- LOGIN
- MemberShip
- 2025-12-20 13:24:36
- Policy
- Smokers at 54.5 times higher risk of SCLC than non-smokers
- by Lee, Tak-Sun Aug 12, 2025 06:13am
- A study has found that 30-year smokers have a 54.5 times higher risk of developing small cell lung cancer than non-smokers. The National Health Insurance Service (NHIS) Health Insurance Policy Research Institute announced on the 11th the results of a comparative analysis of cancer incidence risk and attributable risk by cancer type among individuals with the same level of polygenic risk score (PRS) and similar living environments, focusing on major cancers with high incidence rates in Korea. The study found that the risk of developing small cell lung cancer among current smokers (30 years or more, 20 pack-years or more) was 54.5 times higher than that of non-smokers, significantly higher than colorectal cancer (1.5 times), liver cancer (2.3 times), and stomach cancer (2.4 times higher). T Cancer risk according to smoking history (hazard ratio) This study was jointly conducted by the Health Insurance Policy Research Institute (Director Seongin Jeong) and Yonsei University Graduate School of Public Health (Professor Seon-ha Ji's research team). The study analyzed data from 136,965 participants who underwent health screenings at 18 private screening centers nationwide from 2004 to 2013, linking health screening results, polygenic risk scores (PRS), central cancer registry data, and health insurance eligibility data, and followed the participants until 2020. The analysis of cancer incidence risk showed that even when general characteristics, living environments, and PRS were at the same level, the incidence risk of smoking-related cancers (small cell lung cancer, squamous cell lung cancer, and squamous cell laryngeal cancer) was higher in the smoker group than that of other cancers. Compared to non-smokers, current smokers with a smoking history of “30 years or more and 20 pack-years or more” had a significantly higher cancer incidence risk: 54.5 times higher for small cell lung cancer, 21.4 times higher for squamous cell lung cancer, and 8.3 times higher for squamous cell laryngeal cancer. However, the risks were 2.4 times higher for stomach cancer, 2.3 times higher for liver cancer, and 1.5 times higher for colorectal cancer. The extent to which smoking contributes to the development of small cell lung cancer was found to be 98.2%, followed by 88.0% for squamous cell laryngeal cancer, and 86.2% for squamous cell lung cancer, confirming that smoking is the primary cause of the cancers targeted in the tobacco litigation. In contrast, the extent to which smoking contributes to the development of colorectal cancer is 28.6%, stomach cancer 50.8%, and liver cancer 57.2%, indicating that smoking contributes significantly less to these types of cancer compared to the cancers subject to litigation, and that other factors play a major role. Genetic factors contribute only 0.4% to the development of squamous cell lung cancer, whereas they account for 7.3% of colorectal cancer and 5.1% of stomach cancer, indicating that genetic factors have a significantly greater influence on these cancers compared to squamous cell lung cancer, with respective differences of 18.3 times and 12.8 times. Sunmi Lee, Director of the Health Insurance Policy Research Division at the Health Insurance Policy Research Institute, emphasized, “The results of this study show that smoking contributes significantly more to the development of lung cancer and laryngeal cancer compared to other types of cancer, while genetic factors play an extremely minor role. This further clarifies the causal relationship between smoking and the development of lung cancer and laryngeal cancer.”
- Company
- Gilead’s Livdelzi receives orphan drug designation in KOR
- by Eo, Yun-Ho Aug 11, 2025 06:05am
- The new primary biliary cholangitis drug ‘Livdelzi’ has received the orphan drug designation in Korea. The Ministry of Food and Drug Safety announced the news in a public notice on the 4th. Specifically, the designated indication is “the treatment of primary biliary cholangitis (PBC) in adults who have an inadequate response or intolerance to ursodeoxycholic acid.” Livdelzi (seladelpar) was also designated as a candidate for the Global Innovative Products on Fast Track (GIFT) program in June. PBC is a rare, intractable autoimmune disease characterized by chronic inflammation and destruction of the bile ducts in the liver, leading to bile stasis and liver damage, which can then potentially progress to cirrhosis and liver failure. While ursodeoxycholic acid (UDCA) is currently used as the first-line treatment, there has been an ongoing need for new treatment strategies for patients who have an inadequate response to UDCA or experience intolerance. Seladelpar is an oral selective peroxisome proliferator-activated receptor (PPAR) delta agonist. This drug demonstrated efficacy in a Phase III RESPONSE study. The RESPONSE study was a randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of seladelpar compared to placebo in adult PBC patients with inadequate response to UDCA or intolerance to UDCA. Study results showed that 62% of patients in the seladelpar treatment group achieved the primary endpoint (composite biochemical response, defined as improvement in alkaline phosphatase (ALP) levels and total bilirubin levels) at 12 months, demonstrating statistically significant superiority over the 20% in the placebo group. Notably, 25% of patients in the seladelpar treatment group achieved normalization of alkaline phosphatase (ALP) values, demonstrating significance. The efficacy of seladelpar was also confirmed in the improvement of pruritus, a key secondary endpoint. Compared to baseline, at 6 months, the seladelpar treatment group showed an average reduction of 3.2 points in pruritus scores among patients with moderate-to-severe pruritus, demonstrating a statistically significant improvement compared to the placebo group's reduction of 1.7 points. Meanwhile, Livdelzi was granted accelerated approval by the U.S. FDA in August last year and was approved in Europe in February this year.
- Company
- First TYK2i Sotyktu shows effect in Asian psoriasis patients
- by Son, Hyung Min Aug 11, 2025 06:04am
- BMS Korea The psoriatic arthritis treatment Sotyktu, which is jointly marketed in Korea by BMS Korea and Yuhan Corp, has established itself as a personalized oral treatment option for psoriatic arthritis patients in Korea, accumulating data from patients in East Asia. Sotyktu has been proven effective even in difficult-to-treat areas such as the scalp and nails, and is evaluated as having increased patients' access to early treatment through its inclusion in the national health insurance reimbursement list. According to industry sources on the 8th, BMS recently released clinical data of Sotyktu on East Asian patients, which included Koreans. Sotyktu is a first-in-class selective TYK2 inhibitor that, unlike other JAK inhibitors, has an allosteric mechanism that binds to the inactive regulatory domain. Psoriasis is a chronic disease that is difficult to cure and involves repeated improvements and worsening of symptoms, requiring long-term and continuous treatment. In particular, there remained a therapeutic need for new alternatives due to the diverse treatment options required based on individual disease characteristics and treatment preferences. Sotyktu is a once-daily oral treatment that can be taken regardless of meal intake and without dose adjustment, expanding the treatment options for psoriasis patients who previously had only biological agents as an alternative to conventional treatments. Sotyktu has demonstrated its efficacy and safety profile as a psoriasis treatment in numerous global and Asian clinical trials. In the POETYK PSO-3 study, which included Asian patients including Koreans, the proportion of patients who showed a 75% or greater reduction in the Psoriasis Area and Severity Index score (PASI 75) at Week 16 was 68.8%, and the rate of achieving a score of 0/1 on the static Physician’s Global Assessment (sPGA) was 55.6%, showing significant improvement compared to the placebo group. The results of three recent analyses conducted in Japan also supported the efficacy and safety of Sotyktu in East Asian patients. New data was released this year, including a post-hoc analysis of the POETYK PSO-4 Phase III clinical trial, a three-year efficacy and safety analysis of Japanese patients through the POETYK PSO-1, 4, and LTE studies, and an analysis of Japanese patient reports from POETYK PSO-4. In the post-analysis of POETYK PSO-4 in Japanese patients, the PASI 2 or less rate at week 16 of treatment in the Sotyktu group was 46.0%, and at week 52, the PASI 2 or less rate was 68.3% and the PASI 1 or less rate was 47.6%. In the patient-reported outcome (PRO) analysis of the same study, the rate of patients achieving a DLQI (Dermatology Life Quality Index) of 0/1 at week 52 reached 66.1%. Major symptoms such as erythema, scaling thickness, and severity of scaling began to improve from week 1 of treatment, with over 80% improvement by week 16, and approximately 90% of the effect was maintained or improved by week 52. Kiheon Jeong, Professor of Dermatology at Kyung Hee University Hospital (Insurance Director of the Korean Society for Psoriasis (KSP)), stated, “Areas such as the scalp and palms/soles, which are visible and cause daily discomfort, often have low patient satisfaction even with mild symptoms. We anticipate that oral medications with proven efficacy in these special areas will expand treatment options.” Health insurance coverage greatly reduces treatment costs and improves access to the latest treatments Sotyktu showed consistent efficacy even in patients with a variety of previous treatment histories, demonstrating its potential as a long-term treatment option. In a three-year post-hoc analysis including POETYK PSO-1, PSO-4, and long-term extension (LTE) studies, 65.6% of 125 Japanese patients had prior treatment experience, and 20.8% of these had a history of biological agent use. In the analysis of Japanese patients with PSO-1, the PASI achievement rate was 88.9% in the first year and remained at 87.5% in the third year, and the sPGA 0/1 response rate also showed a long-term response, from 74.1% in the first year to 66.7% in the third year. Similar results were observed in patients who switched from placebo to Sotyktu. This suggests that consistent clinical responses are possible over a long period of time, regardless of previous treatment experience. Sotyktu was approved in Korea in 2023 and was reimbursed the following year, greatly improving patient access and reducing their burden of cost compared to existing treatments. According to the health insurance reimbursement criteria, it can be administered to adult patients aged 18 years or older with chronic severe plaque psoriasis that has persisted for at least six months. Sotyktu was approved by the Ministry of Food and Drug Safety in 2023 and became eligible for reimbursement by health insurance eight months later. Reimbursement is available for adult patients aged 18 years or older with chronic severe plaque psoriasis that has persisted for six months or longer and who have ▲symptoms on BSA (body surface area) of 10% or more ▲PASI score of 10 or higher, and who have not responded to or cannot continue treatment with methotrexate or cyclosporine for at least three months due to efficacy or adverse effects, or have not responded to or cannot continue photochemotherapy or narrowband ultraviolet B therapy for at least three months due to efficacy or adverse effects. Professor Jeong said, “The accumulation of evidence from East Asian patients is encouraging for the psoriasis treatment environment in Korea. With reimbursement, the burden of treatment costs has been reduced, enabling quick and economical treatment even for patients who do not qualify for the special calculation benefits. This will provide practical treatment opportunities to more patients.”
- Company
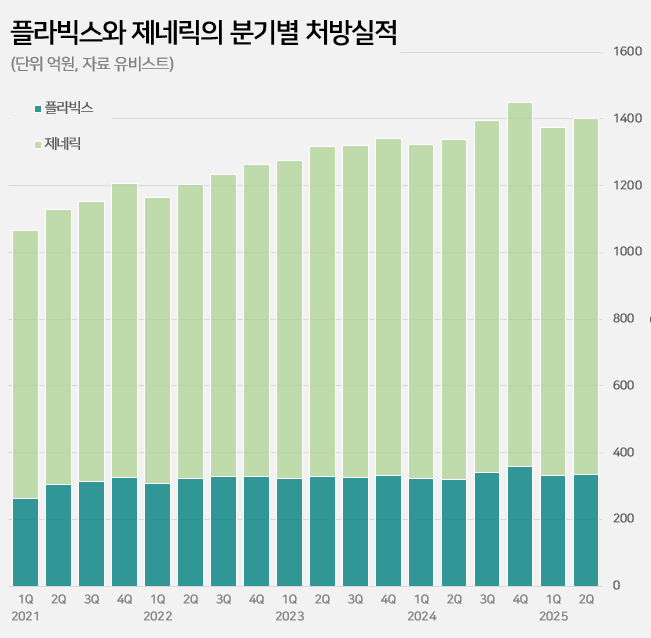
- Plavix’s sales continue to grow 27 years into its release
- by Kim, Jin-Gu Aug 11, 2025 06:04am
- Sales of the original drug ‘Plavix’ continue to rise in its 27th year of release in the clopidogrel-based antiplatelet agent market. It has maintained its lead with a 23% market share in the annual KRW 550 billion market. Meanwhile, major generic products are showing signs of slowing down. Samjin Pharmaceutical's ‘Platless’ saw a decrease in prescriptions compared to the same period last year, while sales of Dong-A ST's ‘Plavitor’ only increased by 2%. Sales of Chong Kun Dang’s ‘Pregrel' saw a 10% increase over the past year, but it still falls short of its performance before sales were suspended. Plavix’s sales in the first half of the year reach KRW 64.1 billion... Sales still on the rise even after 27 years on the market According to the pharmaceutical market research institute UBIST on the 9th, prescriptions for clopidogrel-containing antiplatelet drugs in the first half of this year reached KRW 264 billion, a 5% increase from the KRW 252.3 billion in the same period last year. The market leader is Handok’s Plavix. Sanofi launched Plavix in Korea in 1999. Since 2006, it has been manufactured by Handok. In 2016, a combination product called “Plavix A (Plavix+aspirin)” was added. Even after 27 years since its launch, sales of Plavix continue to grow in the outpatient prescription market. After the Plavix patent expired in 2007, 145 companies were approved to sell generic versions and entered the market. This is in contrast to the general trend of a decline observed in the prescription sales of original drugs after the launch of generic drugs. In fact, the combined prescription amount for Plavix and Plavix A exceeded KRW 100 billion for the first time in 2019 and continued to increase for 5 consecutive years thereafter. Based on the prescription amount rendered in the first half of the year, it increased by 29% in five years compared to KRW 49.8 billion in the first half of 2020. Quarterly prescriptions of Plavix and its generic versions The background behind Plavix's long-term growth lies in its market expansion strategy. Clopidogrel is mainly used to prevent the recurrence of ischemic cardiovascular diseases such as myocardial infarction and stroke, and has maintained its status as a standard therapy in domestic and international treatment guidelines along with an increase in the number of patients. The trust and prescription preference of medical professionals for the original drug have also contributed to its steady growth. Major generic products such as Samjin’s Platless and Dong-A ST's Plavitor show sluggish growth Meanwhile, sales of major generic products have recently shown signs of a slowdown. Samjin Pharmaceutical's Platless, the second-largest product in the market, recorded prescription sales of KRW 40.8 billion in the first half of this year. This represents a 4% decrease from the KRW 42.5 billion in the first half of last year. Dong-A ST's Plavitor, the third-largest product, saw a 2% increase in sales from KRW 15.3 billion to KRW 15.6 billion. Sales of Chong Kun Dang’s ‘Pregrel’ increased by 10% from KRW 11 billion to KRW 12.1 billion. However, it has yet to recover its pre-suspension sales performance. Pregrel’s sales were suspended from April to July 2021 after it was revealed that it had arbitrarily used additives. Before the suspension, Pregrel’s prescription sales exceeded KRW 7 billion every quarter, but since sales resumed, it has remained in the KRW 5 to 6 billion range. However, overall generic prescription sales increased by 4% from KRW 202.1 billion to KRW 210.7 billion in one year. This is believed to be the result of strong performance by products other than the top-ranked generics. Sales of Daewoong Pharmaceutical's 'Cloart' increased by 7% from KRW 10.4 billion to KRW 11.1 billion, and Hanmi Pharmaceutical's 'Pidogul' increased by 5% from KRW 9.7 billion to KRW 10.1 billion. Other products, such as JinYang Pharm’s Krivix (11%), Teragenix's Pravixen (14%), and Dasam Pharmaceutical's Clpigrel (54%), also recorded relatively high growth rates. Generic companies' clopidogrel-aspirin combination drugs are not faring so well in the market. HK Inno.N, Jeil Pharmaceutical, and Myungin Pharmaceutical have launched combination drugs containing the two ingredients since 2011. The combined prescription sales of these combination drugs decreased by 3% from KRW 13.8 billion in the first half of last year to KRW 13.4 billion in the first half of this year.
- Company
- The launch of Mounjaro is imminent
- by Moon, sung-ho Aug 11, 2025 06:03am
- The launch of Eli Lilly's 'Mounjaro,' which is called a 'miracle weight-loss drug' and garnered global attention alongside Wegovy, in Korea, is imminent. With the domestic launch of Mounjaro scheduled for mid-August, competition in the obesity treatment market, besides diabetes, is entering a new phase. There is significant interest in the sales and marketing strategies that Eli Lilly Korea will employ to challenge Novo Nordisk's Wegovy, which was launched in the domestic market approximately a year earlier. According to pharmaceutical industry sources on August 7, Eli Lilly Korea is set to launch the GIP/GLP-1 dual agonist Mounjaro Prefilled Pen Inj (tirzepatide), hereafter referred to as Mounjaro, in 2.5 mg and 5 mg dosages for patients with type 2 diabetes and obesity in mid-August. Mounjaro is the first and only GIP (Glucose-dependent Insulinotropic Polypeptide)/GLP-1 (Glucagon-like Peptide-1) receptor dual agonist. It is a single-molecule injectable designed to selectively bind to and activate GIP and GLP-1 receptors, allowing for once-weekly administration. It helps lower blood sugar by promoting insulin secretion, improving insulin sensitivity, and reducing glucagon concentration. Additionally, it aids in weight loss by delaying gastric emptying, which in turn reduces food intake. In Korea, Mounjaro is approved as an adjunct drug to diet and exercise for improving glycemic control in adult patients with Type 2 diabetes (as monotherapy or combination therapy). It is also approved as an adjunct to a low-calorie diet and increased physical activity for chronic weight management in obese adults (initial BMI≥30kg/m2) or overweight adults (initial BMI≥30kg/m2) with at least one weight-related comorbidity (hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease). For both indications, the recommended starting dose is 2.5mg once a week (intended for treatment initiation and not for glycemic control or weight management). After four weeks, the dose is increased to 5mg once a week. If further dose adjustment is needed, the dose is increased by 2.5 mg after at least four weeks at the current dose, with a maximum dose of 15 mg once a week. Meanwhile, Eli Lilly Korea has decided to initially launch the 'pre-filled pen' formulation, which was first approved in 2023, amid delays in the approval of the vial and QuickPen formulations. This is interpreted as a strategy first to release the pre-filled pen formulation and subsequently launch the other formulations once their ongoing approval process is complete. Following the launch of Mounjaro, Eli Lilly Korea plans to implement a comprehensive sales and marketing strategy, which will include hosting symposium for medical professionals at major hospitals and clinics starting in late August. Consequently, the clinical community's attention is focused on Mounjaro's pricing. Since obesity treatment is not reimbursed, Mounjaro's supply price will be a critical factor. According to the pharmaceutical industry, Mounjaro is expected to be supplied at around KRW 278,000 for the 2.5mg dose (4PEN) and approximately KRW 369,000 for the 5mg dose (4PEN). This pricing is interpreted as a strategic move, considering that Novo Nordisk Pharma's Wegovy (semaglutide) was priced at KRW 372,025 per month for all dosages when it was launched in Korea last year. It's believed the 5mg dose was strategically priced to compete with Wegovy. Consequently, it's expected that doctors will also consider the drug's supply price when setting the average monthly price for non-reimbursed treatments. For reference, patients typically pay an average of KRW 500,000 to 600,000 per month for Wegovy as a non-reimbursed treatment at clinics. Given this, the non-reimbursed price for Mounjaro 2.5mg is likely to be lower than that of Wegovy, while the 5mg dose may be similar to Wegovy's monthly cost. Cheol Jin Lee, Chairman of the Korean Society for the Study of Obesity (Good Family Clinic), stated, "The vial and QuickPen formulations need approval, but it seems that the Ministry of Food and Drug Safety's (MFDS) approval is delayed." Lee added, "If the vial formulation is also launched in Korea, it would offer a significant pricing advantage. As far as we know, Mounjaro will initially be released in a pre-filled pen format, which is a disposable injectable form." Lee noted, "Since the efficacy of this drug was confirmed in clinical trials, if it is launched, it will likely be used primarily for new patients." He explained, "This is because it would be difficult for patients who are already on high-dose Wegovy to switch to Mounjaro, which starts at a 2.5mg dose. When considering efficacy and other factors, we would likely consider Mounjaro for new patients if it is launched." With Mounjaro's launch, the focus shifts to its competition with Wegovy in clinical practice. Novo Nordisk is also actively ramping up its sales and marketing efforts as the first anniversary of Wegovy's domestic launch approaches. In addition to its in-house sales team of around 80 people, the company is reportedly considering a joint sales and marketing strategy with a domestic pharmaceutical company. Chong Kun Dang is a strong candidate being discussed within the pharmaceutical industry as a domestic sales partner. However, Novo Nordisk's position is that while they are pursuing collaboration with a domestic pharmaceutical company, nothing has been finalized. At the same time, Novo Nordisk is also seeking to expand Wegovy's indication to adolescents aged 12 and older. If this becomes a reality, it would give Wegovy a differentiated competitive edge over Mounjaro in domestic clinical practice, primarily because adolescent obesity has emerged as a significant social issue. Eli Lilly Korea has also established its own sales force, and like Novo Nordisk, is open to collaboration with domestic pharmaceutical companies. Although several companies, including Hanmi Pharm, are being mentioned, Lilly is expected to focus on Mounjaro's launch for the time being. In this context, the pharmaceutical industry is also closely monitoring Eli Lilly Korea's efforts to secure reimbursement for Mounjaro's diabetes indication. There is great interest in whether it will be recognized as an innovative new drug, alongside its competitor, Ozempic. This contrasts with Novo Nordisk's strategy, which involves applying different indications for a single product as part of its reimbursement strategy. An official said, "We have applied to the Health Insurance Review & Assessment Service (HIRA) for national health insurance coverage and are currently awaiting consideration from the DREC." They added, "As the first GIP/GLP-1 receptor dual agonist and a new treatment for type 2 diabetes, we expect Mounjaro to provide differentiated clinical value. Therefore, we are doing our best for it to be the first chronic disease drug to receive flexible application of the ICER value for innovative new drugs." A pharmaceutical industry official, who requested anonymity, expressed doubt about whether Mounjaro could meet the government's definition of innovation. They said, "Of course, this doesn't mean Mounjaro has no innovative value. However, we need to verify whether it meets the criteria and definition of innovativeness that the government has established. Ultimately, the question of whether it can be applied will inevitably follow." An official also said, "We are curious about how the drug price will be set if one treatment is covered for diabetes but not for obesity," and added, "With Ozempic also pursuing reimbursement with a target of the first half of next year, this issue will become a new topic of discussion."
- Company
- New pulmonary hypertension drugs are competing for approval
- by Moon, sung-ho Aug 11, 2025 06:03am
- Global pharmaceutical companies are introducing new treatment options for pulmonary hypertension, utilizing novel mechanisms, which are being successively approved in the Korean market. These drugs are expected to change the clinical treatment paradigm. It's anticipated that they will establish a new market alongside the currently reimbursed treatments. According to the pharmaceutical industry source on July 28, the Ministry of Food and Drug Safety (MFDS) recently approved Winrevair (sotatercept) from MSD Korea and Opsynvi Tab (macitentan-tadalafil) from Janssen Korea as treatments for pulmonary hypertension. Pulmonary hypertension is a rare, severe, intractable disease where the walls of the pulmonary arterioles abnormally thicken and narrow, causing pressure to rise. As the disease progresses, symptoms like shortness of breath, chest pain, and fainting appear, severely limiting all aspects of daily life. Additionally, the increased pressure in the right ventricle due to the narrowed pulmonary arteries gradually weakens the function of the right side of the heart, leading to a high risk of sudden death. First, MSD Korea's Winrevair is an 'Activin Signaling Inhibitor (ASI), the first-in-class (as of July 2025)' to be approved in the field of pulmonary hypertension. It is a new treatment mechanism that has emerged after 20 years. Its mechanism directly addresses the fundamental cause of the disease by blocking the excessive proliferative signals of the protein complex 'activin,' which causes cell proliferation within pulmonary artery blood vessels, and by restoring the balance with anti-proliferative signals that inhibit cell growth, thereby inducing reverse remodeling to normalize the deformed vascular structure. MFDS approved Winrevair for use in combination with existing treatments to improve the exercise capacity of adult patients (18 years or older) with pulmonary hypertension (WHO Group I) who are in WHO Functional Class II-III. The three existing treatments are endothelin receptor antagonists (ERA), PDE-5 inhibitors (PDE-5i), and prostacyclin analogues (PCA). With the addition of this new mechanism, the Activin Signaling Inhibitor, which differs from existing treatments, has further broadened the options for pulmonary hypertension patients. The STELLAR clinical trial, which served as the basis for approval, evaluated the efficacy and safety of Winrevair in 323 adult patients with pulmonary hypertension in WHO-FC II or III. During the 24-week study period, patients received either Winrevair or a placebo in combination with their existing treatment once every three weeks. Winrevair increased the 6-minute walk distance by 40.8m compared to placebo at the 24-week mark (95% CI, 27.5-54.1; P
- Company
- Jaqbo’s substance patent extended to 2040
- by Kim, Jin-Gu Aug 08, 2025 06:03am
- Onconic Therapeutics announced on the 7th that the patent term for Jaqbo (zastaprazan), a new drug for gastroesophageal reflux disease in the P-CAB (potassium-competitive acid blocker class, has been extended by four years and two months. According to the company, the Korean Intellectual Property Office recently extended the patent term of Jaqbo’s substance patent, “Imidazo[1,2-a]pyridine derivatives, methods for preparing the same and use thereof” from July 5, 2036, to September 13, 2040, for approximately 4 years and 2 months. The KIPO recently made an official decision on the extension registration and published it in the official gazette. The patent term extension registration system is designed to compensate for the reduction in the actual patent term due to issues such as the time taken for marketing authorizations. If certain requirements are met, the patent term can be extended for up to five years, which is considered a key mechanism for protecting the intellectual property of new drug development companies and securing market competitiveness. Jaqbo Onconic Therapeutics’ new drug, which was approved in April last year as the 37th domestically developed new drug. It is a P-CAB gastric acid secretion inhibitor and is evaluated to have faster efficacy and superior nighttime gastric acid control compared to existing PPI (proton pump inhibitor) type treatments. Recently, it has been approved for use in treating not only gastroesophageal reflux disease but also gastric ulcers. Jaqbo’s sales surpassed KRW 10 billion in quarterly prescriptions less than a year after its launch in October last year. The company expects that the patent extension will accelerate sales growth in line with its strategy to expand the indications for Jaqbo and advance into global markets. An official from Onconic Therapeutics said, “With this patent extension, Jaqbo will be able to maintain its exclusive position in the domestic market until 2040,” adding, “As the rights to our independently developed new drug have been strengthened, we plan to focus our capabilities on the development of subsequent pipelines.”
- Policy
- Will RSV vaccines be included in the NIP?
- by Whang, byung-woo Aug 08, 2025 06:03am
- With the RSV (respiratory syncytial virus) vaccine being released in Korea this year, interest in its inclusion in the National Immunization Program (NIP) has been gaining attention. According to the National Assembly's legislative information system, on the 6th, Rep. Yong-ki Jeon of the Democratic Party of Korea introduced a bill titled “Partial Revision of the Infectious Disease Control and Prevention Act,” which contained provisions on including RSV in the NIP. Rep. Jeon explained, “The current law stipulates diseases subject to mandatory vaccination for the prevention of infectious diseases with high prevalence rates and frequent outbreaks. For influenza, a representative disease subject to mandatory vaccination, the prevalence rate of respiratory viruses from 2015 to 2019 was 11.7% to 21.5%.” He went on to point out that “Even though the prevalence rate of respiratory syncytial virus (RSV) and acute respiratory infections caused by RSV during the same period was 11.7% to 20.1%, indicating a risk level similar to that of influenza, mandatory vaccinations are not currently being administered for RSV.” Accordingly, the legislative intent of the bill is to contribute to improving public health through infectious disease prevention by stipulating that mandatory vaccinations be administered for acute respiratory infections as well. If the bill is passed, it is expected to accelerate the inclusion of RSV vaccines into the National Immunization Program (NIP). Currently, Sanofi's Beyfortus, an RSV preventive antibody for infants and young children, and GSK's Arexvy, an RSV vaccine for adults aged 60 and older, have been approved and released in South Korea. Currently, the KDCA maintains that the inclusion of RSV vaccines in the NIP requires sufficient discussion and review. During last year's government audit, Rep. Myeong-ok Seo (People Power Party) of the National Assembly Health and Welfare Committee questioned the KDCA about the necessity of including RSV vaccines in the NIP, and the KDCA responded that it would “conduct a thorough review in the mid- to long-term through expert and vaccination committee deliberations.” In particular, the NA’s inquiry referred to Beyfortus, the only preventive antibody approved for RSV at the time. Still, the KDCA also mentioned that Beyfortus is not classified as a vaccine. The KDCA explained, “The NIP includes vaccines, so to introduce new formulations such as preventive antibodies into the program, there must be social consensus on the NIP itself.” RSV preventive antibodies work by injecting passive antibodies into the human body to trigger an immune response. Although the goal and mechanism are similar to those of vaccines, strictly speaking, they are not traditional vaccines. Under these circumstances, it appears that the KDCA is taking a cautious approach to determining whether a drug of a different class can be incorporated into such existing programs. In this case, if the bill is passed, it is likely that Arexvy, which has been approved as a vaccine, will be approved. According to the medical community, the Korean Society of Infectious Diseases is reportedly discussing guidelines for adult RSV vaccination. However, if the proposed amendment is passed, discussions will likely take place within the broader framework of contributing to the promotion of public health through the prevention of infectious diseases. With the support of the legislation, it could also have a positive impact on securing the budget necessary for NIP discussions. However, the proposed amendment includes provisions regarding the submission of a cost estimation report, which may require the submission of economic evaluation data for the vaccine in the future.
- Company
- Rapid growth in RSV prevention market for infants
- by Whang, byung-woo Aug 08, 2025 06:02am
- After the COVID-19 pandemic, the importance of preventing infectious diseases has been re-emphasized. A new anti-RSV (Respiratory Syncytial Virus) antibody injection for infants, Beyfortus, is gaining attention as a potential turning point in the vaccine market Korea. Attention has been focused on whether a presidential pledge to support this antibody injection, which marks a global shift in RSV prevention strategy away from only high-risk groups, will lead to its inclusion in the National Immunization Program (NIP). According to the '2024 Vaccine Industry Trend Report' published by the Ministry of Food and Drug Safety (MFDS), the global vaccine market size in 2023 was approximately $34 billion, showing a steep growth from 2022 and marking the most significant increase in the last five years. Analysis suggests that the primary reason for this is attributed to the increased focus on the entire vaccine industry, as infectious disease response capabilities emerge as a national competitive asset following the COVID-19 pandemic. The RSV prevention options market, in particular, is experiencing significant growth. The two RSV prevention options recently launched in Korea are Sanofi's 'Beyfortus' and GSK's 'Arexvy.' Arexvy can be administered to adults aged 60 and over, while Beyfortus is for all newborns and infants entering their first RSV season. Children up to 24 months of age who are at high risk for severe RSV disease during their second RSV season can also receive it. The MFDS report projects that the RSV market, which began with approvals in 2023, will grow rapidly to $4.5 billion to $ 7.5 billion by 2027. However, the growth trajectory is expected to vary slightly depending on the indication, for instance, pediatric vs. adult. Due to factors such as adjusted recommended ages in the U.S., Arexvy's cumulative global sales decreased by 37% year-over-year by Q3 2024. In contrast, Beyfortus recorded cumulative sales of approximately KRW 1.2609 trillion during the same period, representing a 516% year-over-year increase. This growth is interpreted as a significant expansion in demand for infant and newborn RSV prevention options, as their immature bronchi and lungs can worsen to bronchiolitis or pneumonia, leading to hospitalization. RSV is a common respiratory virus that infects 90% of infants and young children under the age of two. It's a leading cause of hospitalization for respiratory infections in this age group during late autumn and winter, requiring special attention from families with young children. The issue is the lack of a clear prevention option for RSV infection until recently. Before Beyfortus was launched, an antibody injection called Synagis was only used for high-risk infants and young children, such as those who were premature or had underlying conditions like heart or lung disease. However, persistent criticism has been raised about the existence of a preventive blind spot, as approximately 80% of infants who visited hospitals for RSV were healthy full-term babies with no underlying conditions. Consequently, there has been a high unmet need for a preventive option for all newborns and infants. The introduction of the RSV preventive antibody injection, Beyfortus, has started to address these unmet needs. Launched in Korea in February of this year, Beyfortus is an RSV preventive antibody injection that can be administered to all newborns and infants entering their first RSV season, as well as high-risk children under 24 months. Since it provides continuous protection for at least five months with a single injection, either before or during the season, garnering significant interest from parents of infants and young children since its launch. Dr. Ki Wook Yun, a professor in the infectious diseases division of the Department of Pediatrics at Seoul National University College of Medicine, explained, "RSV is a virus that infects 90% of infants and young children under the age of two, and in some infants, symptoms can worsen to bronchiolitis or pneumonia, leading to hospitalization." This response is also confirmed in the global market. In places like Galicia, Spain, and Queensland, Australia, Beyfortus has been included in their National Immunization Programs (NIPs) and regional immunization programs, leading to a reduction in RSV-related hospitalizations. Notably, in Queensland, Australia, the importance of vaccination is being re-emphasized, as evidenced by the fact that there were no RSV hospitalizations in infants who received Beyfortus over a four-week period. In Korea, however, the Beyfortus vaccination is paid for entirely out of pocket. Although it's a preventive option for RSV, which is known to infect two out of three infants under one year old, its cost burden means only a few families can afford it. This raises concerns about health equity. However, the current administration's presidential pledge included support for an anti-RSV antibody injection for infants and young children, which has led to growing expectations for future policy changes. If this policy is implemented through measures such as including Beyfortus in the NIP, all newborns and infants in Korea will be able to receive Beyfortus without incurring the cost burden. In this regard, Yun commented, "Although only out of pocket vaccination is currently possible in Korea, I hope that support for the anti-RSV antibody injection will be expanded as part of the government's low birth rate and infant health policies."
- Company
- Immunosuppressant market rapidly expands in 3yrs in KOR
- by Kim, Jin-Gu Aug 08, 2025 06:02am
- The market for major immunosuppressants such as tacrolimus, cyclosporine, and mycophenolate has rapidly expanded over the past three years. The related market grew relatively slowly at less than 5% until 2022, but since 2023, it has expanded by more than 10% a year, showing a sharp growth trend. The tacrolimus and mycophenolate markets also showed continued growth in the first half of this year. By company, Chong Kun Dang’s performance is standing out in all three ingredients. In the cyclosporine and mycophenolate markets, Chong Kun Dang’s Cipol-N and My-Rept are dominating with over half of the market share. In the tacrolimus market, the company has been ranking second. The once-dormant tacrolimus immunosuppressant market grows rapidly from 2023 According to a report by the pharmaceutical market research institution UBIST on the 8th, the outpatient prescription sales of tacrolimus-containing immunosuppressants in the first half of this year amounted to KRW 34.9 billion. This represents a 10% increase from the KRW 31.7 billion in the first half of last year. Tacrolimus is a calcineurin inhibitor class immunosuppressant. It is primarily used to suppress rejection reactions after liver, kidney, and bone marrow transplants, as well as to treat autoimmune diseases such as rheumatoid arthritis and lupus nephritis. In the outpatient prescription market, prescriptions for the treatment of autoimmune diseases account for the majority of sales. Quarterly tacrobel prescriptions (Unit: KRW 100 million, Source: UBIST) This market had been growing steadily until 2022. The tacrolimus immunosuppressant market, which was worth KRW 45 billion in 2020, increased by 7% to KRW 48.4 billion the following year. It then increased by 6% to KRW 51.2 billion in 2022. However, growth accelerated after 2023. In 2023, prescriptions reached KRW 59.3 billion, a 16% increase from the previous year. Last year, the market grew by an additional 10% to KRW 65.2 billion. The market also grew 10% in the first half of this year, continuing the recent high growth trend. Compared to the first half of 2022, before the growth rate accelerated, the market has expanded by 44% from KRW 23.7 billion to KRW 34.9 billion in just three years. Cyclosporine market up 25% in three years... Mycophenolate jumps 67% Another calcineurin inhibitor immunosuppressant, cyclosporine, also showed a similar trend. Cyclosporine has similar indications to tacrolimus and is widely used for suppressing rejection reactions after organ transplants in the liver, kidney, heart, lung, and pancreas, as well as for autoimmune diseases such as psoriasis, rheumatoid arthritis, aplastic anemia, and nephrotic syndrome. Eye drops containing the same ingredient are used to treat conjunctivitis. However, they were excluded from this survey that researched immunosuppressants. This market also saw rapid growth after 2023. Until then, prescriptions had remained in the KRW 30 billion range, posting KRW 28,4 billion in 2020, KRW 30 billion in 2021, and KRW 29.3 billion in 2022. However, it increased by 9% year-on-year to KRW 31.8 billion in 2023, and further expanded by 13% to KRW 35.8 billion last year. In the first half of this year, prescription sales reached KRW 17.7 billion. Compared to KRW 14.2 billion in the first half of 2022, this represents a 25% increase in three years. Quarterly cyclosporine prescriptions (Unit: KRW 100 million, Source: UBIST) Mycophenolate, an anti-metabolite class immunosuppressant, also showed a sharp growth trend around 2023. The mycophenolate market showed fluctuations until 2022, posting KRW 12.7 billion in 2020, KRW 11.7 billion in 2021, and KRW 12.8 billion in 2022. However, in 2023, it increased by 20% year-on-year to KRW 15.4 billion, and last year, it increased by 19% to KRW 18.4 billion. In the first half of this year, it recorded KRW 10.1 billion in prescriptions. Compared to KRW 6 billion in the first half of 2022, it increased by 67% in three years. Quarterly mycophenolate prescriptions (Unit: KRW 100 million, Source: UBIST) Chong Kun Dang leads the immunosuppressant market... The combined prescription sales of the three products reached KRW 28.3 billion in the first half of the year By company, Chong Kun Dang is making a presence in all three immunosuppressant ingredient markets. Chong Kun Dang’s combined prescription sales of Tacrobel, Cipol-N, and My-Rept in the first half of this year reached KRW 28.3 billion, a 5% increase from the same period last year. In the tacrolimus market, Chong Kun Dang’s Tacrobel ranked second after Astellas's Prograf. Tacrobel's prescription sales for the first half of this year reached KRW 8.7 billion, a 7% increase from the KRW 8.2 billion in the same period last year. During the same period, Astellas' Prograf sales increased by 13%, from KRW 16.3 billion to KRW 18.4 billion. In the cyclosporine market, Chong Kun Dang’s Cipol-N dominates the market with over half of the market share. Cipol-N’s prescription sales for the first half of the year were KRW 12.8 billion, maintaining a similar level to last year. Its market share stands at 73%. Novartis' Sandimmune Oral recorded prescription sales of KRW 2.6 billion in the first half of the year. In the mycophenolate market, Chong Kun Dang’s My-Rept also maintained its dominant position with a 67% market share. My-Rept’s first-half prescription sales reached KRW 6.8 billion, a 12% increase from the KRW 6.1 billion recorded in the same period last year. During the same period, Roche's CellCept increased by 15% from KRW 1.6 billion to KRW 1.9 billion, while sales of Novartis' Myfortic rose from KRW 800 million to KRW 1 billion.