- LOGIN
- MemberShip
- 2025-12-22 16:20:32
- Company
- GC Cell sells its Japanese bio stock to associated companies
- by Cha, Jihyun Mar 19, 2025 06:02am
- GC Group's subsidiary, GC Cell, has transferred some shares of its Japanese cell therapy company, which it acquired seven years ago, to a holding company that specializes in gene analysis within the group. The company decided that entrusting a business to a local subsidiary with business experience in the Japanese market would be more efficient. GC Group aims to enter the Japanese market for cell therapy on a full scale.다. According to the Financial Supervisory Service on March 18, GC Cell sold 50,685 stake of GC Lymphotec for KRW 2.6 billion in Q4 of last year. Lymphotec is a company founded by Dr. Sekine, who worked as a researcher at the National Cancer Center Research Institute of Japan, in 1999. Dr. Sekine has been recognized as the leading researcher in the field of immune cell therapy, developing proprietary immune cell culture methods and publishing numerous research articles. The company primarily manufactures and sells cell therapies and culture reagents in Japan. As of last year, the company generated KRW 5.1 billion in sales and achieved KRW 600 million in operating profit. Previously, Green Cross Cell Corp acquired Lymphotec in 2018. In addition to its existing 14.5% stock of Lymphotec, Green Cross Cell Corp acquired an additional 68.8% of the stock, thereby becoming the largest shareholder. After that, Green Cross LabCell and Green Cross Cell Corp merged to form a unified entity, GC Cell, and Lymphotec was incorporated into GC Cell's subsidiary. With the recent share sale, GC Cell's stake in Lymphotec has been reduced from 83.3% to 24.4%, and the company has reclassified Lymphotec from a subsidiary to an affiliate. Under accounting principles, a firm must hold more than 50% of the shares to be considered a subsidiary. The transaction was made to Green Cross Holdings and a subsidiary specializing in gene analysis, s. The amount of stock sold by each company has not been disclosed GC Cell reasoned that divesting shares to related parties such as Green Cross Holdings and GC Genome was a strategy of choice and focus. The company believes that entrusting operations to a local holding company with business experience in the Japanese market will lead to more efficient management. GC has been targeting the Japanese market with its 'Hunterase,' a Hunter syndrome treatment designed as intraventricular use. In early 2021, the company became the first in the world to receive marketing approval from Japanese regulatory authorities for Hunterase ICV. This form replaces the conventional intravenous administration with direct intraventricular injection. In addition, GC is collaborating with Tottori University in Japan to develop an oral chaperone therapy for GM1-gangliosidosis. Centering the company's capacity around its holding company, GC Group plans to enter the Japanese cell therapy market on a full scale. Leveraging Green Cross Holdings' local network and market insights, the group will likely take a more strategic approach. Moreover, collaborations among affiliates within the group are expected to strengthen connections across R&D, clinical development, and manufacturing. This is expected to accelerate GC Genome's ongoing IPO efforts further. In November of last year, the company submitted a preliminary review application for a KOSDAQ listing to the Korea Exchange, planning to list 22.5 million shares in addition to 2.94 million shares to be offered to the public. Moreover, GC Genome secured A ratings from the designated professional evaluation agencies in October, passing the first hurdle for a technology-special listing. Using Lymphotec's established network of Japanese medical institutions and partners, GC Genome plans to introduce the AI-based multi-cancer early detection test, 'ai-CANCERCH,' to the local market. The company will also strengthen local clinical research and technology development through partnerships with major Japanese hospitals and research centers. GC Group said, "Given that Green Cross Holdings has experience conducting business in the Japanese market, holding the Lymphotec stock and managing it will enable the company to operate a local business effectively," adding, "Lymphotec will provide a connecting role for the company's business in Japan."
- Company
- A company that cares about employee well-being
- by Whang, byung-woo Mar 19, 2025 06:02am
- Medtronic, which was founded in Minneapolis, Minnesota, USA in 1949, has been expanding its influence in Korea for 37 years since it opened a liaison office in Korea in 1987 and established a corporation in 2000. Medtronic is currently focusing on four areas: cardiovascular, neuroscience, medical-surgical, and diabetes, with 500 Korean employees and executives. In particular, the company's mission statement explicitly states that it “recognizes the personal worth of all employees.” This means that the company must create an environment where its employees and the local community can grow together for the company's growth. Youngil Moon, HR Director of Medtronic Korea Medtronic conducts a biannual “Organizational Health Survey” for all Medtronic employees worldwide and then improves its organizational culture and working methods by reflecting the opinions of its employees. Thanks to these efforts, in February, the company was honorably selected as one of the “Best Companies to Work for in Korea” for the sixth consecutive year by the global consulting firm Great Place to Work Institute (GPTW). Medtronic Korea places an emphasis on an inclusive workplace where the best talent can grow together. Dailypharm met with Youngil Moon, HR Director of Medtronic Korea, to learn about the company's organizational culture and working environment. - The company's HR vision emphasizes an inclusive and friendly workplace. Is there a reason for this? =Due to our company’s characteristics, we have a wide range of stakeholders as the company carries out diverse products and conducts different businesses. For this reason, each employee needs to develop their own expertise and independence to carry out business efficiently. One might think that a professional and inclusive workplace are two different things, but a flexible organizational culture that can accommodate various goals and directions is important for improving the competitiveness of each employee's expertise and growth. -I am curious about what efforts the company is making to create an inclusive workplace. =Our company has a matrix structure, and many employees work with managers in regions or globally, so rather than following what is required from above, a culture that respects various departments and jobs has naturally been formed. There are some parts that need to be abided by the organization, but we guarantee flexibility in various areas, such as working hours and workplaces, to make the most of each employee's expertise -The company’s vision should also be reflected in your type of talent. What type of talent do you seek? =Since the company is composed of about 15 independent business units, employees are often exposed to situations where they have to communicate directly with the business units at the headquarters level and make decisions in the necessary direction. For this reason, we value the ability to become the best expert in one's work, not just in terms of technical knowledge, but also in the ability to make quick decisions in a situation. We also have a matrix organizational structure, so a culture and capacity to work organically with other teams and sometimes discuss different opinions, rather than receive instructions, is important. In this respect, it is also important to have talented people who are open and inclusive, who perform well and communicate well. - It seems important to select employees who fit the talent profile. What is the hiring process like? One of the major differences is that the organization's structure and individual job positions are planned in advance from the moment a one-year budget is set. The overall organization's job positions and hiring scale are determined by considering a series of business plans and the priorities of various businesses, rather than making decisions based on the situation as the work progresses. However, given the company's situation, large-scale hiring does not occur on a regular basis. In order to recruit good talent, the company provides information on how to apply and how to get to know the company. The global recruitment team independently selects candidates through a fair process. After that, the actual recruitment process involves interviews with three to four key stakeholders, including managers. - As a global company, language skills must also be important = The level of language proficiency required varies depending on whether the job involves working more with domestic stakeholders or working with global or other countries. There is a minimum standard for language proficiency, but since English is not the native language in Korea, there are bound to be limitations, and since language proficiency can often be developed through actual work, we recommend that you actively apply. Also, since it is a global company, you may experience unfamiliar situations due to cultural differences, etc., when working with people from other countries in addition to language differences, but the company helps you to quickly get used to these aspects. -How does the company specifically help employees to adapt? = Basically, we provide language skills training, such as English learning, in a similar way to what other global companies offer. Recently, we have been focusing on two programs to develop competencies in coping with cultural differences and work styles, not just English. First, we provide opportunities to experience work in other jobs and other countries through the 'Excel with Experience Café' program. Moving jobs is not an easy decision for employees, and opportunities do not come often, so we are running a program that allows employees to experience different projects for a short period of time. There is also a program called 'Talent Xperience' that allows employees to go to the country for a certain period of time and work with the local team. Last year, we visited Medtronic Japan to gain experience, and we also had the opportunity to invite and work with employees from Singapore and the Philippines. -So, can these programs lead to actual overseas jobs? =Already, many employees from Medtronic Korea are successfully working in various countries. In particular, unlike many companies that send employees to overseas branches or regions at the leadership level, Medtronic provides opportunities for young talent, by sending employees to overseas branches at mid-career. It is true that many global companies do not have many young employees or opportunities due to their structure of training and hiring specialists for specific jobs. However, Medtronic is open to providing opportunities for young employees, and employees in their 20s to account for about 20% of the total number of employees at Medtronic. -Your company has been selected as a 'Great Place to Work' in Korea for 6 consecutive years. What kind of employee welfare does the company pride itself on? =We have a system called '4 Weeks from Anywhere' that allows employees to work from anywhere in the world for one month. There are systems that give long-term employees long vacations for a certain period of time, but there are pros and cons, such as in cases where no one can take over the person’s job for a short period of time. This system provides an option that minimizes the burden by allowing employees to stay in another region and work during the day while spending their weekends and evenings with their families and having unique experiences. When the system was first implemented, I thought there might be some people who would like it and some who would not, but it has become a unique and helpful program that allows employees to go abroad together during their children's school breaks. -Lastly, I'd like to know about the company's plans for this year. While specific programs and one-time events are important in terms of organization, it is important to establish and implement various visions in a sustainable manner and in a form that suits individual teams and employees to ensure that they are well and palpably established. While it may sound too grand to coin it as an “organizational culture,” our plan for this year is to create and develop a good organizational culture that employees can feel through small words and actions.
- Company
- Takeda Korea appoints Kwang-gyu Park as new General Manager
- by Whang, byung-woo Mar 19, 2025 06:02am
- Kwang-kyu Park, new General Manager of Takeda Pharmaceuticals KoreaTakeda Pharmaceuticals Korea announced on the 18th that it has appointed Kwang-kyu Park as its new General Manager as of March 17, 2025. Park is a pharmaceutical industry expert with more than 20 years of experience in the industry and strong expertise and leadership in Specialty Care and Oncology (hematologic malignancies and immunotherapy). Park graduated from Kyung Hee University with a degree in Pharmacy and earned an MBA from the Graduate School of Business at Seoul National University. Since then, he has demonstrated leadership in global companies such as BMS Korea, AstraZeneca Korea, and MSD Korea, leading the introduction of new drugs, organizational innovation, and stakeholder collaboration. Most recently, he served as the Senior Director of Gilead Sciences Korea’s Liver Disease Business Unit, playing a pivotal role in improving the treatment environment for liver diseases in Korea and realizing patient-centered medical innovation. Park said, “Takeda Pharmaceuticals Korea has pursued continuous growth through patient-centered innovation amid the rapidly shifting medical environment. We will continue to accelerate the introduction of innovative treatments and seek multi-faceted solutions to ensure that both the healthcare professionals and patients can have optimal treatment opportunities.” Park added, “We will strengthen cooperation with domestic bio and pharmaceutical companies to improve the treatment environment and contribute to the continuous development of the domestic pharmaceutical industry.” Mahender Nayak, Senior Vice President of Growth and Emerging Markets at Takeda Pharmaceuticals said, “We are very pleased to welcome Mr. Park to Takeda Pharmaceuticals as we seek to advance our 6 innovative late-stage pipelines. I expect His expertise, which includes his extensive business experience and strategic leadership, will serve a pivotal role in the continued success of Takeda Pharmaceuticals Korea.”
- Policy
- Drug briefing on Enhertu's NSCLC reimb has been requested
- by Lee, Tak-Sun Mar 19, 2025 06:02am
- Product photo of Enhertu It has been reported that Daiichi Sankyo, which is aiming for Enhertu (trastuzumab deruxtecan)'s expanded reimbursement for HER2-mutant non-small cell lung cancer (NSCLC), will hold a drug briefing session with the Health Insurance Review & Assessment Service (HIRA). In April 2024, Enhertu was added to the reimbursement listing in South Korea as a treatment for HER2-positive metastatic breast cancer and gastric cancer. It has garnered attention as the drug received benefits for exceeding the incremental cost-effectiveness ratio (ICER) value, an index for economic evaluation. According to industry sources on March 17, Daiichi Sankyo recently requested a drug briefing regarding Enhertu's HER2-mutant NSCLC treatment. Enhertu, an antibody-drug conjugate (ADC) jointly developed by Daiichi Sankyo and AstraZeneca, was approved by the U.S. Food and Drug Administration (FDA) in 2019. In December 2024, Daiichi Sankyo applied for insurance reimbursement of the ADC Enhertu's two additional indications. The company had applied for Enhertu's indication to treat ▲Patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who have previously undergone systemic therapy in the metastatic setting or relapsed within 6 months of completing adjuvant chemotherapy and hormone receptor-positive (HR+) patients who have received or are unsuitable for endocrine therapy ▲Patients with unresectable or metastatic NSCLC with activated HER2 (ERBB2) mutations who have previously received systemic therapy, including platinum-based chemotherapy. Enhertu was added to the reimbursement listing as a treatment for HER2-positive metastatic breast cancer and gastric cancer in April of last year. The ceiling price of the drug was KRW 1.43 million per vial. The Ministry of Health and Welfare (MOHW) explained that after the reimbursement of Enhertu, "Patients with HER2-positive metastatic breast cancer who spent approximately KRW 83 million annually for administration cost will now only pay KRW 4.17 million with the health insurance coverage." It has been reported that the health authority offered benefits for Enhertu's inclusion in the reimbursement list, considering the ICER value. Enhertu reportedly generated sales of approximately KRW 20 billion last year. Analysis suggests that if the reimbursement is applied to NSCLC with many patients, reimbursement claim amount will increase. As a result, whether the reimbursement will be expanded will depend on pharmaceutical company's share of financial burden. However, expanded reimbursement of Enhertu is expected to provide significant therapeutic benefits for patients, given that Enhertu has demonstrated clear efficacy. Enhertu demonstrated an anti-tumor response for the second-line treatment of HER2-mutant metastatic NSCLC through the DESTINY-Lung02 study. The clinical results demonstrated that Enhertu had Blinded Independent Central Review (BICR)-assessed confirmed ORR of 49%, complete response (CR) of 1%, and partial response (PR) of 48%. Daiichi Sankyo is expected to present these efficacy data to persuade reviewers at the HIRA. The drug briefing session was introduced in 2010 to enhance transparency and objectivity in evaluations by facilitating the mutual exchange of information about new drugs between pharmaceutical companies and evaluators. These sessions are typically held within 1-2 months after a drug evaluation application is submitted. If additional data are requested, the session is scheduled once the supplementary materials are reviewed. Attendees include HIRA personnel and related deputy directors responsible for setting the standards and reimbursement evaluations for new drugs. Daiichi Sankyo previously utilized a drug briefing session during the initial listing of Enhertu, which reportedly positively impacted its reimbursement approval, leading them to apply for another session. "Drug briefing sessions provide accurate drug information and, through two-way communication, allow for sharing any supplemental details, which can help expedite the reimbursement review process," said an industry employee.
- Company
- Ilaris can be prescribed in 7 hospitals in Korea
- by Eo, Yun-Ho Mar 19, 2025 06:01am
- The ultra-rare disease treatment 'Ilaris' may now be prescribed in more hospitals in Korea. According to industry sources, Novartis Korea's hereditary recurrent fever syndrome drug Ilaris (canakinumab) has passed the drug committees (DCs) of tertiary hospitals such as Seoul National University Hospital, Seoul St. Mary's Hospital, and Sinchon Severance Hospital as well as regional medical institutions such as Pusan National University Yangsan Hospital, Jeju National University Hospital, and Pyeongchon (Hallym Univ. Sacred Heart Hospital. The drug’s landing procedures are also underway at Chung-Ang University Hospital. Ilaris, which was approved in Korea in 2015, has been on the reimbursement list since August last year. The company quickly accepted the conditional reimbursement decision made by the MFDS’s Drug Reimbursement Evaluation Committee in April and then promptly concluded the difficult drug pricing negotiation process. However, due to the small number of patients who were eligible to use the drug, there were not many hospitals that could prescribe it. The number of patients who could be prescribed Ilaris is extremely small. At the time of Ilaris's approval, the number of patients in Korea was estimated to be around 10. In fact, some of Ilaris' indications do not even have disease codes or have only recently been registered. In this situation, it is encouraging news that the number of medical institutions that can prescribe the reimbursed drug has increased to seven. Ilaris is an interleukin-1 (IL-1) inhibitor recommended for treating CAPS in the 2021 international guidelines of the European Congress of Rheumatology and the American College of Rheumatology. In a Phase III study, the drug demonstrated significant clinical benefit in remission after a single dose and remission rate at 6 months and can be administered every 8 weeks in patients with CAPS, improving the quality of life for patients and their caregivers. In the CLUSTER study, which included 46 patients with TRAPS and 63 patients with colchicine-resistant FMF, 45% (n=10/22) of TRAPS patients treated with Ilaris 150 mg and 61% (n=19/31) of colchicine-resistant FMF patients achieved a complete response at week 16. Ilrais is indicated for the following diseases in Korea: ▲Periodic fever syndromes (PFS), cryopyrin-associated periodic syndromes (CAPS), tumor necrosis factor receptor-associated periodic syndrome (TRAPS), hyperimmunoglobulin D syndrome (HIDS)/mevalonate kinase deficiency (MKD), and familial Mediterranean fever (FMF) ▲Active systemic juvenile idiopathic arthritis (Systemic JIA). For CAPS, the indication can be further categorized into the following symptoms: ▲Familial cold autoinflammatory syndrome (FCAS)/ familial cold urticaria (FCU) ▲Muckle-Wells syndrome (MWS) ▲Neonatal onset multisystem inflammatory disease (NOMID)/chronic infantile neurological, cutaneous and articular syndrome (CINCA).
- Company
- Vadanem for renal anemia makes its 'third attempt at reimb'
- by Nho, Byung Chul Mar 18, 2025 05:57am
- Mitsubishi Tanabe Pharma Attention has been drawn to the oral renal anemia drug Vadanem as the company completed meeting procedural requirements and attempts again to obtain reimbursement listing. According to industry sources, Mitsubishi Tanabe Pharma recently submitted Vadanem's drug pricing application to the Health Insurance Review and Assessment Service (HIRA). After obtaining approval from the Ministry of Food and Drug Safety (MFDS) in March 2023, Vadanem is making a third attempt at insurance reimbursement. After Vadanem obtained FDA approval in March 2024, it was listed in Germany for its demonstrated safety and efficacy. In January, it also secured a prescription recommendation from the U.K health authority As a result, Vadanem secured all medical health technology evidence required by the Korean health authority's listing criteria. Therefore, it is anticipated to be accepted. Mitsubishi Tanabe Pharma suggests a weighted average price of the substitute as about KRW 1 million to 1.2 million. Because the suggested price is about KRW 300,000 to 500,000 lower than the conventional EPO injectables, it has been evaluated to contribute significantly to saving the National Health Insurance finance. The efficacy of the drug was demonstrated through comparative effects (non-inferior) to a control drug. The side effect of the drug was reported to be similar to conventional drugs. Thus, it is being regarded as a new treatment choice. Anemia is caused by decreased kidney function and EPO production capacity, followed by lowered hematopoiesis. Renal anemia is accompanied by oxygen depletion due to decreased red blood cells, and it is commonly accompanied by in tiredness, decreased appetite, insomnia, and depression. It reduces the quality of life and impacts the mortality rate of patients. There are over 700 million patients with chronic renal kidney disease worldwide, and 1 out of 7 patients experience anemic symptoms. According to the National Health Insurance Service (NHIS) data, the number of patients with chronic kidney disease in Korea increased 36.9%, from 206,061 patients in 2017 to 282,169 patients in 2021. The increase was particularly steep in patients in their 80s, up 82.8%. Dialysis patients are also on the rise exponentially. Listing of competitive and new drugs may be justified as the National Health Insurance finance spent on approximately 100,000 patients amounts to KRW 3 trillion. Considering that these drugs are priced 30% less than injectable formulations and have improved convenience of administration, with equivalent effectiveness, it would be a significant loss in national finance. Meanwhile, the EPO formulation, developed 30 years ago, is the only available treatment for renal anemia. Third generation injectables with extended intervals of administration have been launched recently. However, many patients do not respond to these treatments, experience changes in blood pressure, and have adverse reactions, such as nausea and vomiting. Thefore, there is a need for treatments with new mechanism. Vadanem is a hypoxia-inducible factor (HIF) prolyl hydroxylase (PH) enzyme (HIF-PH) inhibitor. It works by improving hemoglobin levels by activating 'erythropoietin (EPO)' and reducing 'hepcidin,' a hormone responsible for iron metabolism. The domestic market for renal anemia drugs has been dominated by erythropoiesis-stimulating agents (ESAs). Hypoxia-inducible factor prolyl hydroxylase enzyme (HIF-PH) inhibitors are demonstrating potential after AstraZeneca·JW Pharmaceuticals·Mitsubishi Tanabe Pharma obtained approval from the MFDS for Evrenzo Tab (roxadustat)·Enaroy Tab (Enarodustat)·Vadanem Tab (vadadustat) in 2021·2022·2023, respectively. During this process, AstraZeneca withdrew from the launching of Evrenzo Tab in South Korea due to substantially lower drug pricing than the prime cost. According to a document on pharmaceutical distribution performance, external sales of renal anemia drugs amount to KRW 100 billion. The overseas market for all types of HIF-PHI prescriptions is worth KRW 10 trillion.
- Company
- Alteogen signs 2 licenses out agreements with AZ subsidiary
- by Cha, Jihyun Mar 18, 2025 05:56am
- Alteogen has signed two licensing-out agreements with AstraZeneca’s subsidiaries. According to the Financial Supervisory Service on the 17th, Alteogen has signed 2 exclusive license agreements with MedImmune, a subsidiary of AstraZeneca's bio R&D, for its subcutaneous (SC) formulation modification platform 'ALT-B4' based on recombinant human hyaluronidase enzyme. The company signed agreements with the UK subsidiary of MedImmune and the US subsidiary of MedImmune, respectively. The non-refundable upfront payment with the UK entity was KRW 36.4 billion. The technology fee (milestone payments) for development and commercialization is KRW 1.547 trillion, with separate sales royalties upon successful commercialization. Alteogen will receive sales royalties equivalent to a certain percentage of the net sales generated after the first commercial sale of products using ALT-B4. The upfront payment for the contract signed with the US corporation is KRW 29.1 billion. The milestone payment is 843.8 billion won, with separate sales royalties. The total upfront payment for the two technology export contracts signed by Alteogen is KRW 65.5 billion. The total milestone payment for the 2 contracts is KRW 1.8985 trillion. Alteogen's ALT-B4 technology can change an intravenous (IV) formulation to a subcutaneous (SC) formulation by hydrolyzing hyaluronic acid under the skin. Unlike the IV formulation that patients have to receive for 4-5 hours in the hospital, the SC formulation allows patients to self-inject the treatments at home in 5 minutes. Since 2019, Alteogen has signed a series of technology transfer agreements with global pharmaceutical companies including Merck (MSD) in the United States, Intas Pharmaceuticals in India, and Sandoz in Switzerland. Alteogen has licensed out its ALT-B4 technology to the global pharmaceutical company GPC for USD 1.373 billion in 2019, MSD for USD 3.865 billion in 2020, Intas for USD 109 million in 2021, and Sandoz for USD 145 million in 2022. Among these, the contract with MSD was extended by 4 months in February last year, increasing the contract amount by USD 432 million to USD 4.317 billion. The contract with Sandoz, signed in 2022, was replaced by a contract to develop an SC formulation biosimilar through the joint development of a new hyaluronidase in July. The specific terms and conditions of the contract and development strategy were not disclosed.
- Opinion
- [Reporter’s View] Being a global clinical trial powerhouse
- by Kim, Jin-Gu Mar 18, 2025 05:56am
- Korea is a global clinical trial powerhouse. According to the Korea National Enterprise For Clinical Trials, Korea's share of global clinical trials was 4.04% as of 2023. It is in 4th place after the United States (22.02%), China (13.59%), and Spain (4.09%). By city, Seoul has been in first place since 2017. Korea’s outlook is also positive. Korea’s global clinical trial market share rank rose one step each year, from 6th in 2021 to 5th in 2022 and 4th in 2023. The share gap with Spain is only 0.05%, and if the current trend continues, Korea is expected to rise to become one of the Top 3 clinical trial countries in the world. However, there are some drawbacks. Although Korea has established itself as a clear clinical trial powerhouse in terms of quantity, there are indications that it is not so in terms of quality. The most straightforward example is the clinical trials related to antibody-drug conjugates (ADCs), which have recently emerged as a new trend in drug development. According to the Korea National Enterprise For Clinical Trials, as of May last year, more than 600 ADC-related clinical trials were registered on ClinicalTrials.gov worldwide, but there is not a single ADC clinical trial registered or being conducted in Korea. This cannot be simply attributed to Korea’s small population. This is because 27 ADC clinical trials are underway in Taiwan, which has a population of about half that of South Korea. It is time to improve the quality of Korea’s clinical trials. In particular, improving regulations related to clinical trials may be a way to improve the quality of clinical trials. From this perspective, it is encouraging that the first step has recently been taken in the development of “decentralized clinical trials (DCT)”. DCTs refer to de-hospitalized clinical trials that collect clinical data using wearable or mobile devices and deliver test drugs by mail, etc. Patients can participate in clinical trials without having to visit a medical institution in person. A representative success story of decentralized clinical trials is Moderna, which developed the COVID-19 vaccine. The industry has consistently called for the introduction of decentralized clinical trials. In response, the government has decided to launch a pilot project for decentralized clinical trials starting this year. The government plans to conduct 6 decentralized clinical trials during the pilot project period until 2027, and then institutionalize the system based on these trials. Korea’s high-quality infrastructure is considered the main reason why Korea has grown into a global clinical trial powerhouse. This includes excellent medical staff, hospital facilities, and the high level of education of clinical trial participants. This has enabled Korea to become a clinical trial powerhouse in terms of quantity. However, there is still much that needs to be done in terms of quality. Now is the time to improve the quality of clinical trials based on excellent infrastructure. Regulatory improvement is the surest way to improve the quality of clinical trials. This can foster an environment in which clinical trials of advanced drugs such as ADC can be conducted in Korea. Only when innovative new drugs can be developed in Korea will Korea be able to go beyond being a clinical trial powerhouse and become a pharmaceutical powerhouse.
- Company
- Can the myelofibrosis drug Ojjara pass CDDC review in KOR?
- by Eo, Yun-Ho Mar 18, 2025 05:56am
- Industry attention is rising on whether GSK’s new drug for myelofibrosis, Ojjara, will be able to make progress in its reimbursement journey and be listed in Korea. According to industry sources, GSK's myelofibrosis treatment Ojjara (momelotinib) will be presented for review to the Health Insurance Review and Assessment Service's Cancer Disease Deliberation Committee tomorrow (19th). Specifically, the indication being reviewed is its use as a ‘treatment for adults with anemia at intermediate or high risk of myelofibrosis.’ The drug is currently indicated for primary myelofibrosis, post-polycythemia vera myelofibrosis, or post-essential thrombocythemia myelofibrosis. GSK recently launched Ojjara as a non-reimbursed drug. It remains to be seen whether the application will lead to the introduction of a covered treatment option that will significantly improve anemia, which has remained an unmet need in the treatment of myelofibrosis in Korea. Ojjara has a unique triple-inhibition mechanism that blocks JAK1, JAK2, and ACVR1 (Activin A Receptor Type 1). In the treatment of myelofibrosis, inhibition of JAK1 and JAK2 can contribute to the improvement of systemic symptoms and reduction of splenomegaly in patients, while inhibition of ACVR1 can help alleviate anemia by inducing a reduction in hepcidin expression. Managing anemia is one of the unmet needs in the treatment of existing patients with myelofibrosis. Anemia, which increases the need for blood transfusions, causes more than just dizziness, and depending on the severity, it can lead to a serious condition that can be life-threatening. The Phase III SIMPLIFY-1 and MOMENTUM studies have shown that, regardless of prior treatment with JAK inhibitors, Ojjara can significantly improve the main symptoms of splenomegaly and transfusion dependence in the treatment of patients with myelofibrosis with anemia. In the SIMPLIFY-1 study, which confirmed the clinical efficacy and safety of Ojjara compared to that of JAK inhibitors in the first-line treatment of patients with myelofibrosis who had no prior experience with JAK inhibitors, Ojjara demonstrated non-inferiority to JAK inhibitors in the primary endpoint of spleen volume response at Week 24 of treatment. The proportion of transfusion independence in each patient group was 66.5% for the Ojjara arm and 49.3% for the ruxolitinib arm, indicating that Ojjara showed significantly less transfusion dependency. “While JAK inhibitors, which were used in the treatment of myelofibrosis, showed effects in alleviating splenomegaly and systemic symptoms, they worsened anemia or increased the need for blood transfusions, which left an unmet need,” said Seo-Yeon Ahn, Professor of Hematology & Oncology at Chonnam National University Hwasun Hospital, “Ojjara has confirmed its significant clinical value in managing anemia, which is closely related to the prognosis of patients with myelofibrosis, and we expect that its launch in Korea will contribute to improving the treatment outcomes and quality of life of more patients.”
- Company
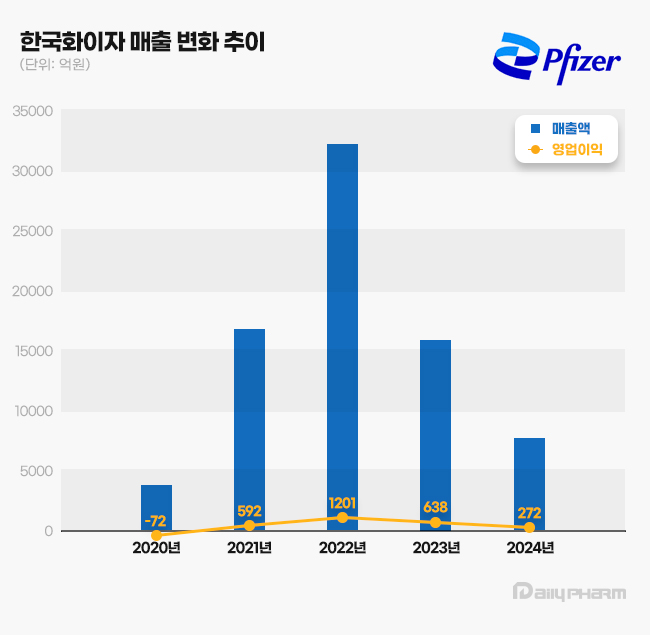
- Pfizer distributed KRW 160 billion dividend over 2 years
- by Chon, Seung-Hyun Mar 18, 2025 05:56am
- Pfizer Korea has decided on a large-scale dividend payout for the second consecutive year. While the company maintained an annual dividend policy of KRW 12.48 million, it has distributed KRW 160 billion to its parent company over the past two years. Large-scale profits during the COVID-19 pandemic enabled this high dividend. According to the Financial Supervisory Service (FSS) on the March 15, Pfizer Korea’s total dividend payout last year amounted to KRW 60.01 billion. It included an interim dividend of KRW 60 billion and an additional annual dividend of KRW 12.48 million. Pfizer Korea’s 2024 interim dividend was applied a 6,501% face value dividend rate, meaning shareholders received over 60 times the face value of KRW 5,000 per share. The interim dividend was distributed to 172,104 common shares, totaling KRW 55.9 billion, and 12,480 preferred shares, amounting to KRW 4.1 billion. Pfizer Korea made the second consecutive year of large-scale dividend payments after 2023. In 2023, the company distributed an interim dividend of KRW 100 billion and an annual dividend of KRW 12.48 million. The 2023 interim dividend was applied at a 10,835% face value dividend rate. Pfizer Korea The largest shareholder of Pfizer Korea is Pfizer’s subsidiary in the Netherlands, 'PF OFG South Korea 1 B.V.,' which holds a 99.99% stake in the company. Pfizer Korea significantly increased its dividend payouts as its sales surged during the COVID-19 pandemic. In 2020, Pfizer Korea recorded KRW 391.9 billion in sales. The sales more than quadrupled to KRW 1.694 trillion in 2021 and further skyrocketed to KRW 3.2254 trillion in 2022, an eightfold increase in sales over two years. Although its 2023 sales fell to KRW 1.6018 trillion, roughly half of the previous year's, it still was a fourfold increase compared to 2020. In 2020, Pfizer Korea reported an operating loss of KRW 7.2 billion and a net loss of KRW 21.2 billion. However, in 2022, its operating profit and net profit surged to KRW 120.1 billion and KRW 119.5 billion, respectively. In 2023, the company reported KRW 59.2 billion in operating profit and KRW 84.9 billion in net profit. Pfizer began its messenger RNA (mRNA) vaccine development in March 2020 in collaboration with BioNTech, using BioNTech's mRNA technology and Pfizer's extensive experience in global clinical trials. Within less than a year of COVID-19 spread, Pfizer successfully developed Comirnaty, a vaccine with 95% preventive effect. The U.S. Food and Drug Administration (FDA) granted it Emergency Use Authorization (EUA) in December 2020, and it received EUA approval in Korea in March 2021, leading to its full-scale supply through Pfizer's Korean subsidiary. Pfizer later developed the COVID-19 antiviral treatment Paxlovid. The Korean government used Paxlovid as a COVID-19 treatment. Pfizer Korea continued implementing a unique dividend policy, consistently paying KRW 12.48 million in annual dividends. The company calculates its dividend payout based on a 20% dividend rate applied to preferred share capital, which totals KRW 62.4 million. Pfizer Korea's total capital stands at KRW 922.92 million, with KRW 860.52 million in common shares (172,104 shares) and KRW 62.4 million in preferred shares (12,480 shares). Since 2005, Pfizer Korea has maintained a consistent dividend payout of KRW 12.48 million annually, based on a 20% preferred share dividend rate, except for four instances in the past 20 years. However, in 2017, the dividend payout was set at KRW 79.794 billion, exceeding net profit. At that time, the dividend rate was set at 660% of the face value (KRW 5,000 per share) for both common (2,455,520 shares) and preferred shares (12,480 shares), resulting in a significantly expanded payout. In 2008, the company allocated KRW 190 billion in dividends despite posting a KRW 600 million deficit that year, setting a 3045% dividend rate based on the face value. However, whether Pfizer Korea's high dividends will continue remains uncertain. In 2023, revenue fell 51.1% year-over-year to KRW 783.7 billion. Operating profit declined 57.4%, from KRW 63.8 billion to KRW 27.2 billion. The company's 2023 revenue and operating profit decreased 75.7% and 77.4%, respectively, compared to 2022, mainly due to the impact of entering endemic and declining COVID-19-related sales.