photo of MounjaroAccording to pharmaceutical industry sources on the 25th, Eli Lilly Korea recently submitted a reimbursement application for Mounjaro as a 'treatment for adult type 2 diabetes' to the Health Insurance Review & Assessment Service (HIRA). Mounjaro was initially approved for its indication in June 2023 as an adjunct therapy to diet and exercise to improve glycemic control in adults with type 2 diabetes. In August of last year, it was approved as a once-weekly subcutaneous injection as an adjunct therapy to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients. Mounjaro can be used in▲obese patients with an initial Body Mass Index (BMI) of 30 kg/m² or higher, or ▲overweight patients with an initial BMI of 27 kg/m² or higher but less than 30 kg/m², who have at least one weight-related comorbidity (e.g., hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease). In other words, Mounjaro received indications for both diabetes and obesity treatment. Eli Lilly Korea has decided to prioritize the launch of the 'pre-filled pen' formulation, which was first approved in 2023, due to delays in obtaining approvals for the vial and quick-pen formulations. This is interpreted as a strategy to launch the pre-filled pen first, while continuing to pursue domestic approvals for the vial and quick-pen formulations. Concurrently, Eli Lilly Korea plans to accelerate its efforts to secure reimbursement for Mounjaro for adult type 2 diabetes. For reference, 'Ozempic Prefilled Pen (Ozempic), a semaglutide diabetes treatment from Novo Nordisk Pharma Korea that could compete in clinical settings, is also being re-pursued for reimbursement. Novo Nordisk Pharma Korea attempted to get Ozempic reimbursed in 2023 but withdrew its application during the drug price negotiation process with the National Health Insurance Service, which is considered the final stage. Although it had received conditional reimbursement approval from HIRA's Drug Reimbursement Evaluation Committee (DREC) in the preceding stage and had even agreed on a price with the NHIS, uncertainties regarding product supply in Korea hampered its progress. However, having overcome the supply issue, active reimbursement discussions are now possible. In this situation, Eli Lilly Korea plans to seek reimbursement for Mounjaro by having it recognized as the first 'innovative new drug' for a chronic disease. This means it aims to be the first chronic disease treatment to be listed through the innovative new drug reimbursement process, which has primarily focused on anticancer drugs such as Gilead's triple-negative breast cancer treatment Trodelvy (sacituzumab govitecan). An Eli Lilly Korea official stated, "We have applied for National Health Insurance reimbursement with HIRA, and are currently awaiting deliberation by the DREC." They added, "We expect Mounjaro, a new type 2 diabetes treatment and the first GIP/GLP-1 receptor dual agonist, to provide differentiated clinical value. Therefore, we are doing our best to ensure it can be recognized as the first chronic disease drug to receive flexible application of the ICER value for innovative new drugs." However, the pharmaceutical industry is offering a cautious interpretation regarding the possibility of Mounjaro's reimbursement as an innovative new drug. This implies that there are limitations in applying Mounjaro, a chronic disease treatment, to the innovative new drug eligibility criteria established by the government. For reference, HIRA revised the 'Detailed Evaluation Criteria for Negotiable Drugs (New Drugs)' in August last year, specifying the 'innovativeness' evaluation criteria. This provided a standard for evaluating the appropriate value of new drugs by specifically defining the meaning of 'innovativeness' as one of the ICER value evaluation factors. Consequently, a new drug's innovativeness is recognized only when it satisfies all three of the following conditions: ▲there is no available alternative or therapeutically equivalent product or treatment; ▲significant clinical improvement in final outcome measures, such as extended survival, is recognized ▲it is a new drug approved through MFDS's expedited review (as per Article 35-4, Paragraph 2 of the Pharmaceutical Affairs Act) or a comparable drug recognized by the Committee. A pharmaceutical industry official, who remained anonymous, questioned, "It's uncertain whether Mounjaro can meet the definition of innovativeness established by the government." They added, "Of course, this doesn't mean Mounjaro has no innovative value. However, we need to consider whether it fits the currently established criteria and the government's definition of innovativeness. Consequently, it seems that questions will inevitably follow regarding whether it can be applied."
- Policy
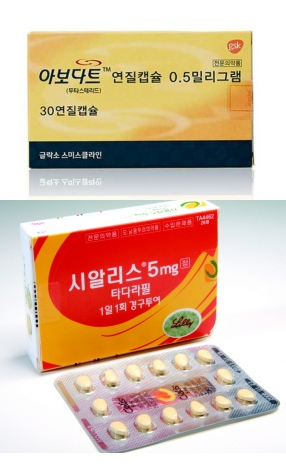
- Will the Korean Avodart+Cialis combos be reimbursed?
- by Lee, Tak-Sun Jul 29, 2025 06:04am
- There is growing interest in whether domestic pharmaceutical companies will succeed in securing reimbursement for the world's first dutasteride and tadalafil combination drug. Currently, this drug is undergoing a new drug reimbursement evaluation process rather than the standard combination drug assessment, as tadalafil is not a reimbursed ingredient. Pharmaceutical companies are seeking the best strategies to provide patients with the treatment options they need. According to industry sources on the 29th, reimbursement reviews are underway for domestically developed dutasteride+tadalafil combination drugs that were approved in January this year. With Dongkoook Pharmaceutical serving as the lead sponsor, Dongkooks’s ‘Uresco Tab,’ Dong-A ST’s ‘Dutana Tab,’ Shinpoong Pharmaceutical’s ‘Avocial Tab,’ DongKoo Bio&Pharma’s ‘Uroguard Tab,’ were approved through a joint clinical trial. The drugs are indicated for the treatment of moderate to severe symptoms of benign prostatic hyperplasia. Normally, combination drugs composed of previously approved ingredients are priced based on the reimbursement rates of their components. However, since tadalafil is a non-reimbursed ingredient, the dutasteride + tadalafil combination drug is excluded from the standard pricing process and is instead undergoing reimbursement evaluation as a new drug. The combination drugs that undergo standard pricing procedures are eligible for reimbursement 3 months after application, but new drugs require additional time for reimbursement due to cost-effectiveness reviews. Furthermore, as the joint developers had expected reimbursement through the standard pricing process, they did not have enough time to prepare the materials required for a new drug review. Nevertheless, the four companies are joining forces to knock on HIRA's door for their drugs’ reimbursement. However, it seems that more time would be required to submit all the necessary materials, as HIRA recently requested additional supplementary materials. As a result, the concerns of pharmaceutical companies are growing. The joint developers have stated that they will closely coordinate with each other regarding the drug’s domestic launch to establish an optimal strategy. A representative from a related pharmaceutical company stated, “We are doing our best to prepare the material and provide this treatment to patients in need in Korea.”
- Company
- "A paradigm shift in ulcerative colitis treatment"
- by Whang, byung-woo Jul 29, 2025 06:04am
- The treatment strategy for ulcerative colitis is rapidly evolving with the emergence of new global drugs. Global guidelines recommend a rapid transition to advanced therapies (ATs) early on if 5-ASA treatment fails. In line with these recommendations, Korea also needs a shift in its treatment paradigm. However, the early utilization of high-efficacy treatments is limited due to factors such as insurance reimbursement criteria. In a meeting with DailyPharm, Professor Sang-Bum Kang of Daejeon St. Mary's Hospital (Chairman of the Korean Association for the Study of Intestinal Diseases' Insurance Committee) emphasized the need for re-establishing ulcerative colitis treatment standards and improving the reimbursement system. Ulcerative Colitis Treatment Paradigm Shifting from 'Sequential Therapy' to 'Treat-to-Target' Approach Ulcerative colitis is a chronic inflammatory disease of unknown cause that primarily affects the large intestine, potentially spreading inflammation from the rectum upwards. Professor Sang-Bum Kang of the Department of Internal Medicine at Daejeon St. MaryNotably, uncontrolled ulcerative colitis can be associated with an increased risk of colectomy and hospitalization. Therefore, in moderate and severe cases, strategies involving the early use of treatment options, such as biologics or small-molecule drugs, are being considered to achieve remission. Professor Kang explained, "Ulcerative colitis symptoms have a vast spectrum, and the course of the disease, including the extent of inflammation and disease activity, can continuously change, making it difficult to determine a patient's condition with fragmented numerical values definitively." According to Professor Kang, ulcerative colitis treatment usually begins with 5-ASA agents. However, if a patient has high severity at the time of diagnosis and multiple risk factors, it is a principle to use Advanced Therapy (AT) from the early stage. In this process, Professor Kang emphasizes a personalized treatment strategy tailored to the patient's specific condition. Previously, a 'step-up' approach was typical, where patients who did not respond to conventional treatments, such as 5-ASA, would progressively move to steroids, immunosuppressants, and then ATs. However, recently, the standard has shifted to 'Treat-to-Target (T2T),' which involves setting clear treatment goals from the beginning and pursuing aggressive treatment accordingly. Professor Kang explained, "The current ultimate goal of ulcerative colitis treatment is 'mucosal healing,' confirmed endoscopically. Patients who achieve mucosal healing have a lower risk of relapse and can expect a good long-term prognosis. Therefore, rapid achievement of mucosal healing and maintenance of a relapse-free remission state are key indicators of treatment success." The problem is that conventional drugs alone have limitations in achieving mucosal healing. In contrast, ATs are considered core options in ulcerative colitis treatment due to their higher mucosal healing rates and better response and maintenance effects in mucosal healing. Indeed, the American Gastroenterological Association (AGA) also, in its latest guideline revision, recommended the early use of proven high-efficacy treatments rather than dose escalation if 5-ASA treatment fails, explicitly naming Zeposia, an S1P modulator, as one of the high-efficacy treatment options. "Paradigm shift in ulcerative colitis treatment, potential for oral therapy zeposia" Based on this evidence, Professor Kang emphasized the importance of the initial treatment strategy. He stated, "Realistically, it's not possible to try every treatment sequentially, and it's meaningless to look back after failing the first treatment and think 'what if I had used something else first'." He stressed, "Above all, it's crucial to make an accurate judgment in initial treatment to select the optimal drug and achieve maximum effect." Some reports indicate that 70-80% of all ulcerative colitis patients can achieve symptom control with first-line treatment alone, suggesting the need for a strategy that deploys the best "weapon" tailored to the patient's characteristics. During the treatment paradigm shift, the once-daily oral new drug Zeposia (ozanimod) is also gaining attention as a new alternative for ulcerative colitis treatment. Zeposia is a sphingosine 1-phosphate (S1P) receptor modulator, featuring an innovative mechanism that inhibits inflammation by blocking the migration pathways of inflammatory immune cells. Professor Kang stated, "Zeposia is expected to be effective when used as a first-line treatment for moderate ulcerative colitis patients who have failed conventional therapies like 5-ASA agents." He added, "This efficacy was proven in Zeposia's Phase 3 clinical trials, and consistent results have been confirmed in long-term follow-up and real-world data." Professor Kang also shared an experience where a patient who had previously used biologics but showed insufficient efficacy was switched to Zeposia, and their prognosis significantly improved within 8 weeks. He also said, "Among various AT options, Zeposia is evaluated as a less burdensome drug that can be used long-term. When considering treatment effects, patients who are typically young and actively engaged in socioeconomic activities are suitable for Zeposia treatment." "Diversified ulcerative colitis treatment options require reimbursement system support" While the emergence of new drugs like Zeposia has further diversified treatment options for ulcerative colitis, there's a growing call for the insurance reimbursement environment to support their practical utilization. Regarding this, Professor Kang pointed out, "In Korea, reimbursement is only approved for ATs if patients have tried steroids or immunosuppressants and shown no effect or experienced side effects," and added, "The currently applied reimbursement criteria were established 20 years ago, creating a significant disparity from both the latest treatment trends and clinical reality." Currently, domestic reimbursement criteria stipulate that patients must score 6 points or more out of 12 on the Mayo Scoring System for Assessment of Ulcerative Colitis Activity. However, experts evaluate that some assessment indicators, such as stool frequency and the presence of bloody stools, rely heavily on patient statements and exhibit significant variations in physician assessment, resulting in a lack of objectivity. Professor Kang emphasized, "Ulcerative colitis, an unpredictable, intractable disease, requires flexible treatment access. However, due to rigid reimbursement conditions, the latest treatments cannot be utilized appropriately." He stressed, "While it's impossible to accommodate all demands with limited finances, reimbursement criteria must be adjusted to match current clinical standard so that severe patients can receive high-efficacy treatment on time." In line with these demands, the Korean Association for the Study of Intestinal Diseases is working on a revision of its ulcerative colitis treatment guidelines to reflect the evolving treatment landscape. Professor Kang stated, "We have formed a TF team within the association and are working on new guidelines." He added, "We plan to prepare more practical treatment guidelines by differentiating recommendation grades and evidence levels, similar to the AGA." He explained that they are particularly considering factors like the national health insurance reimbursement system to tailor the recommendations to the Korean context. Professor Kang said, "It has already been 5 years since the ulcerative colitis guidelines were released, and during that time, various new drugs have emerged, significantly changing the treatment environment. I believe now is the opportune time to re-establish treatment standards and push for improvements in the reimbursement system." Finally, Professor Kang said, "In the future, research on personalized treatment should be strengthened, selecting the optimal treatment for each patient based on biomarkers such as intestinal microbes or genetic information." He concluded, "We plan to strive not only for the introduction of the latest treatments but also for the advancement of precision medicine and the training of next-generation specialized personnel."
- Company
- Fewer drugs reimbursed 5 years into pricing reform
- by Chon, Seung-Hyun Jul 29, 2025 06:04am
- Over the past five years, 4,500 drugs have been removed from the reimbursement list in Korea. The number of new drugs entering the market has decreased significantly with the introduction of generic drug price reforms and joint development regulations. The number of prescription drug approvals has decreased by more than 80% compared to 5 years ago. Pharmaceutical companies rushed to enter the generic market ahead of the tighter regulations, and new market entries plummeted after changes were made to the approval and drug pricing systems. With more products being withdrawn than entering the market, the total number of listed prescription drugs began to decline. Reimbursed drugs down 17% in 5 years... Decline continues after drug price reform According to the Health Insurance Review and Assessment Service on the 28th, as of the 1st of this month, a total of 20,277 drugs were listed on the health insurance reimbursement list. Although this is an increase of 44 from the 21,983 last month, it is a decrease of 1,000 from 23,027 in July last year. This means that the number of drugs listed on the reimbursement list has decreased by an average of 83.3 per month over the past year. Approvals for prescription drugs decreased by 84% this year compared to 5 years ago... Joint development regulations accelerate sharp decline in approvals The number of approvals for prescription drugs has significantly decreased since the reform of the drug pricing system. As of June this year, the number of approvals for prescription drugs was 315, with a monthly average of 52.5. Although this is 4.2 drugs more than last year's monthly average of 48.3, it is 23.8 fewer than the 76.3 in 2023, marking a decrease of 23.8 in 2 years. In the first half of 2020, a total of 2,015 prescription drugs were approved, averaging 335.8 per month. This is an 84.4% decrease in the monthly average number of prescription drug approvals over 5 years. The average number of prescription drugs approved per month in 2021 and 2022 was 133.3 and 93.2, respectively, which was significantly higher than the average number approved this year. No. of prescription drugs approved every month (Source: HIRA) The analysis is that the attempts to enter the generic drug market, which accounts for the largest share of new entries in the prescription drug market, have decreased significantly. The rise in the regulatory barriers for approval significantly dampened the market entry momentum. Since July 2021, the revised Pharmaceutical Affairs Act has limited the number of incrementally modified and generic drugs that can be approved on a single clinical trial. The new regulations, known as the “1+3 rule,” restrict the number of incrementally modified drugs and generics that can be approved based on a single clinical trial. If a pharmaceutical company manufactures its product at the same facility with the same formulation and manufacturing process as the product for which it conducted its own bioequivalence study, the use of that data is limited to three. In other words, only four generic drugs in total can be approved based on a single bioequivalence study. Similarly, clinical trial data can be shared with and applied to only 3 other products besides the one directly conducted by the company. In the past, when one pharmaceutical company obtained approval for a generic drug after bioequivalence testing, dozens of other pharmaceutical companies could obtain approval for their own generic drugs using the same data. However, due to the joint development regulations, “unrestricted replication of generic drugs” is no longer possible. The number of prescription drugs, which had been increasing annually, turned to a decline. According to the MFDS, the number of prescription drugs approved last year was 15,893, a decrease of 739 from 1,6632 in 2023. This means that 739 more products had their approvals expire than those approved as prescription drugs in a single year. The number of prescription drug items had increased every year until 2023, since recording 9,572 in 2010. During that period, the number of new products entering the market exceeded the number of products withdrawn from the market every year. However, due to the decrease in prescription drug approvals, an unusual situation occurred last year, with the total number of items decreasing. The number of prescription drug approvals has increased explosively since 2019, but has turned to a decline since 2020. In 2018, 1,562 prescription drugs were approved, averaging 130 per month, but in 2019, the number jumped to 4,195, averaging 350 per month, more than double the previous year. In May 2019 alone, 584 prescription drugs were approved in that single month. From October 2018 to July 2020, more than 100 prescription drugs were approved each month, but in August 2020, the number of approvals fell below 100 for the first time in 23 months. Since January 2023, when 216 prescription drugs were approved, the number of prescription drugs approved each month has fallen below 100 for two years and five months. Companies indiscriminately entered the market in 2019 and 2020 before the introduction of tightened regulations... Repeated mass withdrawals due to non-production and non-claiming The surge in prescription drug approvals in 2019 and 2020 has been attributed to government policies. The government's move to tighten regulations on generics led to a surge in generic approvals. In 2018, 175 items containing the high blood pressure drug valsartan were banned from sale due to excessive impurities. At that time, the MOHW and MFDS formed a “Generic Drug System Improvement Council” and began to develop measures to curb the proliferation of generics. When the government signaled its intention to strengthen regulations, pharmaceutical companies moved to secure generic products in advance, leading to a temporary surge in generic drug approvals. The number of generic drug approvals surged in response to the government's plans to tighten regulations, but returned to previous levels after the system was revised. At the time, there were numerous cases of pharmaceutical companies withdrawing their generic products from the market without selling them after indiscriminately obtaining approval. In November last year, over 1,000 drug items were removed from the health insurance reimbursement list due to non-production and non-claims. Health authorities will remove drugs from the reimbursement list if there have been no insurance reimbursement claims in the last 2 years or no production or import reports in the last 3 years. This means that 1,000 items were removed from the reimbursement list despite being approved by the Ministry of Food and Drug Safety and being listed for reimbursement because they had no production or sales for a certain period of time. No. of reimbursement discontinuation drugs due to lack of claims or production by year of approval as of November 2024 (Source: MFDS): Many of the drugs that were removed from the reimbursement list in November last year were approved in 2019 and 2020. Among the 1,000 drugs removed from the reimbursement list in November last year, 334 were approved in 2000, and 187 in 2019. Products approved in 2019 and 2020 accounted for more than half of all products removed from the reimbursement list, being 521 products in total. This means that more than half of the drugs whose reimbursement was revoked were new products that had been on the market for less than 5 years. Among the drugs whose reimbursement was discontinued due to lack of claims or production, 47 were approved in 2015, and 39 were approved in 2016 and 2017, which was significantly lower than in 2019 and 2020. Only 24 products approved in 2018 were removed from the reimbursement list, compared to how the number of products withdrawn from the market skyrocketed among drugs approved in 2019 and 2020, Pharmaceutical companies indiscriminately obtained generic approvals in response to the government's strengthened regulatory measures, but many products ended up disappearing from the market without being sold. The companies pursued an indiscriminate policy of securing as many generic products as possible before the government strengthened its regulations, leading to an unusual phenomenon where products were withdrawn from the market in large numbers after a certain period of time.