- LOGIN
- MemberShip
- 2025-12-20 13:50:13
- Company
- Mounjaro set for launch in Korea this month
- by Son, Hyung Min Aug 08, 2025 06:02am
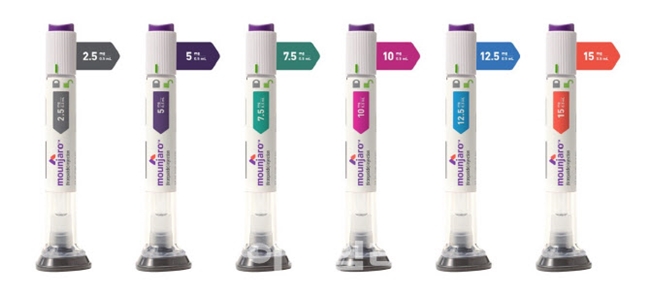
- Eli Lilly Korea's diabetes and obesity treatment, Mounjaro, is set to launch in mid-August. Therefore, it will compete against Novo Nordisk's Wegovy. Lilly plans to handle Mounjaro's distribution directly, a strategy that differentiates it from Novo Nordisk, which is currently seeking co-promotion partners. According to the distribution industry on August 8, Lilly Korea plans to launch Mounjaro in Korea this month. The company has organized its own sales and marketing teams and is entering the Korean market through its existing network of distributors. The launch is expected to take place in the third week of August. The domestic supply price for Mounjaro is reportedly around KRW 278,000 for the 2.5mg dose, KRW 369,000 for the 5mg dose, and KRW 521,377 for the 7.5mg and 10mg doses. The initial launch will include only the 2.5mg and 5mg formulations. Mounjaro is a once-weekly subcutaneous injection. Treatment begins with a 2.5mg dose, with the dosage increasing every four weeks. The maximum dose is 15mg. An official from Lilly Korea stated, "We are preparing for the launch of Mounjaro in Korea in the third week of August. We plan to release only the 2.5mg and 5mg doses initially, but we will supply higher-dose formulations, including 7.5mg, 10mg, 12.5mg, and 15mg, without disruption to meet patient demand." Obesity drugs Wegovy, Zepbound, and Saxenda Lilly Korea and Novo Nordisk have adopted different approaches to distributing Mounjaro and Wegovy. Lilly Korea has recently begun contract discussions with over 30 pharmaceutical distributors, including the largest domestic distributor, Ji-O-Young. These negotiations are reportedly in the final stages, with discussions underway with 12 to 15 distributors in the Seoul metropolitan area and 10 to 14 in other regions. For Mounjaro's distribution, Lilly Korea has selected a model in which distributors directly supply healthcare institutions. The company will only be involved in setting the supply price, leaving the actual transaction price to the discretion of the distributors. However, a specific price guide has been established to ensure the product isn't sold "too expensively or too cheaply." Having prior experience in supplying GLP-1 drugs, such as Trulicity, in Korea, Lilly Korea believes it can maintain its competitive edge with this direct distribution model. Novo Nordisk, on the other hand, currently entrusts the nationwide distribution of Wegovy to a foreign-based pharmaceutical distributor, Zuellig Pharma, with supplies also handled by different distributors, such as Bluemtech. Recent reports suggest that Novo Nordisk and domestic pharmaceutical company Chong Kun Dang may enter a co-promotion agreement for Wegovy. Chong Kun Dang has previous experience selling the obesity drug Qsymia from Alvogen Korea. However, according to the company, no final decision has been made. The market is focused on how much Mounjaro will impact Wegovy's dominance. Even with delays in its official domestic launch, Wegovy rapidly captured the market through various channels. According to market research firm IQVIA, Wegovy's sales in the first quarter of this year reached KRW 79.4 billion, accounting for a 73.2% share of the total obesity drug market. Wegovy, a GLP-1 monotherapy containing semaglutide, gained immediate attention following its launch in Korea in October 2024. Despite its high price, demand for prescriptions surged due to its significant weight loss effects. Wegovy quickly rose to the top of the obesity drug market with sales of KRW 60.3 billion in the fourth quarter of last year. The obesity drug market size in the third quarter of last year was KRW 47.4 billion, but it skyrocketed by 97.9% to KRW 93.8 billion in just one quarter with the launch of Wegovy. Mounjaro Demonstrates Superior Weight Loss Effectiveness Over Wegovy Mounjaro is a new diabetes drug developed by Lilly. This treatment, a GIP/GLP-1 receptor dual agonist, works by acting on both Glucose-dependent Insulinotropic Polypeptide (GIP) receptors and Glucagon-like Peptide-1 (GLP-1) receptors. This mechanism promotes insulin secretion, improves insulin resistance, and reduces glucagon secretion, resulting in a decrease in blood sugar levels before and after meal. Major causes of reduced incretin in diabetic and obese patients are decreased GLP-1 secretion and impaired GIP insulin-stimulating effect. GLP-1 and GIP are hormones responsible for two-thirds of the post-meal insulin response. Treatments for diabetes and obesity In Korea, Mounjaro is approved as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. It is also approved for chronic weight management in obese adults (initial BMI ≥ 30 kg/m²) or overweight adults (initial BMI ≥ 27 kg/m² to < 30 kg/m²) with at least one weight-related comorbidity (e.g., hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease), as an adjunct to a reduced-calorie diet and increased physical activity. Mounjaro has the advantage of not only controlling blood sugar but also demonstrating excellent weight loss effects. Mounjaro proved its weight loss efficacy through the Phase 3 SURMOUNT-1 clinical trial, conducted in obese or overweight adults with at least one comorbidity but without diabetes. Notably, Mounjaro demonstrated superior weight loss effects in SURMOUNT-5, a direct comparative clinical trial against Wegovy. The results of the Phase 3 trial showed that the mean weight loss percentage at 72 weeks for the Mounjaro treatment group (10mg or 15mg) was 20.2%, which was a greater improvement compared to the 13.7% for the Wegovy treatment group (1.7mg or 2.4mg). Having confirmed its weight loss effects in clinical trials for Mounjaro, Lilly launched Zepbound, an obesity treatment with the same active ingredient, in the U.S. market in November 2023. In Korea, both the diabetes and obesity indications will be marketed under the single product name, Mounjaro. Lilly Korea plans to apply for insurance reimbursement for its Mounjaro indication for type 2 diabetes.
- Company
- Donepezil ↑8%, memantine 10%↑…replaces choline alfoscerat
- by Kim, Jin-Gu Aug 07, 2025 06:10am
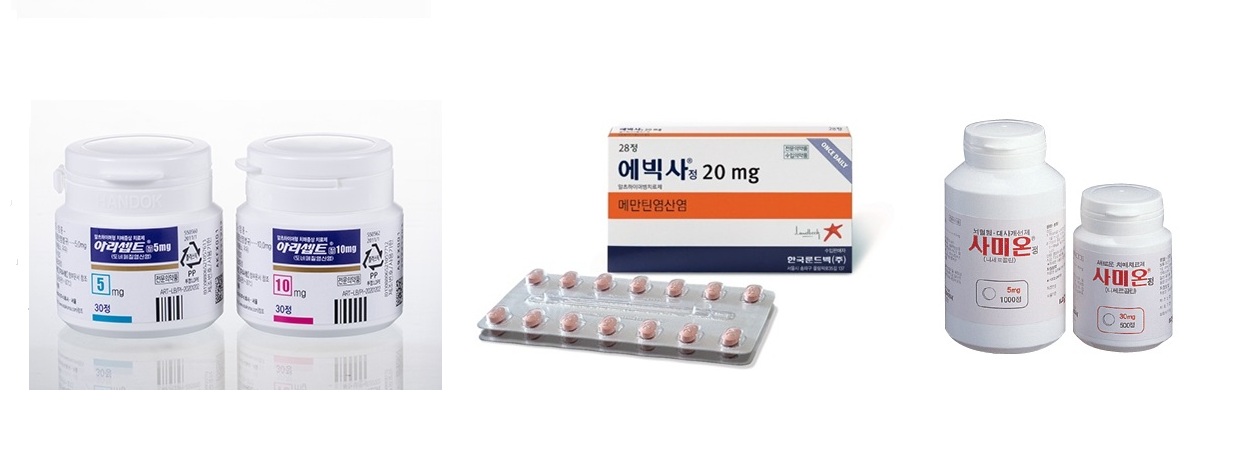
- Aricept(donepexil), Ebixa(memantine), Sermion(nicergoline) products that are considered alternatives to choline alfoscerate Sales of donepezil, memantine, and nicergoline-based dementia treatments, which have emerged as alternatives to choline alfoscerate formulations, are growing. In the first half of this year, donepezil’s sales recorded KRW 162.6 billion, an 8% increase from the previous year. Sales of memantine-based formulation increased by 10% year-on-year, and nicergoline-based formulations also increased by 36%. Prescription market for the choline alfoscerate alternative ‘donepezil’ grows from KRW 151.1 billion to KRW 162.6 billion in one year According to the pharmaceutical market research institution UBIST on the 6th, the outpatient prescription sales of donepezil-based formulations in the first half of this year amounted to KRW 162.6 billion. This represents an 8% increase from the KRW 151.1 billion in the first half of last year. Donepezil is used to treat symptoms of Alzheimer's dementia. The original product is Hanok's Aricept. In August 2000, Daewoong Pharmaceutical imported the finished product from the original developer and began domestic production and supply. Subsequently, the domestic marketing rights were transferred to Hanok. Sales are handled by Eisai Korea. In 2019, the indication for ‘vascular dementia’ was removed following a clinical reevaluation, but it has had little impact on prescription sales. On the contrary, it has continued to grow by around 5-8% annually since 2020. Prescription sales of donepezil-based formulations increased from KRW 243.6 billion in 2020 to KRW 259.8 billion in 2021, KRW 171.5 billion in 2022, KRW 291.9 billion in 2023, and to KRW 313.9 billion last year. Considering the upward trend in prescriptions in the first half of this year, its sales are expected to continue to increase at the same level as previous years. In particular, sales of generic products have shown marked growth. While the sales of the original product Aricept increased by only 2% from KRW 52.2 billion in the first half of last year to KRW 53.4 billion in the first half of this year, sales of generic products increased by 11% from KRW 98.9 billion to KRW 109.3 billion during the same period. Sales of Daewoong Bio's Beacept increased 14% from KRW 14.9 billion to KRW 17 billion, Samjin Pharmaceutical's Neutoin increased 27% to KRW 6.4 billion in a year, and Whan In Pharm’s Donegil increased 88% to KRW 3.8 billion. The rise of donepezil preparations is analyzed to connected to the crisis of choline alfoscerate products. Choline alfoscerate, which were previously the most widely used in the field of dementia prevention, are facing market withdrawal due to reduced reimbursement for their indications and clinical reevaluations. Initially, choline alfoscerate drugs had three indications: ▲secondary symptoms and degenerative or degenerative brain syndrome caused by cerebrovascular defects; ▲emotional and behavioral changes; ▲senile pseudo-depression. During the clinical reevaluation process, two of the three indications were removed, excluding its use as a treatment of ‘secondary symptoms and degenerative or degenerative brain disorders caused by cerebral vascular defects.’ A separate clinical reevaluation to verify the drug’s safety and efficacy is also underway. If pharmaceutical companies fail to prove efficacy in the clinical reevaluation process, their drugs will have to be completely withdrawn from the market. In addition, they must return 20% of the prescription amounts accrued during the clinical trial period to the health authorities. Given this situation, the pharmaceutical industry has been intent on finding alternatives to replace choline alfoscerate. In this process, donepezil, which has similar indications to choline alfoscerate, has emerged as one of the main alternatives. Although it has been used consistently for dementia prevention, there has been a series of new product approvals since the threat of choline preparations being withdrawn from the market. In fact, since June 2020, when the Ministry of Food and Drug Safety requested companies with choline alfoscerate-based products to submit clinical trial data, 39 pharmaceutical companies have received new approvals for 50 donepezil preparations. Quarterly prescriptions of donepezil products (Unit: KRW 100 million, Source: UBIST) The rise in sales of donepezil-based formulations is analyzed as linked to the crisis of choline alfoscerate drugs. Choline alfoscerate-based formulations, which were previously the most widely used ingredient in the field of dementia p #Sales of memantine-based formulations rise from KRW 27.7 billion to KRW 30.4 billion in one year... Sales of nicergoline preparations also jumped 36% The same situation goes for memantine and nisergoline-based products. These two ingredients are considered major alternatives to donepezil and choline alfoscerate. Prescription of memantine-based products increased 10% in the first half of this year, reaching KRW 30.4 billion, compared to the KRW 27,7 billion in the same period last year. Memantine is indicated for the treatment of moderate-to-severe Alzheimer's disease. The original product is Lundbeck's ‘Ebixa,’ which was approved in 2003. Like donepezil-based formulations, new product approvals have skyrocketed since June 2020. Twenty-seven pharmaceutical companies have been approved for 37 products in the last five years. One in three of all memantine products (109) on the market has been approved since the controversy arose over the efficacy of choline preparations. At the end of last year, approvals for new combination products that contain memantine and donepezil followed one after another. After HyundaiPharm received approval for ‘DM Duo,’ eight companies obtained approval for 14 additional products. Related products collectively achieved prescription sales of KRW 500 million in the first half of this year. Among choline esterase inhibitor alternatives, nicergoline-based formulations have had the most new product approvals in the industry. Nicergoline is approved for the ‘primary treatment of dementia symptoms such as memory impairment, concentration disorders, judgment disorders, and lack of initiative associated with primary degenerative vascular dementia and mixed dementia.’ The original product is Ildong Pharmaceutical’s Sermion. Ildong Pharmaceutical received approval for this product in 1997. Until 2022, no subsequent products were approved, excluding those for export. However, since 2023, new product approvals have been granted one after another. Following Hanmi Pharmaceutical's approval of ‘Nicegoline’ in January 2023, 39 companies have received approval for 53 products as of recently. Prescription sales of nicergoline formulations increased by 36% from KRW 3.3 billion in the first half of last year to KRW 4.5 billion in the first half of this year. In particular, the growth of new follow-on products that were recently released to the market has been rapid. The combined prescription sales of the follow on products, which amounted to only KRW 400 million in the first half of last year, increased more than threefold to KRW 1.5 billion in just one year. Sermion’s sales also increased slightly from KRW 2.9 billion to KRW 3 billion. Sales of existing choline preparations recorded KRW 294 billion in the first half of this year. Although this represents a slight decrease from the KRW 301.4 billion in the first half of last year, it still boasts prescription sales of nearly KRW 300 billion, demonstrating its continued popularity. Daewoong Bio and Chong Kun Dang, which hold the top two positions in the existing choline alfoscerate market, are also actively seeking alternatives. Daewoong Bio's ‘Gliatamin’ and Chong Kun Dang's ‘Chongkundang Gliatirin’ accounted for more than half of the total choline preparation market in the first half of the year. In preparation for the withdrawal of its choline alofscerate products, Daewoong Bio has received approval for ‘Beacept,’ which contains donepezil, ‘Glibixa’ containing memantine, and ‘Daewoong Nicergoline’ containing nicergoline. Chong Kun Dang has obtained approval for ‘Neuromanthyn’ containing memantine and ‘Nexcholine’ containing nicergoline. Additionally, at the end of last year, it added ‘Neurocept Duo,’ a combination of donepezil and memantine.
- Company
- Yuhan’s Leclaza to receive ₩100B in milestone payment
- by Chon, Seung-Hyun Aug 07, 2025 06:06am
- With Yuhan Corp’s new anticancer drug Leclaza approved in China, the company announced that it will receive KRW 63 billion in technology fees. Added to the technology fees secured at the end of last year for the drug’s approval in Europe, over KRW 100 billion in additional milestone payments are expected to be paid to the company. As a result, Yuhan Corp has secured a total of over KRW 400 billion in technology fees and sales royalties for Leclaza. 1 According to industry sources, the Chinese National Medical Products Administration (NMPA) recently included Leclaza in its list of newly approved drugs. Leclaza has been approved as a first-line treatment for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation in combination with Johnson & Johnson's Rybrevant. The drug is a non-small cell lung cancer treatment that was approved as the 31st domestically developed new drug in January 2021. Yuhan Corp exported the technology for Leclaza to Janssen Biotech in November 2018. Yuhan Corp will receive an additional $45 million in milestone payments from Janssen when sales of Leclaza begin in China. The combination therapy of Leclaza and Rybrevant was approved by the US Food and Drug Administration (FDA) in August last year and received approval from the European Commission (EC) at the end of last year. Japan's Ministry of Health, Labour and Welfare approved the combination therapy in March. Yuhan Corporation has not yet received the USD 30 million milestone payment for Leclaza's European approval. The fee will be paid once Leclaza goes on sale in major European countries. This means that Yuhan has secured a total of USD 75 million (approximately KRW 100 billion) in technology fees from China and Europe. Yuhan Corp has received a total of USD 225 million from Janssen since signing the licensing out deal for Leclaza. Yuhan Corp received USD 50 million in November 2018 as an upfront payment for Leclaza’s licensing out deal. Yuhan Corporation then received a milestone payment of USD 35 million from Janssen in April 2020. At that time, Johnson & Johnson paid Yuhan an additional milestone payment as it began clinical trials of Rybrevant in combination with Leclaza. Johnson & Johnson paid Yuhan an additional milestone payment of USD 65 million in November 2020 as it began recruiting subjects for clinical trials. Then, the company received an additional USD 60 million in milestone payments last year with the approval of Leclaza in the United States. In May, it received an additional KRW 15 million with the launch of Leclaza in Japan. Yuhan Corporation posted KRW 25.5 billion in milestone payments in the second quarter from the Japanese approval. With the addition of payments to come from China and Europe, Leclaza's technology fees will total near USD 300 million (KRW 420 billion). Yuhan Corporation receives a percentage of Leclaza's overseas sales as sales royalties. In other words, the more Leclaza's global sales expand, the more its revenue expands. According to Johnson & Johnson, the combination therapy of Leclaza and Rybrevant recorded sales of USD 320 million in the first half of this year. Forty percent of the technology fee revenue secured by Yuhan Corp will be paid to Oscotec, the original developer. Yuhan Corporation acquired the rights to develop Leclaza, which was in the preclinical stage, from Oscotec and its subsidiary Genosco in 2016. The total contract value was worth KRW 1.5 billion. Oscotec received KRW 1.289 billion in sales royalties from Leclaza in the first quarter of this year.
- Policy
- Gov’t sets criteria for drug shortage prevention
- by Lee, Hye-Kyung Aug 07, 2025 06:06am
- Prime Minister Min-seok Kim chaired the 7th Biohealth Innovation Committee at the Government Complex Seoul on the 5th The government will improve the criteria to raise the prices of drugs essential for patient treatment. Specifically, it will establish detailed evaluation criteria for price adjustment requests submitted by pharmaceutical companies that deem the current insurance price ceiling unreasonable. Prime Minister Min-seok Kim chaired the 7th Biohealth Innovation Committee at the Government Complex Seoul on the 5th and discussed measures to foster Korea into a biohealth powerhouse with government and private sector members. The meeting covered major issues such as improving drug price standards, revising the cost calculation method for exit prevention drugs, and expanding support for late-stage clinical bio venture funds. First, the criteria for designating drugs subject to drug shortage prevention measures will be revised upward to align with the changing pharmaceutical market environment in consideration of how their stable supply would affect the population as well as the fiscal expenditures required from the national health insurance. There have been calls from the field that the current criteria for designating drugs subject to drug shortage prevention measures, which have been in place since 2017, lack practicality, and there is a consensus that the minimum threshold amount needs to be revised to reflect factors such as inflation rates. In response, the government plans to publicly disclose by the end of the year a price adjustment criteria for such essential medicines, which will comprehensively consider factors such as the medical necessity, availability of alternatives, the number of suppliers of the same formulation (including whether the product is de facto the only drug supplied), and the supply situation of the drug, when evaluating price increases for drugs whose maximum prices have been announced and deemed significantly unreasonable by pharmaceutical companies. The government stated that through this, it expects to realistically adjust the minimum benchmark amount for selecting drugs to prevent withdrawal from the market by reflecting factors such as inflation rates, and to evaluate whether to adjust the maximum price based on drug price adjustment criteria that comprehensively consider actual supply conditions, thereby improving the profitability of essential drugs for patient care. Additionally, based on results of a recent study on improving the system for drug shortage prevention drugs, the government will revise part of the Standards for the Determination and Adjustment of Drug Prices (Appendix 5) to improve the cost calculation method for such medications, with the revised standards to be implemented in the first half of next year. The system improvements that will be made through revisions to the relevant regulations are expected to ensure preservation of appropriate production costs for drug shortage prevention drugs, leading to an increase in drug prices and enhanced supply stability for such drugs. Discussions on support for biotech companies were also held. The government is considering the establishment of a specialized fund for companies with ongoing or complete Phase III clinical trials that possess candidate substances or pipeline for innovative new drugs and biobetters. This is expected to enable domestic pharmaceutical and venture companies with excellent capabilities to secure sustained investment for Phase 3 clinical trials, which require significant time and costs, thereby increasing the likelihood of developing blockbuster new drugs. The government also plans to establish a basic-specialized-advanced step-by-step support system to help domestic companies enter the global market in response to the rapidly changing global pharmaceutical environment and provide customized consulting services. In addition, the government plans to provide specialized information through workshops on approval and licensing of advanced pharmaceutical and biotech products, produce online educational videos, and publish expert articles. Discussions were also held on expanding customized cost support in response to regulatory tightening in advanced countries such as the United States and the EU. To prepare for the tight regulations in advanced countries such as the FDA and MDR, the government plans to adjust the schedule for announcing customized cost support programs (from March 2024 to January 2025) to extend the support period for selected companies in the same year. Also, the government plans to change the project operation method to allow continuous cost support without additional project solicitations after monitoring the first-year performance when selecting this year's support recipients. Through these measures, domestic companies will be able to alleviate the financial burden and human resource shortages associated with certifications for the U.S. FDA and European MDR. In order to respond to the rapidly changing global pharmaceutical environment, the government plans to establish a step-by-step (basic-specialized-advanced) support system for domestic companies to advance into the global market and provide customized consulting services. Discussions were also held on a project being carried out by the Korea Disease Control and Prevention Agency (KDCA). The KDCA’s project supports the development of mRNA vaccines for pandemic preparedness in order to secure a vaccine platform that can be developed at a rapid pace in preparation for future pandemics. This project is a large-scale research project with a total budget of KRW 505.2 billion, which will support research and development tasks from preclinical to Phase III clinical trials over four years (2025-2028) with the goal of obtaining approval for a COVID-19 mRNA vaccine by 2028. The preliminary feasibility study was exempted in 2024, and the total budget and project period were finalized in March this year through a review of the appropriateness of the project plan. The KDCA confirmed the selection of four non-clinical trial project institutions in April and is currently supporting research and development to enter Phase I clinical trial in December of this year. To ensure the smooth implementation of the project, the KDCA's mRNA Vaccine Development Support Team and the Korea Health Industry Development Institute are planning the project and managing the performance targets. If the COVID-19 mRNA vaccine is developed through the project, it will not only ensure a stable supply of vaccines for high-risk groups but also enable the rapid development of vaccines within 100 to 200 days using Korea’s own mRNA vaccine technology in the event of a future pandemic.
- Policy
- H1 Pharma exports amounted to $5.38B
- by Lee, Hye-Kyung Aug 07, 2025 06:05am
- In the first half of this year, healthcare industry exports increased by 13.2% compared to the same period last year, reaching $13.79 billion, an all-time high for a half-year period. By sector, exports were led by cosmetics at $5.51 billion (+14.9%), pharmaceuticals at $5.38 billion (+20.5%), and medical devices at $2.91 billion (△0.6%). The Korea Health Industry Development Institute (KHIDI, President: Soon-do Cha) announced these export figures for pharmaceuticals, medical devices, and cosmetics for the first half of 2025 on August 6. Exports of pharmaceuticals increased by 20.5% year-over-year to $5.38 billion, driven by a surge in biopharmaceutical and vaccine exports. Biopharmaceutical exports (accounting for approximately 63.4% of total pharmaceutical exports) recorded $3.41 billion, a 27.4% increase year-over-year. Notably, exports saw a significant increase to the United States ($980 million, +41.4%), Hungary ($520 million, +26.8%), Germany ($470 million, +66.7%), Switzerland ($460 million, +76.9%), and the Netherlands ($250 million, +719.8%). H1 2025 Healthcare Industry Exports (unit: $1 million, %) Vaccine exports ($170 million, +53.3%) grew significantly in Sudan ($20 million, +397.3%), South Sudan (from $0 in H1 2024 to $10 million in H1 2025), and Congo (from $0 in H1 2024 to $10 million in H1 2025). According to Lee Byung-kwan, head of KHIDI's Biohealth Innovation Planning Division, "In the first half of 2025, healthcare industry exports were driven by all-time high half-year performances in the cosmetics and pharmaceutical sectors," and added, "In the second half, export growth is also expected to continue, driven by the expanding global demand for key products like biopharmaceuticals and basic cosmetics." Lee emphasized, "As external uncertainties such as changes in the U.S. tariff policy persist, it is crucial to monitor market trends and respond with caution and strategy carefully."
- Company
- 'Brintellix', stable position in antidepressant drugs mkt
- by Eo, Yun-Ho Aug 07, 2025 06:05am
- Product photo of Brintellix 'Brintellix' has established a stable presence in the antidepressant drugs market over the past 10 years. Its presence has not been disturbed by issues such as generic entries. According to industry sources, Lundbeck Korea's Brintellix (vortioxetine) has been growing in the market for antidepressant drugs for the past 10 years. In Q1 20125, Brintellix ranked No.2 in sales in the antidepressant market, based on the IQVIA data. Brintellix, first introduced in Korea in 2015, is differentiated from existing antidepressants, such as Selective Serotonin Reuptake Inhibitors (SSRIs) or Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs), which work by blocking the reuptake of serotonin or norepinephrine to produce an antidepressant effect. Brintellix is a new antidepressant with a mechanism that acts on various serotonin receptors while also inhibiting serotonin reuptake. Through this, it not only balances various neurotransmitters in addition to serotonin but also shows an effect in improving depressive symptoms and cognitive symptoms (such as concentration, attention, learning ability, and executive function) in patients with Major Depressive Disorder (MDD). Brintellix has been proven to significantly improve cognitive symptoms such as reduced attention, lack of concentration, and memory impairment that are observed in MDD patients. Furthermore, this drug was confirmed to significantly improve symptoms of reduced motivation and lack of energy in depressed patients, and it also statistically and meaningfully improved emotional blunting. These improvements in cognitive and emotional symptoms were not limited to short-term treatment. Brintellix demonstrated sustained antidepressant effects through a long-term study lasting 52 weeks and was reported to reduce the relapse rate in MDD patients by approximately 50% compared to placebo. Additionally, efforts to enhance the administration convenience for Brintellix are underway. Lundbeck is currently conducting clinical trials to compare the safety and pharmacokinetic properties of a salt-changed product of Brintellix upon administration. An official of Lundbeck stated, "As we celebrate the 10th year of Brintellix launch in Korea, Lundbeck has a variety of plans to help more patients based on its years of clinical experience and reliable products. We plan to contribute to treating depression with a patient-centered treatment option."
- Company
- COVID-19 vaccines transitioned to NIP
- by Whang, byung-woo Aug 06, 2025 06:10am
- COVID-19 vaccines, which were previously contracted through a pre-purchase model with pharmaceutical companies, will transition to the National Immunization Program (NIP) system. The Korea Disease Control and Prevention Agency (KDCA) announced on August 5 that it has signed a procurement contract for the supply of vaccines for the 2025-2026 seasonal COVID-19 immunization program. Unlike the previous method (2020-2024), where contracts were signed through a pre-purchase model with pharmaceutical companies using full government funding, this COVID-19 vaccine contract was signed through a government procurement model, transitioning it to a local government-subsidyzed project (matching local government funds), which is the same as the existing NIP system. The COVID-19 vaccines to be supplied to Korea for the 2025-2026 season are vaccines with the LP.8.1 strain, which have been recommended for use by the WHO (World Health Organization), EMA (European Medicines Agency) on May 16, and FDA (U.S. Food and Drug Administration). The decision was made by the Korea Expert Committee on Immunization Practices (KECIP). The total vaccine volume to be procured is 5.3 million doses (3.28 million from Pfizer and 2.02 million from Moderna), and contracts were signed through their respective COVID-19 vaccine distributors (companies with exclusive sales rights) in Korea. The domestic distributor for the Pfizer vaccine is HK inno.N, and Boryung Biopharma is the domestic distributor for Moderna. Regarding this contract, the KDCA explained, "The COVID-19 vaccine supply contract in Korea was signed using a private contract method, rather than a competitive bid, to ensure stable supply. However, based on a survey of local government demand, we incorporated price competitiveness elements and an additional reserve volume (5%) for each pharmaceutical company, aiming for both stable vaccine supply and budget savings." To minimize vaccine waste, vaccines nearing their expiration date during the project period can be exchanged to ensure continuous use throughout the vaccination period. After the project concludes, remaining vaccines can be returned within a 5% limit of the contracted volume. To minimize vaccine waste, vaccines nearing their expiration date during the project period can be exchanged to ensure continuous use throughout the vaccination period. After the project concludes, remaining vaccines can be returned within a 5% limit of the contracted volume. Meanwhile, Moderna announced that it will strive to protect high-risk groups aged 65 and over in Korea through the 2025-2026 seasonal NIP. Moderna's COVID-19 vaccine has accumulated extensive real-world vaccination data in Korea, with approximately 29.18 million doses administered from the beginning of the pandemic until now, confirming its efficacy and safety. According to a study conducted by the KDCA, the Moderna vaccine was found to have the lowest breakthrough infection rate among the vaccines used in Korea during the early stages of the pandemic. Since 2021, Moderna has collaborated with Samsung Biologics to produce the only mRNA COVID-19 vaccine in Korea. In addition, Moderna is making efforts to strengthen mRNA technology capabilities through various collaborations, including joint research on a vaccine candidate for Severe Fever with Thrombocytopenia Syndrome (SFTS) with the National Institute of Infectious Diseases under the KDCA, and a partnership with KAIST and Yonsei University K-NIBRT for training mRNA research talent. Kim Sang-pyo, General Manager of Moderna Korea, said, "We are deeply grateful and find it meaningful that Moderna's efforts over the past few years to protect high-risk groups from the COVID-19 can continue through this year's NIP," and added, "As COVID-19 remains an infectious disease with a high severity rate, especially in adults aged 65 and over, we will put utmost efforts to protect more high-risk individuals in line with the government's vaccination plan."
- Company
- Imfinzi wins nod as pre- and post-operative adjuvant therapy
- by Whang, byung-woo Aug 06, 2025 06:10am
- Product photo of Imfinzi AstraZeneca announced on August 4 that it has received approval for its Imfinzi (durvalumab) as pre- and post-operative adjuvant therapy for patients with muscle-invasive bladder cancer from the Ministry of Food and Drug Safety on July 30. With this approval, Imfinzi has become the first and only immunotherapy in Korea to be approved for pre- and post-operative adjuvant therapy in the treatment of muscle-invasive bladder cancer. Imfinzi's specific approved indication is for 'the treatment of patients with muscle-invasive bladder cancer as a neoadjuvant combination therapy with cisplatin and gemcitabine or as Imfinzi monotherapy as adjuvant therapy after radical cystectomy.' Imfinzi's specific approved indication is for 'the treatment of patients with muscle-invasive bladder cancer as a neoadjuvant combination therapy with cisplatin and gemcitabine or as Imfinzi monotherapy as adjuvant therapy after radical cystectomy.' The previous standard therapy was neoadjuvant chemotherapy followed by radical cystectomy. However, this left an unmet need due to high recurrence rates and poor prognosis even after treatment. It is known that approximately 50% of muscle-invasive bladder cancer patients experience recurrence within three years. The expanded indication is based on the Phase 3 NIAGARA clinical study, which evaluated the clinical efficacy and safety of Imfinzi as a pre- and post-operative adjuvant therapy for muscle-invasive bladder cancer. In this study, patients were divided into two groups. The experimental group received Imfinzi and chemotherapy (gemcitabine-cisplatin) in combination as neoadjuvant therapy for 4 cycles at 3-week intervals, followed by surgery and then Imfinzi monotherapy as adjuvant therapy for 8 cycles at 4-week intervals. The control group underwent chemotherapy (gemcitabine-cisplatin) as neoadjuvant therapy, followed by surgery only. The study results showed that Imfinzi's pre- and post-operative adjuvant therapy significantly improved one of the key primary endpoints, event-free survival (EFS), compared to the control group. The 2-year event-free survival rate was 67.8% for the Imfinzi pre- and post-operative adjuvant therapy group and 59.8% for the control group. The Imfinzi pre- and post-operative adjuvant therapy reduced the risk of disease progression, recurrence, failure to undergo radical cystectomy (surgery), and death from all causes by 32%. In terms of the safety profile, Imfinzi's results were consistent with the individual safety profiles of previously confirmed Imfinzi and chemotherapy (gemcitabine-cisplatin). Based on these clinical benefits, Imfinzi is a Category 1 preferred recommendation for pre- and post-operative adjuvant therapy for patients with muscle-invasive bladder cancer in the National Comprehensive Cancer Network (NCCN) guidelines. Furthermore, the European Society for Medical Oncology (ESMO) has rated it with an A-grade, the highest rating in their Magnitude of Clinical Benefit Scale (MCBS) framework. Professor Byong Chang Jeong of the Department of Urology at Samsung Medical Center (President of the Korean Urological Oncology Society) explained, "The previous standard therapy for muscle-invasive bladder cancer had limitations in improving patient survival due to high recurrence rates," and added, "The newest approval of an immunotherapy as a pre- and post-operative adjuvant therapy is a favorable change in the clinical field." Jeong added, "By administering Imfinzi, we can expect to increase the success rate of surgery through neoadjuvant therapy and reduce the recurrence rate through adjuvant therapy, ultimately improving the survival rate of patients with muscle-invasive bladder cancer in Korea."
- Company
- US drug price 3.9 times higher than in Korea
- by Kim, Jin-Gu Aug 06, 2025 06:09am
- With the US government pushing forward the introduction of a Most Favored Nation (MFN) policy to lower drug prices, an analysis has revealed that US drug prices are 3.9 times higher than those in South Korea. The Korea Biotechnology Industry Organization cited an analysis by the US public policy research institute Rand Corporation on the 4th on this finding. The data was released in February 2024 and is based on drug prices in 33 OECD countries, including the United States. According to the data, the overall drug price level in the United States is 2.8 times higher than the average of 32 OECD countries. Compared to South Korea, it is 3.9 times higher. In other words, drug prices in South Korea are equivalent to 25.6% of those in the United States. In addition, US drug prices are 3.5 times higher than Japan’s, 3.3 times higher than France’s, 2.9 times higher than Germany’s, and 2.7 times higher than the United Kingdom’s. Among OECD countries, Turkey has the largest price disparity, with prices 10.3 times higher than those in the United States. Also, a significant price difference was observed between brand-name (original) drugs and generic drugs. The price of brand-name drugs was 4.2 times higher in the US than the OECD average. In particular, the top 60 drugs in terms of sales in the US are 5.0 times higher than the OECD average. Among brand-name drugs, biopharmaceuticals are 3.6 times higher than the OECD average. On the other hand, the price of generic drugs in the US is lower – around 66.8% of the OECD average. The US President Donald Trump has been strengthening trade pressure on pharmaceuticals by imposing tariffs and most-favored-nation (MFN) policies. While announcing plans to impose tariffs on pharmaceuticals, he is also targeting drug prices in other countries as a means of exerting trade pressure. As part of this effort, President Trump signed an executive order in May to lower prescription drug prices in the U.S. to the lowest prices among major developed countries (MFN). The main point of the order is to compare drug prices in OECD countries with a per capita GDP of 60% or more than that of the US, and lower US drug prices to the lowest among them. The most-favored-nation treatment policy applies only to products that do not have biosimilars or generics. The US Department of Health and Human Services plans to first encourage pharmaceutical companies to voluntarily lower drug prices and then force them to do so. If the policy is fully implemented, the prices of new drugs entering the US market are expected to be set lower than those in other countries, such as the EU and Canada. It is speculated that this may automatically lower the prices of domestic pharmaceutical companies' drugs in the US. In particular, new drug development companies are expected to be affected. If drug prices in the US drop to European or Canadian levels, pharmaceutical companies' expected sales are likely to decrease, and the value of their new drug licenses is expected to decline. On the other hand, biosimilars and generics are not expected to be directly affected, as the policy targets products without existing biosimilars or generics. However, these products may still face pressure on profitability in the future. If the prices of original drugs decrease, the benchmark prices for generics and biosimilars could also decline, reducing sales incentives.
- Opinion
- [Reporter's View] Can NA resolve the drug shortage issue?
- by Kim, Jin-Gu Aug 06, 2025 06:09am
- Whether the four bills presented for amendments to the Pharmaceutical Affairs Act aimed at resolving the shortage of medicines will be discussed at the National Assembly's Health and Welfare Committee in August is gaining attention. The amendments to the Pharmaceutical Affairs Act were proposed by Rep. Jeong-ae Han, Rep. Yoon Kim, and Rep. Mi-hwa Seo of the Democratic Party of Korea, and Rep. Sun-min Kim of the Rebuilding Korea Party. Rep Jeong-ae Han’s bill proposes the establishment of a supply management committee involving both the public and private sectors, as well as the designation of “medicines with unstable supply” and the establishment of regulations for emergency production and import orders. Rep Yoon Kim's bill includes medicines with temporary supply shortages or sudden increases in demand as “medicines subject to stable supply management,” and allows medical professionals and relevant institutions, and organizations to participate in the National Essential Medicines Supply Stability Council. Rep. Mi-hwa Seo's bill focuses on including medications without alternatives as National Essential Medicines and implementing constant monitoring. Rep. Sun-min Kim’s bill includes medications with temporary supply shortages or sudden increases in demand in the “stable supply management targets” category and seeks to allow medical professionals and institutions/organizations to participate in the National Essential Medications Supply Stability Council. All four amendments were positively received as they presented their own solutions to stabilize the supply of medicines. Considering the repeated medicine shortages and supply disruptions that arose over the past few years, they were also deemed timely. If the bills are submitted and pass the Health and Welfare Committee review, they are likely to be processed through the Legislative and Judiciary Committee and handled during the September regular session of the National Assembly. If this process is realized, it is expected to provide a significant boost to resolving the medicine supply shortage that has been recurring for several years. Resolving the medicine supply shortage was a common campaign pledge made by both the ruling and opposition parties in the last presidential election. Furthermore, except for the extent to which the international nonproprietary name prescriptions should be allowed, there does not seem to be much disagreement between the parties. This means that now is the optimal time to discuss measures to resolve the medicine supply shortage. However, it is necessary to carefully review whether there are any aspects that have not been included in the revised bill. In particular, the pharmaceutical industry has consistently demanded fundamental solutions for increasing the self-sufficiency rate of domestically produced raw materials for pharmaceuticals and strengthening incentives for the production of low-profit essential drugs, which require urgent attention. There are calls for the need to establish practical supply chain strengthening policies that go beyond simply expanding the scope of items subject to supply instability and forming a consultative body. Fundamental measures are needed to overcome low productivity and a weak supply structure. The government should also consider expanding public reserves and establishing a digital-based real-time monitoring system. Overseas cases are also worth noting. The United States operates a Drug Shortage Task Force to maintain an early warning and supply substitution system. The European Union (EU) operates a system to jointly manage and stockpile about 200 essential drugs. Japan is known to operate a system to reevaluate the prices of low-profit products that cause deficits, focusing on essential drugs. These countries share the common feature of active government intervention to expand supply and promote corporate participation. The current parliamentary discussion presents an opportunity to address critical issues that can no longer be postponed. It must serve as the starting point for developing practical solutions. This rare opportunity must not be wasted. Instead of half-hearted measures, a clear direction that ensures supply stability that both citizens and the pharmaceutical industry can feel must be presented this time.