- LOGIN
- MemberShip
- 2025-12-20 05:04:39
- Policy
- IV formulation of Korea’s first IL-23 drug Tremfya approved
- by Lee, Hye-Kyung Aug 25, 2025 06:05am
- The intravenous injection formulation of Tremfya, the first interleukin-23 (IL-23) inhibitor in Korea, was recently granted approval, paving the way for the supply of all Tremfya injection formulations in Korea. On the 21st, the Ministry of Food and Drug Safety approved Janssen Korea's Tremfya Intravenous Injection (guselkumab, recombinant DNA). In addition to receiving approval for ‘Tremfya Prefilled Syringe’ in 2018 and ‘Tremfya OnePress Autoinjector’ in 2021, the company has now received approval for the intravenous injection formulation and may now introduce the formulation to Korea. The indications for Tremfya vary depending on the formulation. The pre-filled syringe formulation is indicated for plaque psoriasis, palmoplantar pustulosis, psoriatic arthritis, ulcerative colitis, and Crohn's disease. The intravenous injection recently approved is used for the induction therapy of ulcerative colitis and Crohn's disease. The 200mg intravenous injection is administered intravenously over a minimum of 1 hour on weeks 0, 4, and 8, followed by maintenance therapy using Tremfya Prefilled Syringe Injection or Tremfya OnePress Autoinjector. Therefore, the intravenous injection is indicated only for the treatment of moderate-to-severe active ulcerative colitis or Crohn's disease in adults who have not responded adequately, have lost response, or have intolerance to conventional therapies, including biological agents or small-molecule drugs. Meanwhile, Tremfya is the first and only interleukin-23 inhibitor approved in Korea for psoriatic arthritis, and its reimbursement criteria are being continuously expanded. Psoriatic arthritis is a chronic progressive immune disease characterized by joint inflammation, enthesitis (inflammation at the sites where bones, tendons, and ligaments meet), dactylitis (severe inflammation of the fingers and toes), and pain in the hands and feet, commonly occurring in individuals aged 30–50 years. To date, there is no cure for psoriatic arthritis, and despite available treatment options, many patients experience symptoms that impair their ability to perform daily activities. It is estimated that approximately 9% of psoriasis patients in South Korea develop psoriatic arthritis.
- Company
- Lilly seeks Mounjaro’s reimbursement for diabetes in Korea
- by Eo, Yun-Ho Aug 22, 2025 06:08am
- ‘Mounjaro,’ which is causing a roar in the field of obesity treatment, is seeking inclusion in insurance reimbursement for its diabetes indication. According to Dailpharm coverage, Eli Lilly Korea has submitted a reimbursement application for its Mounjaro (tirzepatide), a dual GIP/GLP-1 receptor agonist, and is proceeding with the necessary procedures to present the application for review to the Drug Reimbursement Evaluation Committee of the Health Insurance Review and Assessment Service. Therefore, it remains to be seen whether Mounjaro, a drug for obesity and diabetes, will be listed for reimbursement in Korea. Mounjaro is a single-molecule designed to selectively bind to and activate the GIP receptor and GLP-1 receptor with a once-weekly injection. It helps lower blood sugar by promoting insulin secretion, improving insulin sensitivity, reducing glucagon levels, delaying stomach emptying, and reducing food intake, thereby aiding weight loss. Although its obesity indication is well-known, Mounjaro has achieved significant results in the field of diabetes as well. Beyond just achieving the blood sugar control targets, 6 out of 10 patients achieved normal blood sugar levels without an increased risk of hypoglycemia, reaching the ultimate treatment goals of preventing cardiovascular complications and reducing mortality. It has even demonstrated the potential for “remission” in diabetes. In diabetes, Mounjaro is approved as an adjunct to diet and exercise therapy for improving glycemic control in adults with type 2 diabetes (as monotherapy or as part of a two-drug regimen). The approval of its type 2 diabetes indication approval was based on a randomized, double-blind, placebo-controlled study comparing Mounjaro (5 mg, 10 mg, or 20 mg) with placebo, semaglutide (1 mg), insulin degludec (100 U/mL), and insulin glargine (100 U/mL) in five SURPASS Phase III clinical trials (total of 6,278 participants). Mounjaro significantly improved HbA1c compared to the control group and baseline in all studies. Meanwhile, the top-line results from the Phase III SURPASS-CVOT study, which demonstrated the cardiovascular benefits of Mounjaro, have recently been disclosed. In the study, Mounjaro demonstrated non-inferiority compared to Trulicity in the incidence of 3-point major cardiovascular events (MACE-3), including cardiovascular disease death, myocardial infarction, and stroke, thereby achieving the primary endpoint of the clinical trial. Additionally, it showed improvements in key outcomes, including HbA1c, weight, kidney function, and all-cause mortality.
- Policy
- Blockbuster drugs, Atozet·Rosuzet, get price cuts
- by Lee, Tak-Sun Aug 22, 2025 06:07am
- It has been reported that prices for blockbuster drugs, including the hyperlipidemia combination therapies Atozet (Organon) and Rosuzet (Hanmi Pharmaceutical), are expected to be reduced due to increased usage. These hyperlipidemia combination therapies are frequently subject to annual volume-based drug price negotiations (PVA). Additionally, Celltrion's antibody biosimilar Remsima and Dong-A ST's growth hormone product Growtropin-II Inj are also reported to be on the list for price cuts. On August 21, industry sources reported that reimbursement caps for several products would be adjusted as of September 1, under the 'Type-Da' Price-Volume Agreement. The number of blockbuster products is reported to be included this time. The reimbursement cap for Organon's hyperlipidemia combination therapy, Atozet Tab (atorvastatin-ezetimibe), is expected to be reduced by 3.4% following negotiations. The prices of the 10/10mg, 10/20 mg, 10/40 mg, and 10/80 mg products will be adjusted as follows: 10/10 mg from KRW 951 to KRW 918, 10/20mg from KRW 1,209 to KRW 1,168, 10/40mg from KRW 1,299 to KRW 1,255, and 10/80mg from KRW 1,387 to KRW 1,340. Atozet's outpatient prescription sales last year, based on UBIST data, were KRW 118.7 billion, a 16.3% increase from KRW 102.1 billion in the previous year. The increase in prescription sales alone exceeded KRW 16 billion. This year's negotiation targets for Type-Da Price-Volume Agreement include products whose 2024 claim amount increased by more than 60% compared to 2023, or products that increased by more than 10% with the increase exceeding KRW 5 billion. Based on UBIST data, Atozet meets these criteria. Hanmi Pharmaceutical's hyperlipidemia combination therapy, Rosuzet Tab (rosuvastatin-ezetimibe), is also reported to have undergone a price cut. Rosuzet Tab's reimbursement cap is expected to be reduced by 1.3% to 2.1% depending on the dosage. The prices for the 10/10mg dose are expected to be reduced from KRW 1,103 to KRW 1,087, the 10/20mg dose from KRW 1,111 to KRW 1,093, the 10/5mg dose from KRW 789 to KRW 779, and the 10/2.5mg dose from KRW 727 to KRW 712. Rosuzet recorded outpatient prescription sales of KRW 210.2 billion last year, a 17.6% increase from KRW 178.8 billion in the previous year. HK inno.N's Rovazet and Yuhan Corp.'s Rosuvamibe, which contain the same ingredients as Rosuzet, will also have their reimbursement caps lowered in this negotiation. Rovazet recorded outpatient prescription sales of KRW 47.4 billion last year (up 23%), and Rosuvamibe recorded KRW 89.1 billion (up 14.6%) based on UBIST data. Celltrion's antibody biosimilar , Remsima, used for the treatment of rheumatoid arthritis and other conditions, will also have its price adjusted due to increased usage. Remsima is a biosimilar of Remicade, a blockbuster product with global sales of over KRW 1 trillion. Its reimbursement cap is scheduled to be lowered by 1.2% through an agreement with the NHIS. Dong-A ST's growth hormone product, Growtropin-II Inj, is also on the rise.Growtropin-II Inj's reimbursement cap was adjusted last year due to the Price-Volume Agreement as well. Additionally, Janssen's ADHD treatment Concerta OROS ER Tab is expected to see a 3.9% reduction in its reimbursement cap. Meanwhile, this year's Price-Volume Agreement price reduction list reportedly does not include any of the choline alfoscerate products for cognitive enhancement. These products, which included six items last year, had been consistently on the PVA list. However, after the decision was made to apply selective reimbursement to choline alfoscerate products in a reimbursement re-evaluation, pharmaceutical companies shifted their business to alternative products, which seems to have halted their growth.
- Policy
- ALS drug Qalsody is approved in Korea with a condition
- by Lee, Hye-Kyung Aug 22, 2025 06:07am
- The ALS treatment ‘Qalsody (tofersen)’ has been approved under the condition that the results of its therapeutic confirmatory clinical trial be submitted later. According to the advisory council’s review results regarding the safety and efficacy of Qalsody, which was released by the Ministry of Food and Drug Safety on the 20th, the council saw consensus on the need to grant conditional approval for Qalsody, given how ALS worsens over time and treatment options are limited. Biogen's Qalsody is a nucleic acid therapy that binds to SOD1 mRNA in patients with ALS caused by mutations in the superoxide dismutase 1 (SOD1) gene, reducing the synthesis of mutated proteins (SOD1). It received domestic approval from the MFDS on the 20th of this month. According to the CPAC meeting results, experts noted that ALS is a highly severe disease that can be fatal without treatment, and that there are no specific medications available for the condition at the present. In particular, Qalsody’s indication is limited to ALS patients with SOD1 gene mutations. While the mechanism of action involves binding to mRNA, entering the nucleus, and inhibiting SOD1 expression, there were opinions that it is difficult to directly evaluate or reflect the actual SOD1 secreted externally. A member of the CPAC stated, “Based on the submitted data, the drug seems to show an effect when NfL correction is applied. In neurodegenerative diseases, biomarkers can sufficiently explain the extent of neural damage, and as there is scientific evidence on their effect, conditional approval was deemed appropriate.” Another member explained, “This disease is a rare and severe condition with no available treatments in the country, and existing approved drugs are primarily used for symptom relief. Given the clinical trial results based on the NfL biomarker and its potential to control the disease, we deemed that the benefits are significant and agree to grant a conditional approval.” However, there was also an opinion that the completion date of the conditional Phase III clinical trial should be considered, as patients with SOD1 mutations account for less than 3% of all ALS patients, which may require a longer patient recruitment period. Experts also suggested that conditional approval is necessary to provide treatment opportunities for domestic patients, as the drug is already in use overseas. In other words, the council saw that the safety and efficacy of the drug are deemed acceptable based on the data submitted by the pharmaceutical company, and conditional approval is needed to allow patients to benefit from the treatment as soon as possible. Meanwhile, Qalsody was approved in Korea through the accelerated approval process, the Global Innovative products Fast-Track (GIFT), as the 31st product.
- Opinion
- [Reporter's View] Double standard against natural medicines
- by Kim, Jin-Gu Aug 22, 2025 06:06am
- The government announced the '4th New Natural Product Drug Development Promotion Plan' in May 2024. The plan outlines a joint effort by seven ministries, including the Ministry of Health and Welfare, the Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Environment, Ministry of Oceans and Fisheries, Rural Development Administration, and Korea Forest Service, to build a foundation for natural new drug R&D and promote its industrialization. To achieve this, the plan selected three strategies and six key tasks, and pledged support for customized consulting to enhance clinical success rates, as well as assistance for global expansion. The budget allocated for this was KRW 153 billion, a 2.7% increase from the previous year. However, in the reimbursement sector, the exact opposite policy is underway. A prime example is the ongoing discussion to delist the mugwort extract treatment for gastritis from reimbursement coverage. The government selected the mugwort extract as a target for the 2025 drug reimbursement appropriateness re-evaluation last year. In discussions held this year, the conclusion was that it 'lacks appropriateness for reimbursement.' While an objection procedure remains, the prevailing view, considering past precedents, is that a dramatic reversal of this decision is unlikely. On the one hand, the government is expanding the budget to industrially promote natural medicines, while on the other, it is reducing patient access due to a lack of clinical utility. This contradiction of simultaneously fostering industrial growth and removing a flagship product is seen as a decision that weakens the government's policy consistency. The problem of inconsistent policy is also evident in the differing results from two re-evaluations. The mugwort extract had already been recognized for its usefulness in a reimbursement re-evaluation conducted by health authorities 14 years ago. The Ministry of Health and Welfare reviewed the cost-effectiveness of five therapeutic categories, including cardiovascular and digestive ulcer drugs, as part of the 'Reorganization of the Listed Drugs List' in 2011. At that time, the usefulness of Stiren's 'gastritis treatment' indication was recognized. Conversely, its 'gastritis prevention' indication was questioned, and after a legal dispute over a delay in submitting clinical data, the conclusion was its removal from reimbursement. No issues were raised at the time regarding the usefulness of its gastritis treatment. This amounts to an opposite judgment being made on the same drug. This leaves questions about the consistency of policy not only in clinical settings but also from the perspective of pharmaceutical and biotech companies. A bigger problem is that this double standard has increased overall industry uncertainty and stifled research momentum. In the mid-2000s, major pharmaceutical and biotech companies maintained one or two natural medicine development pipelines; however, this is no longer happening. After Yungjin Pharmaceutical received approval for its atopic dermatitis treatment 'Yutoma' in 2012, no new natural medicines were produced in Korea for 13 years. Yutoma was never even launched due to cost issues, and its approval was canceled in 2022 for failing to submit re-evaluation data. In the same year, Chong Kun Dang received approval for its natural medicine 'G-Tec,' but it has not been launched due to delays in reimbursement listing. A field that was once highlighted as a national strategic project has been halted due to institutional contradictions. The controversy surrounding the mugwort extract extends beyond the survival of a few products and is directly linked to the trajectory of the Korean pharmaceutical and biotechnology industries. While past achievements cannot be uncritically praised, it would be a loss to dismiss the experience and assets accumulated in that process as an anachronistic product. Strengthening regulations to align with global standards is inevitable, but what is needed now is to correct this double standard. The contradiction of simultaneously promoting industrial growth and discussing delisting must be resolved. A balanced policy design that encompasses support for clinical research and commercialization, as well as quality standardization, should follow. Only then can natural medicines re-establish themselves as a competitive asset for the Korean pharmaceutical and bio-industry.
- Company
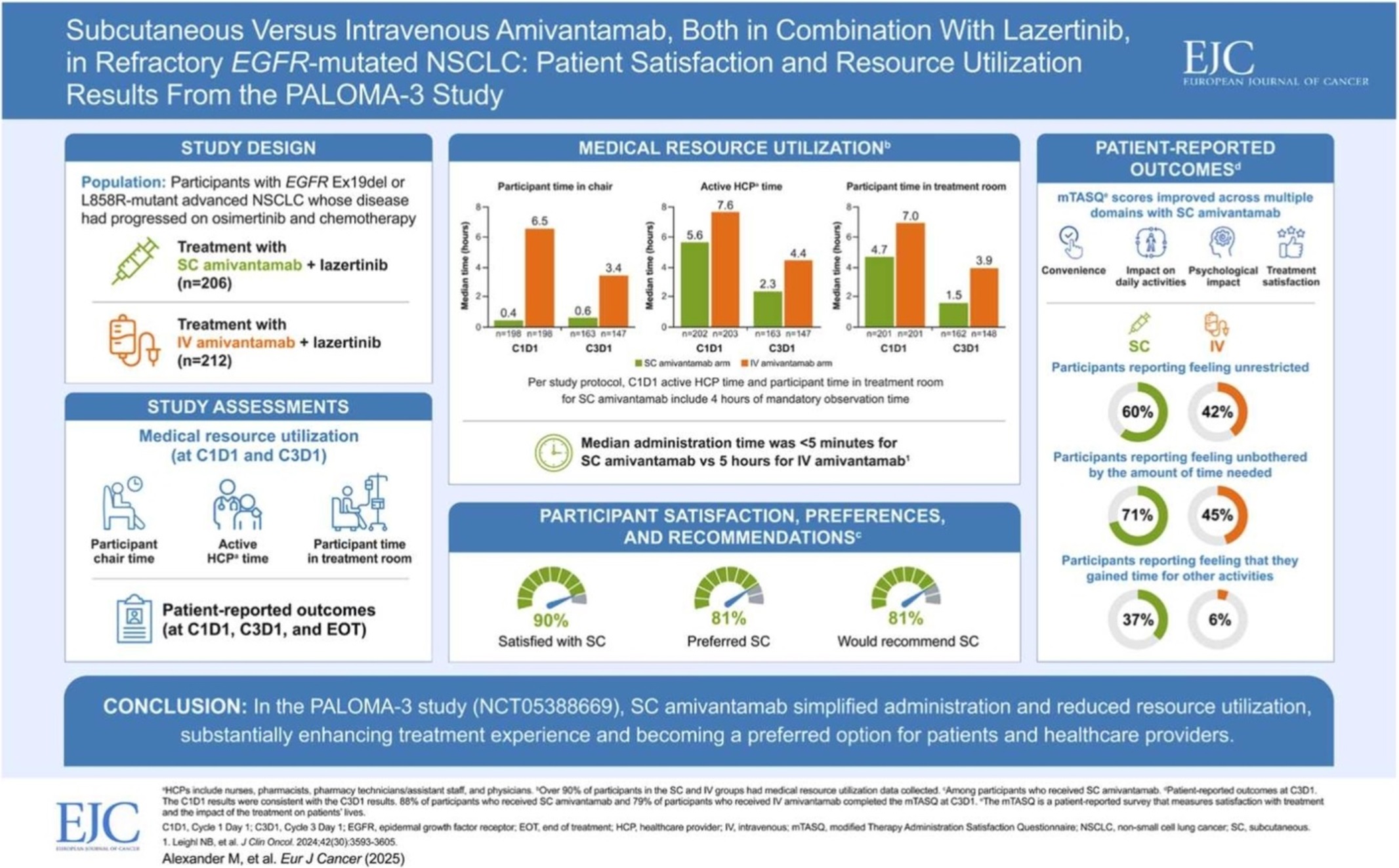
- Leclaza at global crossroads 1 year into FDA approval
- by Moon, sung-ho Aug 22, 2025 06:06am
- One year has passed since Leclaza (lazertinib) received FDA approval in combination with Johnson & Johnson’s Rybrevant (amivantamab). Expanding its influence beyond the United States to Europe and Asia, it has emerged as a global treatment option both in Korea and abroad. In the first half of this year, it recorded an overall survival rate (OS) exceeding 50 months, emerging as a global standard of care for non-small cell lung cancer and contributing to a major shift in the treatment paradigm. # With the FDA’s decision on whether to approve Rybrevant SC set to be determined in the second half of this year, another 'step-up' is also anticipated for Leclaza, depending on the result. According to industry sources on the 18th, the FDA approved the use of Rybrevant in combination with Leclaza (US brand name: Lazcluze) as a first-line treatment for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with EGFR exon 19 deletion or exon 21 L858R substitution mutation, or adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). The combination was also approved in Europe in December of the same year, followed by the UK (March), Japan (March), and Canada (March) in the first quarter of this year, and China (July) in the second quarter. In May, prescriptions began in Japan, indicating the combination is gaining momentum in its expansion into the global market. Among the countries that have approved the combination, the combination’s performance in China is particularly noteworthy. With approximately 1 million new lung cancer diagnoses each year, lung cancer is the No.1 cancer in terms of incidence and mortality in China. This accounts for more than one-third of the 2.5 million new lung cancer cases worldwide. In addition, approximately 85% of lung cancer patients in China have non-small cell lung cancer (EGFR accounts for 40% of non-small cell lung cancer). Furthermore, the OS results of the Phase III (MARIPOSA) study presented at the European Lung Cancer Congress (ELCC 2025) held in Paris, France, in March this year have further accelerated the growth momentum. According to the Phase III MARIPOSA study, the combination therapy arm that received Rybrevant and Leclaza saw a 25% reduction in risk of death compared to the Tagrisso (osimertinib, AstraZeneca) arm (HR=0.75, 95% CI: 0.61–0.92, P
- Product
- Mounjaro arrives at pharmacies..₩310,000–350,000
- by Kang, Hye-Kyung Aug 21, 2025 06:07am
- Mounjaro (tirzepatide), which had been attracting considerable attention even before its arrival in Korea, has now been made available to pharmacies. With prescriptions also beginning on the 20th, pharmacies are bustling with activity. According to local pharmacies, two dosage forms, 2.5mg and 5mg, have begun arriving at pharmacies through wholesalers. Mounjaro Prefilled Pen 2.5mg, 5mg stocked at pharmacies A local pharmacist stated, “The 2.5mg and 5mg doses arrived this morning. Although no prescriptions have been made yet, we are receiving calls asking if the product has arrived. It seems that consumer interest is quite high.” The two main concerns for pharmacies are pricing and medication guidance. Mounjaro is priced in the ₩300,000 range, with the price lowest in Jongno at ₩290,000 ◆Prices vary between pharmacies, with the lowest price at KRW 290,000 = Since Mounjaro is a non-reimbursed drug, the price varies even between pharmacies. For this reason, pharmacies are struggling to set the initial price. According to telemedicine platforms, prices for 2.5mg in-hospital prescriptions at clinics range from KRW 320,000 to KRW 350,000 Pharmacies are pricing the medication between KRW 310,000 to KRW 350,000. While the number of pharmacies stocking Mounjaro is still limited, prices seem to be forming in the early to mid three hundred thousand won range. However, in Jongno, the price is set at KRW 290,000, which is the lowest price. ◆Medication guidance for Mounjaro?= Mounjaro comes in a package containing four single-use pre-filled pens. The outer case states, “Mounjaro is used once a week. Read the instructions carefully before using the medication and keep the instructions with the medication.” Mounjaro was approved as ▲an adjunct to diet and exercise therapy for improving blood glucose control in adult patients with type 2 diabetes; ▲an adjunct to low-calorie diet and exercise therapy for chronic weight management in adult patients; and ▲as an adjunct to a low-calorie diet and exercise therapy for the treatment of moderate-to-severe obstructive sleep apnea (OSA) in obese adult patients with an initial body mass index (BMI) of 30 kg/m2 or higher. When administered for weight management ▲Obese patients with an initial body mass index (BMI) of 30 kg/m2 or higher ▲Overweight patients with an initial body mass index (BMI) of 27 kg/m² or higher but less than 30 kg/m² and one or more weight-related comorbidities such as hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease, may use the treatment. The recommended starting dose is 2.5 mg subcutaneous injection once weekly, increased to 5 mg subcutaneous injection once weekly after 4 weeks, and maintained at that dose. The maximum dose is 15 mg subcutaneous injection once weekly. If a dose is missed, administer the missed dose as soon as possible within 4 days (96 hours) of the missed dose. If more than 4 days have passed, skip the missed dose and administer the next dose on the scheduled day. Mounjaro can be administered at any time of the day, regardless of meals, and should be administered as a subcutaneous injection in the abdomen, thigh, or upper arm. It is recommended to rotate (alternate) the injection site with each dose. The recommended that the injections are refrigerated at 2–8°C. If necessary, each single-use pen can be stored at a temperature not exceeding 30°C for up to 21 days without refrigeration. Do not freeze. If frozen, the medication should not be used and should be stored in its original container to protect from light. The most commonly reported adverse reactions include gastrointestinal disorders such as nausea (very common), diarrhea (very common), and vomiting (common). Generally, the severity of these reactions was mild or moderate, and occurred more frequently during dose escalation, and decreased over time. Meanwhile, Lilly Korea announced that it plans to first release 2.5 mg and 5 mg doses in Korea, but will also supply higher doses of 7.5 mg, 10 mg, 12.5 mg, and 15 mg in accordance with patient demand. Meanwhile, the Ministry of Food and Drug Safety sent a notice to medical organizations and other relevant parties to prevent misuse of the drug following the release of Mounjaro. The MFDS emphasized, “Please prescribe and dispense the drug appropriately in accordance with the approved efficacy, dosage, and precautions for use. To prevent side effects and misuse, please provide accurate information about the drug (efficacy, dosage, and precautions for use) to the patients and instruct them on how to take the drug.”
- Company
- Nubeqa gains flexibility with indication expansion
- by Whang, byung-woo Aug 21, 2025 06:06am
- The influence of Nubeqa (darolutamide) is rising in the market with the company expanding its indication as a treatment for metastatic hormone-sensitive prostate cancer (mHSPC). Experts believe that the drug may settle as a flexible treatment option in Korea’s market as it has broadened its path as a personalized treatment. With the approval, overcoming the reimbursement hurdles is expected to serve as the key to competition in the future. Hyun-ho Han, professor of Urology at Severance HospitalBayer Korea held a media seminar on the 20th to celebrate the indication expansion for Nubeqa, an oral androgen receptor inhibitor (ARi), and highlighted its clinical significance. In June, Nubeqa was approved by the Ministry of Food and Drug Safety as part of a two-drug regimen in combination with androgen deprivation therapy (ADT) for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC). In South Korea, Nubeqa was previously approved for use in combination with ADT and docetaxel for the treatment of mHSPC patients, as well as in combination with ADT for the treatment of high-risk non-metastatic castration-resistant prostate cancer (nmCRPC). With the expanded indication, Nubeqa can now be used not only as a three-drug regimen in combination with ADT and the chemotherapy agent docetaxel but also as a two-drug regimen in combination with ADT. The approval was based on the results of the global Phase III clinical trial ARANOTE, which evaluated the efficacy and safety of the two-drug regimen of Nubeqa in combination with ADT in 669 patients with mHSPC. The study results showed that the Nubeqa combination group significantly reduced the risk of radiographic progression or death by 46% compared to the placebo group, and this improvement in radiographic progression-free survival (rPFS) was consistently observed across all groups, including high-risk and low-risk mHSPC patients. Also, in the secondary endpoint, overall survival (OS), the Nubeqa combination therapy group demonstrated potential survival benefits compared to the placebo group, showing significant delays in disease progression in terms of PSA level, deterioration in quality of life, and pain progression, thereby proving clinically meaningful improvements in quality of life. The most significant aspect of this expanded indication is that Nubeqa is now the only treatment approved for both three-drug regimens with docetaxel and two-drug regimens with ADT in patients with metastatic hormone-sensitive prostate cancer. The introduction of this new option is expected to offer a more flexible treatment approach tailored to the individual condition and treatment goals of mHSPC, including providing treatment options for elderly patients or those who are not suitable for chemotherapy. Hyun-ho Han, professor of Urology at Severance Hospital, who presented at the media seminar, said, “Most mHSPC patients in Korea are elderly, aged 60 or older, and often have comorbidities. It is necessary to devise personalized treatment strategies based on clinical evidence, taking into account the patient's condition and preferences.” Professor Han added, “With the approval of the two-drug Nubeqa + ADT regimen, it is now possible to tailor treatment according to patient characteristics depending on whether docetaxel is used. Patients who require aggressive initial treatment can consider the three-drug combination therapy of Nubeqa + ADT + docetaxel, while patients with CNS concerns, such as the elderly or those with chronic diseases, or patients who are not suitable for docetaxel treatment, can consider the two-drug combination therapy of Nubeqa + ADT." Currently, Nubeqa is approved in over 85 countries worldwide as a treatment for mHSPC and nmCRPC, and as of 2024, it has surpassed annual sales of approximately KRW 2.4 trillion, establishing itself as a blockbuster drug. However, it is still non-reimbursed in Korea, resulting in high costs and limited access for patients. Currently, treatments such as Xtandi, Zytiga, and Erleada are reimbursed as treatments for mHSPC, so reimbursement is essential for competition with these products.. On this, Professor Han emphasized that Korea needs more treatment options when considering the criteria set for reimbursement of the existing drugs in prostate cancer. He said, “There are cases when patients with metastatic prostate cancer or non-metastatic castration-resistant prostate cancer are not eligible for reimbursement coverage. We want to offer cost-effective choices to patients, so any additional covered option that are introduced are welcome on our part.” Considering that existing treatments can only be used in combination with ADT, Professor Han believes that the three-drug regimen of Nubeqa + ADT + docetaxel may offer additional advantages. In addition, MyungKyu Noh, Oncology BU lead at Bayer Korea, said, “Bayer Korea is going through the reimbursement process so that domestic prostate cancer patients can promptly receive our treatment and its reimbursement as soon as possible. We will actively communicate with various stakeholders, including patients, medical staff, and the government, to improve access to treatment for all three indications.
- Company
- Reimb of polycythemia vera drug 'BESREMi' likely in Sept
- by Eo, Yun-Ho Aug 21, 2025 06:06am
- The polycythemia vera treatment 'BESREMi' is expected to be listed on the national health insurance list. The National Health Insurance Service (NHIS) and PharmaEssentia Korea have recently reached a final agreement on the drug price negotiation for BESREMi (ropeginterferon alfa-2b). As a result, if it passes the Health Insurance Policy Review Committee, it can be officially listed in September. BESREMi's company had previously proceeded with the reimbursement process for hydroxyurea-refractory or intolerant polycythemia vera in March 2023. It failed to pass the Health Insurance Review & Assessment Service (HIRA)'s Cancer Disease Review Committee in July of the same year. At that time, the Cancer Disease Review Committeedetermined that there was insufficient evidence to judge the clinical utility of BESREMi as a second-line treatment. PharmaEssentia subsequently supplemented the evidence for this drug's efficacy as a second-line therapy by adding domestic clinical data. It resubmitted the reimbursement application in March of last year, passed the Cancer Disease Review Committee in July of the same year, passed the Drug Reimbursement Evaluation Committee in May, and has now completed the negotiation stage. BESREMii is a next-generation interferon that selectively eliminates the JAK2 mutant gene, which is the cause of polycythemia vera. It was developed to improve the purity and tolerability of existing interferons, allowing for once-every-two-week administration for the initial 1.5 years and once every four weeks thereafter. BESREMi is currently recommended in the National Comprehensive Cancer Network (NCCN) and European LeukemiaNet (ELN) guidelines for the treatment of polycythemia vera, regardless of prior treatment history. Meanwhile, polycythemia vera is a rare blood cancer in which a somatic mutation in the bone marrow abnormally activates bone marrow function, leading to excessive production of red blood cells. According to HIRA data, the number of prevalent patients in Korea is around 5,000, and hydroxyurea is primarily used for more than half of these patients. However, the currently reimbursed drugs are not a fundamental treatment. For patients who fail hydroxyurea treatment, there is no new alternative, making it a disease with high unmet needs.
- Company
- Viatris signs exclusive sales and distribution deal for Brid
- by Whang, byung-woo Aug 21, 2025 06:05am
- Pic of Bridion Viatris Korea announced on the 20th that it has signed an exclusive domestic promotion and distribution agreement for the general anesthesia reversal agent Bridion (Sugammadex) through a strategic partnership with MSD Korea. Under the agreement, Viatris Korea officially took over the domestic promotion and distribution of Bridion as of the 7th of this month Bridion is a general anesthesia reversal agent developed by MSD, which was approved by the Ministry of Food and Drug Safety in October 2012 and launched in Korea in February 2013. Over the past decade, Bridion has established itself as a treatment option that enables rapid and predictable muscle relaxation recovery in clinical settings. Through this strategic partnership, Viatris Korea plans to expand its product portfolio into anesthesia in Korea, enhance patients' access to treatment, and strengthen its market leadership by leveraging its robust sales and marketing capabilities in the general hospital sector. Bill Schuster, Country Manager of Viatris Korea, said, “We are very pleased to add Bridion, MSD's general anesthesia reversal agent that has made many innovative marks in the anesthetic field, to our portfolio. This agreement will further solidify the partnership between the two companies and enable us to better provide treatment access to patients in need of Bridion amid rapidly changing market conditions.” He added, “As a company that empowers people to live healthier lives at every stage of life, Viatris Korea will continue to strive to provide healthcare professionals and patients with more effective and safer treatment options.” Bridion is a rapid and predictable neuromuscular blocking agent reversal drug that selectively reverses the effects of rocuronium or vecuronium, neuromuscular blocking agents used during anesthesia. It completely reverses neuromuscular blockade within an average of three minutes, enhancing the safety and efficiency of anesthesia recovery. Albert Kim, Managing Director of MSD Korea, said, “We expect that surgeons and patients in Korea will be able to continue to benefit from Bridion through our exclusive agreement with Viatris Korea, a company with strong expertise in the field of chronic diseases. Based on our trusted partnership, both companies will do their utmost to ensure a stable supply of Bridion in Korea.”