- LOGIN
- MemberShip
- 2025-12-22 07:30:15
- Company
- Six reimbursement revaluations fail in 4 years
- by Chon, Seung-Hyun Nov 15, 2024 05:49am
- Six items have been removed from the reimbursement list after reimbursement reevaluations during the past 4 years. Silymarin bilberry fruit dried powder, followed by itopride, failed the reimbursement reevaluations. Streptokinase - streptodornase, oxiracetam, and acetylcarnitine were also removed due to reimbursement reevaluation failures. Pharmaceutical companies are facing annual losses of up to KRW 240 billion as a result of the 6 drugs' reimbursement reevaluation failures. According to the Ministry of Health and Welfare on the 14th, 55 itopride-based drugs have been removed from the reimbursement list as of this month. The move follows the government's reimbursement revaluation. The health authorities selected 7 ingredients, including thioctic acid, pranlukast, itopride, sarpogrelate, levodropropizine, mosapride, and formoterol, to be reevaluated this year. As a result, the authorities concluded that itopride lacked clinical efficacy and was removed from the benefit. Three components - thioctic acid, pranlukast, and mosapride - were recognized as clinically useful and will remain on the reimbursement list. In the case of sarpogrelate and levodropropizine, the two will be removed from the reimbursement list but will remain covered if pharmaceutical companies voluntarily reduce their prices. The decision of formoterol was deferred pending clinical reevaluation by the MFDS, with the condition that the NHIS will recollect a portion of their reimbursement costs if the drugs fail to prove clinical efficacy. This means that only itopride fully failed the reimbursement reevaluations, and all products containing itopride will be removed from the reimbursement list. Itopride products from JW Life Science, JW Pharmaceutical, Kuhnil Biopharm, KyungDong Pharm, Kwangdong Pharmaceutical, Kukje Pharm, Nexpharm Korea, Novem Healthcare, NEWGEN Pharma, Daewon Biotech, Daewon Pharmaceutical, Dongsung Pharm, Dongwha Pharmaceutical, Mother’s Pharmaceutical, Medix Pharm, Bukwang Pharmaceutical, Samsung Pharm, Celltrion Pharm, Sinil Pahramcetucial, Shinpoong Pharmaceutical, iCure Pharmacueticals, Ahngook New Pharm, Ahngook Pharmaceutical, Alvogen Korea, SPC, Neo Bio Korea Pharm, Youngil Pharm, Young Poong Pharmaceutical, Yuyu Pharma, Yuhan Corp, Eden Pharma, Reyon Pharmaceutical, Intro Biopharma, Il-Yang Pharmaceutical, Ilwha, Jeil Pharmaceutical, Chong Kun Dang, Jinyang Pharmaceutical, Cosmax Pharma, PharmaKing, Poonglim Pharmatech, Hana Pharm, BMI Korea, Abbott Korea, Union Korea Pharm, Korus Korea, Korea Pharma, Pharmbio Korea, PMG Korea, Hutecs Korea Pharmaceutical, Han Wha Pharma, Whan In Pharm, Huons Meditech, Huons Life Sciences, will be withdrawn from the reimbursement market. Itopride is used to treat digestive symptoms caused by functional dyspepsia. According to the drug research institution UBIST, it generated KRW 23.6 billion in outpatient prescriptions last year. Itopride's prescription volume has decreased 18.7% in 5 years from KRW 29.1 billion in 2019 but has maintained prescription sales of over KRW 20 billion annually. From the pharmaceutical companies’ perspective, the reimbursement cut for itopride means a loss of more than KRW 20 billion a year. During the various reimbursement reevaluations that were conducted since 2021, a total of 6 ingredients were removed from the reimbursement list. In 2021, the health authorities announced a plan to reevaluate the reimbursement adequacy of 5 ingredients, including ▲grape seed extract Vitis vinifera (grape seed and grape leaf extract), ▲avocado soya, ▲ginkgo biloba dried extract, ▲bilberry dry extract, and ▲ silymarin. Among them, silymarin and bilberry dry extract were concluded to be inadequate for reimbursement and were removed from the health insurance reimbursement list. For both silymarin and bilberry dry extract, the prescription market has not been eliminated as some products that have filed administrative lawsuits have retained their reimbursed status. However, the prescription market has shrunk significantly due to the mass breakaway of products that accepted the reimbursement deletion. Silymarin's prescription market size grew 44.6% over 3 years, from KRW 23.6 billion in 2019 to KRW 34.1 billion in 2022, showing a significant increase in demand in the prescription market. Silymarin is an OTC drug used for toxic liver disease, hepatocyte protection, chronic hepatitis, and cirrhosis. However, after failing reimbursement reevaluations, sales fell to KRW 26.7 billion in 2022, down 21.5% from the previous year, and then to KRW 25.7 billion last year, down 24.8% from 2 years ago. Last year, the prescription amount of bilberry dry extract was KRW 13.3 billion, down 30.3% from the previous year. Bilberry dry extract is a drug used to improve retinal degeneration and vascular disorders of the eye caused by diabetes. The prescription market for bilberry dry extract was worth KRW 31 billion in 2021, but after the government deleted its reimbursement status, sales dropped 38.5% to KRW 19.1 billion in 2022 and shrank further last year. Prescriptions for bilberry dry extract last year shrank by 57.1% compared to two years ago. Streptokinase - streptodornase (Strepto preparations), oxiracetam, and acetyl L-carnitine, which were eligible for reimbursement but failed to pass the MFDS’s clinical reassessment, have seen their prescription market decline to the point of extinction. Last year, the prescription market for strepto preparations was KRW 16.1 billion, down 41.0% from the previous year. In 2022, the MFDS determined the strepto preparations lacked clinical efficacy during reimbursement reevaluations. However, given the ongoing clinical reassessment, conditional reimbursement was offered to defer the reimbursement reevaluation results only for items that agreed to refund the reimbursed costs based on the outcome of the clinical reevaluation. Strepto preparations are used for the “relief of acute inflammatory edema caused by ankle surgery or trauma to the ankle” and “difficulty in bile drainage accompanying respiratory diseases.” In 2017, the MFDS ordered a clinical reevaluation of strepto preparations after controversy arose over their efficacy. However, the clinical reevaluation failed to prove their efficacy, and the drug was excluded from the health insurance reimbursement list in December last year, the the indications were also deleted. Oxiracetam and acetyl L-carnitine were selected for reimbursement reevaluations last year but failed clinical reevaluations and were unable to proceed to reimbursement reevaluations. In January, the prescription and dispensing of oxiracetam was discontinued after the clinical reevaluation failed to verify the drug’s efficacy. Oxiracetam was approved for the treatment of cognitive impairment caused by Alzheimer's disease, multiple sclerosis, and temperamental brain syndrome due to brain dysfunction. Oxiracetam generated KRW 23.2 billion in prescription sales in 2022, but its clinical reevaluation failure wiped out the prescription market. Acetyl-L-carnitine was subject to reimbursement reevaluations in 2023 but was not evaluated due to its failure to pass clinical reevaluations. Acetyl-L-carnitine is licensed for use in “primary degenerative diseases” or “degenerative diseases secondary to cerebrovascular diseases.” In 2013, the MFDS ordered a clinical reevaluation of acetyl-L-carnitine preparations. However, in 2022, the indications were removed due to the drug’s failure to demonstrate efficacy in both indications. Acetyl-L-carnitine had a prescription market worth KRW 77.9 billion in 2019, but its removal from both the approval and reimbursement list led to losses for pharmaceutical companies. The 6 ingredients that were removed from the reimbursement list among those subject to reimbursement reevaluations over the past 4 years since 2021 had a combined prescription volume of KRW 242.3 billion in 2019. Pharmaceutical companies have realized an annual loss of KRW 242.4 billion in prescription sales due to the removal of the 6 ingredients.
- Company
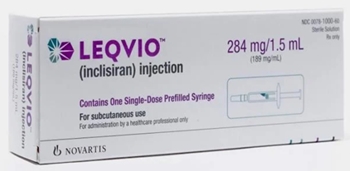
- Leqvio with 'twice-yearly treatment' set for mkt
- by Whang, byung-woo Nov 15, 2024 05:49am
- Leqvio is set to challenge the market with its superior drug tolerance, administered twice yearly, compared to existing treatments. Product photo of Leqvio.According to the pharmaceutical industry on November 15, Novartis Korea launched the siRNA therapy Leqvio (inclisiran) on November 11. Lecvio is the first-in-class siRNA drug approved in South Korea. It is approved as an adjunct to dietary therapy for patients with primary hypercholesterolemia (heterozygous familial and non-familial) and mixed dyslipidemia. Leqvio uses naturally occurring siRNA to block PCSK9 protein production and lower LDL cholesterol levels. It has the advantage of its twice-yearly administration by doctors, reducing the challenges associated with self-injection. Novartis is reportedly working to establish prescription access for Leqvio in general hospitals. Industry sources said that Drug Committee (DC) approvals for Leqvio are proceeding across hospitals nationwide, with some hospitals already completing the process. Novartis' Leqvio was launched in South Korea after the product became available. Novartis is expected to start building its prescription sales as the DC reviews advance within general hospitals. With Leqvio prescriptions anticipated, there is growing interest in how Novartis’ domestic sales strategy will unfold. Although specific details have not yet been announced, it is anticipated that Novartis will consider either utilizing its own sales line or engaging in co-promotion with a Korean company. Leqvio's competing drug, Amgen’s Repatha, is co-marketed with Jeil Pharmaceutical. However, given Leqvio’s potential focus in general hospitals, Novartis may utilize its internal sales team. Will Leqvio lead the prescription trend? It will depend securing reimbursement Experts anticipate that Leqvio will have a market presence despite cost hurdles, as cholesterol management trends evolve to prioritize patient convenience along with clinical effectiveness. For hypercholesterolemia patients, maintaining LDL-cholesterol (LDL-C) at recommended levels consistently and early on is crucial in reducing the risk of atherosclerotic cardiovascular disease (ASCVD). Long-term strategies are anticipated to shift toward minimizing the frequency of medication doses for patients, with an increased focus on injection therapies that allow healthcare providers to monitor patient progress directly. "While statins require daily use and Repatha requires 26 injections annually, Leqvio is administered in twice-yearly injections by a doctor," Dr. Byeong-Keuk Kim, a cardiologist at Sinchon Severance Hospital, said. "Doctors face difficulty when patients who don’t respond well to statins. Now, we could have Leqvio as a treatment option." "Leqvio’s six-month dosing schedule could improve drug adherence, potentially resulting in a sustained decrease in LDL-C levels and lower cardiovascular risk in clinical practice," Dr. Kyung Woo Park, a cardiology professor at Seoul National University Hospital, said. However, with the competing PCSK9 inhibitor Repatha already reimbursed, Novartis' primary aim would be on obtaining reimbursement for Leqvio. Repatha’s reimbursed cost is approximately KRW 121,000 per injection, with recommended dosing every two weeks or once monthly at 420 mg (three dosages), bringing annual treatment costs to KRW 1.45 million. Novartis is working on Leqvio's reimbursement listing. The company aims to continue negotiations with the government to establish reimbursement for Leqvio at about KRW 3 million, aligning with Repatha’s annual price. "While Leqvio may be chosen by elderly patients facing adherence challenges or those with poor prognosis, many others already experience significant improvements by combining existing therapies with regular exercise. For some, even a once-monthly Repatha injection successfully maintains desired levels. In my opinion, balancing high costs against the administration convenience needs further discussion," a cardiology professor at a major hospital in Gyeonggi-do said.
- Company
- Hanmi's new bio drug 'Rolvedon' sales 88%↑ in the US mkt
- by Son, Hyung Min Nov 15, 2024 05:49am
- The U.S. sales of Rolvedon (Korean product name: Rolontis) have substantially increased. Hanmi Pharmaceutical's U.S. partnering company, Assertio, plans to expand market share by securing Rolvedon's indication for the same-day administration. According to Assertio on Novermber 14, Rolveron's sales in the U.S. market in Q3 were US$ 15 million (about KRW 21 billion), up 87.5% Year-over-Year (YoY). Rolvedon has recorded cumulative sales of US$110.30 million (about KRW 155 billion) since its launch in Q4 of 2022. Rolvedon is a treatment for neutropenia developed by Hanmi Pharmaceutical. It is Korea's 33rd new drug, approved in March 2021. After that, Hanmi Pharmaceutical and its U.S. partnering company Spectrum (now Assertio) obtained U.S. Food and Drug Administration (FDA) approval in September of the same year. Rolvedon was outlicensed to the U.S. Spectrum in 2012. After Assertio acquired Spectrum in April last year, it secured the licensing of sales and development of Rolvedon and lung cancer therapy Poziotinib. Rolvedon Since its release in the United States in October 2022, Rolvedon has recorded sales of $10 million within three months. After the product launch, 70 distributors purchased Rolvedon. It was then utilized by the top three community oncology networks, accounting for 22% of the clinic market share. It continued to show strong sales up to Q2 last year. Rolvedon generated sales of $15.6 million in Q1 last year, and in Q2, it recorded $21 million (approx. KRW 28 billion), an increase of 34.6% from the previous quarter. Rolvedon’s sales in Q3 last year slowed down since its launch. It recorded $8 million in Q3 last year, a decrease of 62% from the previous quarter. Regarding sales reduction, Assertio explained that the demand for Rolvedon after applying the reimbursement system was below expectations. Rolvedon successfully rebounded in Q4 last year, generating US$11 million in sales. Rolvedon's sales in Q1 amounted to US$14.50 million and maintained recovery in Q2, recording sales of US$15.10 million. Assertio has high hopes for Rolvedon's same-day administration clinical trial. Currently, Neulasta, jointly developed by Amgen and Kyowa Kirin, is recording over half of the market share in the U.S. market for neutropenia treatment. However, conventional treatments for neutropenia, such as Neulasta, can only be administered 24 hours after cancer therapy, thereby prolonging hospitalization. Assertio plans to gain a competitive edge with its strategy of Rolvedon's same-day administration method. Assertio has recently completed the Phase 1 clinical trial for Rolvedon's same-day administration. The company plans to present the clinical results at the 2024 San Antonio Breast Cancer Symposium (SABCS 2024), held for four days, starting December 10.
- Company
- Lee Hyun-ju named as the representative of ZP Therapeutics
- by Eo, Yun-Ho Nov 14, 2024 05:52am
- Lee Hyun-ju, new representative of ZP Therapeutics Korea Lee Hyun-ju (48), ex-Daiichi Sankyo Korea's Oncology Business Franchise Head, was appointed as the new representative of ZP Therapeutics Korea. Industry sources said Zuellig Pharma has recruited Lee Hyun-ju as the new head of ZP Therapeutics Korea, Zuellig Pharma's commercial services corporation. Lee holds a degree from Sungkyunkwan University's College of Pharmacy, and she has years of experience in the Korean pharmaceutical market with expertise in the anticancer business. Lee started her career in 1999, undertook roles in Handok's marketing and Sanofi's marketing, and worked as Roche Korea's Oncology Cluster Lead and Novartis Korea's Hematology Cluster Lead. Lee moved to Daiichi Sankyo's Oncology Cluster last year and served her role until the new appointment. Meanwhile, ZP Therapeutics Korea is committed to providing a comprehensive solution, encompassing marketing, sales promotion, product launch medical e-detailing, registration & approval, market access, digitalization & data analysis-based sales excellence, adapting needs and changes of the pharmaceutical market. ZP Therapeutics Korea established itself as a commercial solution partner sought by pharmaceutical clients. The company is involved in in-licensing of many prescription drugs and over-the-counter (OTC) drug brands. ZP Therapeutics Korea also provides sales & marketing services, supporting major pharmaceutical companies.
- Company
- Celltrion anticipates Trump admin will bring positive shift
- by Whang, byung-woo Nov 14, 2024 05:51am
- Celltrion anticipates that President-elect Donald Trump's second administration in the United States will positively affect the company's biosimilars and Contract Development and Management Operations (CDMO) services. On November 12, Celltrion presented stockholders with the potential impact on the business under the title 'The Business Impact and Outlook upon President-elect Donald Trump's second administration in the United States launches.' Celltrion cited a report from the Korea Institute for Industrial Economics and Trade, 'The potential impacts of the U.S. presidential election on Korean industry and outlook,' and analyzed that the Trump administration will be friendly towards using generics and biosimilars. Currently, healthcare expenditures in the United States accounted for 17.6% of the country's GDP in 2023. As a solution, Trump's first administration implemented initiatives such as the 'Lowering Drug Prices by Putting America First' administrative orders and the 'American Patients First' plan to lower drug prices. These policies include details regarding biosimilars, including 'Improve Competition' and 'Lowering List Prices.' "When President-elect Trump's second administration launches, the administration is expected to take over the policy during the first administration and pursue healthcare policy," Celltrion said. "We expect the administration will favor expanding biosimilar prescriptions, which is Celltrion's main business." Trump's first administration pursued a policy of regulating drug pricing, proposing a bill to Congress to stop pharmaceutical companies from paying rebates to the top 3 pharmacy benefit managers (PBMs) in the United States. Such a move was said to be friendly to the biosimilar market. Additionally, as the administration pursues the PVM reform to alleviate financial loss, a by-product of economic stimulation, by reducing taxes, implementing a policy that expands the use of biosimilars is expected. Such a move is expected to be favorable to Celltrion. In particular, Celltrion anticipated these policies would positively impact its CDMO service, which will be Celltrion's growth momentum. The 'Biosecure Act' bill, the U.S. Congress initiative, 'prohibits entities that receive federal funds from using biotechnology from a biotechnology company of concern may not contract with any entities that do so.' The Biosecure Act is expected to support establishing a new supply network in US-friendly countries with business competitiveness, including South Korea, Japan, and India. "In line with the U.S. industry trend, we will complete establishing the CDMO corporate entity within this year to seize the opportunity to secure demands from Chinese companies," Celltrion added. "Celltrion will secure a new manufacturing plant, as Celltrion's 100% owned subsidiary, in South Korea or overseas to expand production capacity." Additionally, as Trump's second administration's policy priority is the 'America First' agenda, Celltrion anticipates US dollar strength will likely result in trade disputes and interest rate rises. During the process, Celltrion anticipates its products will remain unaffected by tariff increases, as tariffs on drugs are exempt under the WTO's Pharma Agreement. "Upon Trump's second administration launches, we expect the company will have an opportunity to focus mainly on business aspects, marketing expansion and sales growth, compared to other type of business," Celltrion said. "Celltrion will focus on changes to the U.S. biopharmaceutical industry and generate outcomes by maximizing business opportunity," Celltrion emphasized.
- Company
- ‘Access to bispecific antibody Columvi should be improved’
- by Son, Hyung Min Nov 14, 2024 05:51am
- Dr. Chris Fox, Professor of Haematology at the University of Nottingham, U.K. “Diffuse large B-cell lymphoma (DLBCL) is a disease in which one in four patients experience relapse even after treatment. The bispecific antibody Columvi has demonstrated efficacy in relapsed patients at up to 18 months of follow-up. The clinical performance of Columvi is not just an incremental improvement over existing therapies, but a paradigm shift in the DLBCL treatment environment.” At a recent meeting with Dailypharm, Dr. Chris Fox, Professor of Haematology at the University of Nottingham, U.K., recently described so about Columbo, a bispecific antibody approved for diffuse large B-cell lymphoma in Korea. DLBCL is a disease in which the body's protective “B cells” grow or multiply uncontrollably and is the most common form of non-Hodgkin's lymphoma that accounts for about 40% of all non-Hodgkin's lymphomas. The disease is characterized by aggressive, rapidly progressive staging. The number of DLBCL patients in Korea was 14,183 as of last year, a 36% increase from the 10,428 in 2018. Up to 15% of DLBCL patients fail first-line standard therapy, and 25% of patients experience relapse within 18 months despite achieving a complete response (CR). Patients with relapsed or refractory DLBCL show a characteristically rapidly worsening prognosis as the number of treatment cycles increases. Columvi, the first bispecific antibody targeting CD20XCD3 enters the market...offers the advantage of a fixed dosing period The good news is that a variety of new drugs have emerged for this disease. Roche's Polivy, a representative DLBCL drug, is said to be effective in about two-thirds of patients when used as a first-line treatment. However, this means that about one-third of patients who do not respond to first-line treatment remain in need of further options. Bispecific antibodies and chimeric antigen receptor T-cell (CAR-T) therapy, such as Columvi, are used in such cases of relapse. Columvi, the first bispecific antibody to target CD20xCD3 in DLBCL, was launched without reimbursement in Korea in May and is now on the formulary of more than 10 general hospitals. The drug has a 2:1 structure that binds to two CD20 regions on the surface of malignant B cells and one CD3 region on immune T cells, resulting in a stronger binding. Bispecific antibodies have two targets, each targeting a different cell: one that draws immune T cells closer to malignant B cells and the other that activates the T cells to kill the malignant B cells. Based on this mechanism of action, bispecific antibodies have been shown to be effective in patients who are resistant to conventional antibody therapies or chemotherapy. “Bispecific antibodies and CAR-T therapies have been explored as treatment options for DLBCL, but without head-to-head trials, it is difficult to say which is better. The choice of treatment should be based on the individual patient's state of disease progression. However, one of the side effects of CAR-T in elderly patients, immune effector cell-associated neurotoxicity syndrome (ICANS), is considered when selecting a treatment,” said Dr. Fox. He added, “Columvi has a fixed dosing period. It is designed to be administered for up to 12 cycles (8.3 months), so there is a clear end date for the treatment. It also has the advantage of being an off-the-shelf treatment that can be administered to patients immediately.” Columvi achieves 39% CR rate - still effective after 18 months...“justifies the need for its reimbursement” Columvi demonstrated efficacy in the multicenter, open-label Phase I/II NP30179 trial in patients with relapsed or refractory DLBCL after two or more prior systemic therapies. Trial results showed that Columvi achieved a complete response (CR) of 40% and an overall response rate (ORR) of 52%. Among patients who achieved CR, the median duration of response was 26.9 months, with 67% of patients maintaining CR at 18 months. The study also included about one-third of patients who had received prior CAR-T therapy. “Columvi demonstrated a 40% CR rate in the trial, even in patients who are difficult to treat,” said Dr. Fox. This data alone confirms the efficacy of Columvi, as such data cannot be expected with existing standard treatment options, and Columvi is showing similar results in the real world to the clinical trial,” said Dr. Fox. “In DLBCL, relapse typically occurs within 12 to 18 months, and staging progresses rapidly in relapsed patients. We already have data on Columvi’s use in these patients up to 18 months of follow-up. So we can be confident about Columvi’s efficacy data and maintenance of its effect.” However, Columvi’s reimbursement was rejected in July by the Cancer Disease Review Committee, the first gateway to reimbursement in Korea, due to the lack of long-term data. Roche Korea is aiming to reapply for Columvi’s CDDC review later this year. “Patient access to Columvi has been secured in the UK with reimbursement approval,” said Dr. Fox. “This is because the health authorities have recognized Columvi as an effective treatment in DLBCL.” “Columvi is not just an improvement over existing therapies, but a paradigm-shifting treatment for DLBCL. I want to emphasize that this is a treatment that could have an impact on prolonging the survival of patients with relapsed or refractory DLBCL.”
- Company
- Global CDMOs compete to expand ADC capacities
- by Kim, Jin-Gu Nov 14, 2024 05:51am
- Global competition is heating up in the contract development manufacturing organization (CDMO) market for antibody-drug conjugates (ADCs). Major players include Switzerland's Lonza and Samsung Biologics, the world's top two CDMOs, which are competitively expanding their manufacturing facilities. Lonza recently announced the expansion of a 1,200-liter ADC manufacturing facility, while Samsung Biologics announced the start-up of a 500-liter ADC manufacturing facility within the year. According to KoreaBIO, Lonza announced on Dec. 13 (local time) that it plans to add 2 manufacturing facilities in Visp, Switzerland, to expand its 'bioconjugation' service. An additional 1,200-liter manufacturing facility will be built to produce commercial bioconjugates, including ADCs, in high volumes. At the same time, the company will expand the infrastructure of the existing facility. Construction is expected to be completed and the facility fully operational by 2028. The new manufacturing facility will provide comprehensive end-to-end lifecycle support. This includes drug manufacturing for early-stage clinical development, large-scale manufacturing for commercial supply, and finished product filling services. Lonza has been in the bioconjugate CDMO business since 2006. To date, it has produced more than 1,-00 cGMP batches for more than 70 programs. Christian Morello, Vice President, Head of Bioconjugates, Lonza, said, “We continue to see strong growth in the bioconjugates space as ADCs and other bioconjugated drugs increasingly progress towards commercialization. This investment in our multipurpose commercial bioconjugation capacity addresses the growing market demand, enables us to support the growth of our customers, and offers a flexible and integrated service for manufacturing bioconjugates.” The global CDMO market, including Lonza, has recently been intensely competing to expand capacities around ADC drugs. Samsung Biologics is building a dedicated 500-liter ADC manufacturing facility at its Songdo Biocampus in Incheon, South Korea. The company plans to finalize the construction this year and begin full-scale operation after receiving GMP approval. Lotte Biologics is expanding its ADC manufacturing facility at its Syracuse, USA plant. This is an investment of USD 80 million (approximately KRW 100 billion). The ADC manufacturing facility is currently being expanded and is targeting GMP approval in the first quarter of next year. The company is also in the process of building a related plant in Songdo, Incheon. In addition, Celltrion plans to establish a separate CDMO subsidiary while pursuing ADC drug development. Kyongbo Pharmaceutical is investing KRW 85.5 billion to build an ADC plant. The reason why domestic and foreign CDMOs are rushing to expand production capacity for ADC drugs is due to their marketability and high barriers to entry. ADC is a type of antibody conjugated with a cytotoxic drug (payload) as a linker. They have a high structural complexity compared to conventional antibody drugs, which makes the development and manufacturing process difficult, but they have emerged as the next generation of biopharmaceuticals due to their relatively high therapeutic efficacy and low side effects. Following the success of Daiichi Sankyo's breast cancer drug Enhertu (trastuzumab deruxtecan), research on ADC drugs has increased explosively worldwide. However, facilities for the development and mass manufacture of ADC drugs have not been able to keep pace. This is why an imbalance between ADC-related research and manufacturing is expected in the field. Unlike conventional antibody drug CDMOs, ADC-specific manufacturing facilities require more particular technologies. Unlike antibody drug production facilities, ADC production facilities must incorporate additional design principles because they handle cytotoxic drugs (payloads) and organic solvents. Additional design details include negative pressure design, differential pressure between cleanrooms, and airlocks to prevent the spread of cytotoxic drugs and protect operators.
- Company
- Imfinzi combo drug Imjudo can be prescribed at hospitals
- by Eo, Yun-Ho Nov 13, 2024 05:54am
- Immuno-oncology drug Imfinzi's combination partner Imjudo may now be prescribed in general hospitals in Korea. According to industry sources, AstraZeneca Korea's CTLA-4 inhibitor Imjudo (tremelimumab) has passed the drug committees (DCs) of tertiary hospitals in Korea including Seoul National University Hospital and Seoul Asan Medical Center. For now, however, Imjudo is a non-reimbursed drug. AstraZeneca submitted an application for the reimbursement of the PD-L1 inhibitor Imfinzi (durvalumab) and Imjudo combination for liver cancer in June and is currently awaiting a review by the Health and Insurance Review and Assessment Service’s Cancer Disease Review Committee. Imjudo was approved by the Ministry of Food and Drug Safety in combination with Imfinzi in June last year. The first target indication for the combination is liver cancer and can be prescribed as a first-line treatment for adult patients with advanced or unresectable hepatocellular carcinoma (HCC). The specific regimen is the STRIDE (Single Tremelimumab Regular Interval Durvalumab) regimen, which consists of a single dose of Impinj 1,500 mg plus 300 mg of Imfinzi, followed by an additional dose of Impinj at regular intervals every 4 weeks. At the recent European Society for Medical Oncology (ESMO) Congress 2024, the 5-year overall survival data from the Phase III HIMALAYA trial that demonstrated the efficacy of the Imfinzi and Imjudo combination in hepatocellular carcinoma was presented. In the HIMALAYA trial, patients with inoperable HCC were treated with STRIDE (single dose of Imjudo followed by Imfinzi maintenance therapy), Imfinzi monotherapy, and sorafenib monotherapy. When comparing the results of the Imfinzi and Imjudo combination with sorafenib combination therapy in patients with unresectable HCC, patients who received the STRIDE regimen had a 5-year overall survival (OS) rate of 19.6%, compared with the 9.4% for patients who received sorafenib. The median overall survival was 16.43 months and 13.77 months, respectively, showing a 24% lower risk of death in the Imfinzi-Imjudo combination arm. “ The Imfinzi-Imjudo combination therapy has significant advantages in that it has a much lower risk of bleeding than conventional therapies and does not worsen liver function," said Hong Jae Chon, Professor of Hemato-Oncology at CHA Bundang Medical Center. “In particular, the combination shows potential for longer survival than existing therapies."
- Company
- Treatment-refractory Dravet syndrome calls for new options
- by Whang, byung-woo Nov 13, 2024 05:54am
- Despite increased treatment options for the ultra-rare Dravet syndrome, there are still gaps in care that require attention. Even with the introduction of medical cannabis, cannabidiol, there are patients who do not respond to the drug, which is why improving access to new options should be considered. Dravet syndrome is a rare neurological disorder that begins with fever and convulsions within the first year of life, persists into adulthood, and leaves nearly all young patients moderately to severely disabled after each attack. Although it is known to be a rare disease with an estimated prevalence of 1 to 2 per 10,000 people worldwide, there is no officially investigated prevalence in Korea and was designated as an ultra-rare disease in 2022. Dravet syndrome is characterized by the onset of the first seizure, which is similar to a febrile convulsion that usually occurs with fever at 6 months. The biggest risk factor is “Sudden Unexpected Death in Epilepsy” (SUDEP). While the rate of sudden death in intractable epilepsy is 20-25%, in Dravet syndrome, up to 59% of all deaths are associated with SUDEP. The goal of treatment for Dravet syndrome is to control seizure frequency and non-seizure symptoms to reduce the patient's risk of sudden death and improve quality of life. Initial treatment includes anti-seizure medications and add-on treatments such as the anti-seizure medications stiripentol and cannabidiol are used to treat the “drug refractory” nature of Dravet syndrome. Cannabidiol is a medical cannabis preparation that was previously supplied without reimbursement through the Korea Orphan & Essential Drug Center for urgent use but then has been reimbursed since April 2021. Hoon-Chul Kang, professor of pediatric neurology at Severance Hospital, emphasized that the government's approval of medical cannabis has contributed to improving the treatment environment for Dravet syndrome. Kang said, “The government's decision was based on the desperate voices of parents and caregivers of children with Dravet syndrome, as well as objective data reported in the literature,” he explains. Limitations remain for drug-refractory Dravet syndrome...a fundamental solution is needed However, stiripentol and cannabidiol are only available through the Korea Orphan & Essential Drug Center, and the treatment process from applying for the drugs to meeting the criteria for reimbursement coverage is rather complicated. Hoon-Chul Kang, professor of pediatric neurology at Severance Hospital Hoon-Chul Kang, professor of pediatric neurology at Severance HospitalIn particular, there are still many patients with Dravet syndrome who are refractory to existing medications, leaving a blind spot in terms of seizure management. Unlike Korea, where treatment options are limited, options are increasing overseas with the emergence of new options. In the long run, experts agree that Korea also needs a fundamental treatment for seizures that reduces the quality of life for people with Dravet syndrome and their caregivers. If a new treatment option can significantly improve seizure control while also managing additional comorbidities and disabilities, it would substantially improve the treatment landscape. In addition, despite the limitations of being an ultra-rare disease, there are expectations that Dravet syndrome will benefit from the government's 'fast-track program for serious and rare diseases to reduce the burden of medical expenses’ plan. The industry predicts that the government's interest in pediatric rare and intractable diseases will continue for the time being, given the revised pediatric drug pharmacoeconomic evaluation exemption system last year and the selection of drugs for the first pilot project for the approval-evaluation-negotiation linkage system. A professor of pediatrics at a tertiary hospital said, "The government needs to make another timely decision to improve the treatment environment for Dravet syndrome, an extremely rare disease that is even more neglected than others."
- Company
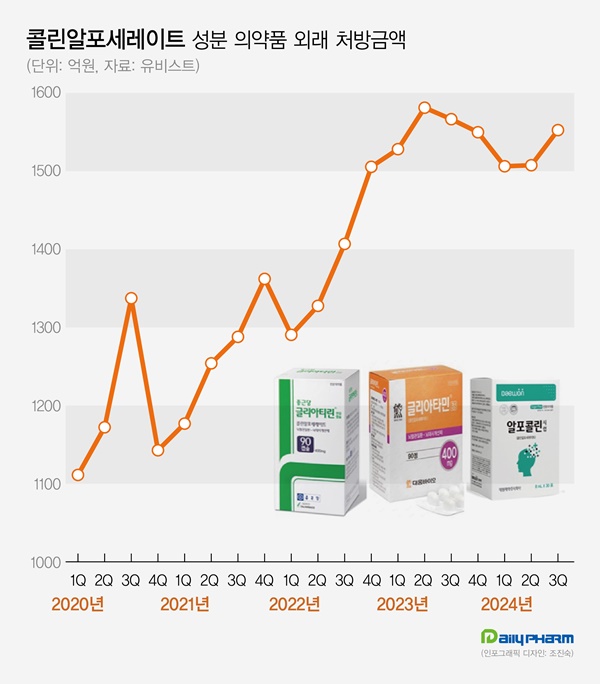
- 'Choline alfoscerate' prescription market continues to grow
- by Chon, Seung-Hyun Nov 13, 2024 05:54am
- The cognitive enhancer 'choline alfoscerate (choline products)' has expanded its presence in the prescription market. Choline products' growth slowed earlier this year but rebounded in Q3, further expanding the market size. Although a few companies withdrew from the market due to the risk of failing clinical re-evaluation, the prescription market continued sales boom. According to the pharmaceutical market research firm UBIST on November 11, the outpatient prescription market for choline products totaled KRW 155.3 billion in Q3. It decreased by 0.9% compared to Q3 of last year but increased by 3.0% compared to the previous quarter. Choline products' prescription sales recorded KRW 158.1 billion in Q2 of 2023. Then they went on a downward slide for three consecutive quarters until Q1 this year. In Q2, the sales slightly increased compared to the previous quarter. In Q3, they showed a strong rebound. Choline products' prescription size for Q3 was recorded as the third highest in history. The slowing growth of choline products in the first half of this year is likely due to steep growth in the past few years. The prescription market for choline products recorded KRW 308.8 billion in 2018. Then, it continued to renew the best record every year. Last year, the market amounted to KRW 622.6 billion, expanding more than twice in five years. Quarterly prescription sales indicate a 46.4% increase over five years from KRW 106.1 billion in Q3 of 2019. Outpatient prescription sales of pharmaceuticals containing choline alfoscerate (unit: KRW 100 million, source: UBIST). Even though choline products are under clinical re-evaluation for efficacy evaluation, clinical practices have continued to have a high demand for choline products. In June 2020, the Ministry of Food and Drug Safety (MFDS) requested companies with choline products to submit their clinical trial documents, and 57 pharmaceutical companies began clinical trials for reassessment. Previously, three indications for choline products had been approved, including ▲secondary symptoms caused by cerebrovascular deficit or degenerative organic brain syndrome ▲emotional and behavioral changes ▲senile pseudodementia. In the re-evaluation process, two out of three indications for choline products were deleted, excluding 'secondary symptoms caused by cerebrovascular deficit or degenerative organic brain syndrome.' Choline products are facing the possibility of reduced reimbursement in addition to the issue of their efficacy. In August 2022, the MOHW issued revised regulations on 'The Criteria and Scope of National Health Insurance,' indicating that patients without prior dementia diagnosis will have a copayment increased from 30% to 80%. After that, two groups of pharmaceutical companies, led by Daewoong Bio and Chong Kun Dang, filed an administrative suit to cancel the MOHW's notification. However, they all lost in the first trial in 2022. Chong Kun Dang also lost in the second trial in May. However, as the suspension of execution filed by pharmaceutical companies has been accepted, reimbursement reductions are on hold. Despite many products being withdrawn from the market after the clinical re-evaluation of choline products, the prescription market continued to grow. According to the MFDS, choline products that received Korean approval total 278 items. Among these, 134 items have been withdrawn from the market due to approval withdrawal or cancelation. Previously, the MFDS ordered the clinical re-evaluation of choline products from 134 companies. 77 companies withdrew from undergoing re-evaluation, resulting in a significant number of withdrawals from the market. More companies that commenced clinical re-evaluation of choline products are withdrawing. In two months from September, Guju Pharmaceutical, Kyongbo Pharmaceutical, PharmGen Science, YooYoung Pharmaceutical, and Medix Pharm voluntarily withdrew choline product approval. Increasing number of companies are exiting the market due to potential retrieval amounts that arise when they fail the re-evaluation for choline products. In 2020, the MOHW issued a national health insurance contract to companies with choline products, entailing 'companies failing clinical trials must return the prescription sales.' Within eight months of the negotiation order, pharmaceutical companies agreed to the term that they would return 20% of the prescription sales from the time they received IND approval to the date of deletion when the indication for the product was deleted due to failed clinical re-evaluation. The retrieval negotiations for choline products are determined by agreements between the MOHW and each pharmaceutical company, resulting in different contract details for each company. While a 20% retrieval rate from prescription sales is commonly applied, the timing of the retrieval rate varies among companies. Sources said that most companies have agreed to increase retrieval rate. For instance, companies may have agreed to set a 10% retrieval rate for this year when they fail the clinical re-evaluation of choline products, then increase to 30% after five years. As the prescription market for choline products continues to grow, companies that agreed to a gradual increase of retrieval rate would end up exponentially expanding retrieval amount due to the market growth. Pharmaceutical companies may have to face increased retrieval amounts as the market for choline products continues to grow if they fail clinical re-evaluation. For these reasons, sources said that more companies are considering exiting the market before completing the clinical re-evaluation. However, analysis suggests that the entire market for choline products continues to grow as other products replace the withdrawn products. Prescription sales by major products indicate that Daewoong Bio's Gliatamin recorded KRW 41.2 billion in Q3, a 4.4% reduction from the previous year. Chong Kun Dang's Chongkundang Gliatirin generated prescription sales of KRW 31.1 billion in Q3, up 10.9% from last year. Arlico's Choliatin recorded prescription sales of KRW 5.1 billion in Q3, a 28.7% reduction from the previous year. Daewon Pharmaceutical's Alfocholine generated prescription sales of KRW 4.9 billion in Q3, down 10.3%. Yuhan's Alfoatilin recorded KRW 3.7 billion, down 16.7% from the previous year. Dongkoo Bio's Glifos' sales increased from KRW 2.7 billion in Q3 of last year to KRW 3.7 billion in a year, up 33.9%. Mother's Pharm's Memoem recorded prescription sales at KRW 1.1 billion in Q3 of last year, then increased to KRW 3.3 billion in a year, an increase of over threefold.