- LOGIN
- MemberShip
- 2025-12-22 09:30:28
- Six reimbursement revaluations fail in 4 years
- by Chon, Seung-Hyun | translator Alice Kang | 2024-11-15 05:49:54
Six items have been removed from the reimbursement list after reimbursement reevaluations during the past 4 years.
Silymarin bilberry fruit dried powder, followed by itopride, failed the reimbursement reevaluations.
Streptokinase - streptodornase, oxiracetam, and acetylcarnitine were also removed due to reimbursement reevaluation failures.
Pharmaceutical companies are facing annual losses of up to KRW 240 billion as a result of the 6 drugs' reimbursement reevaluation failures.
According to the Ministry of Health and Welfare on the 14th, 55 itopride-based drugs have been removed from the reimbursement list as of this month.
The move follows the government's reimbursement revaluation.
The health authorities selected 7 ingredients, including thioctic acid, pranlukast, itopride, sarpogrelate, levodropropizine, mosapride, and formoterol, to be reevaluated this year.
As a result, the authorities concluded that itopride lacked clinical efficacy and was removed from the benefit.
Three components - thioctic acid, pranlukast, and mosapride - were recognized as clinically useful and will remain on the reimbursement list.
In the case of sarpogrelate and levodropropizine, the two will be removed from the reimbursement list but will remain covered if pharmaceutical companies voluntarily reduce their prices.
The decision of formoterol was deferred pending clinical reevaluation by the MFDS, with the condition that the NHIS will recollect a portion of their reimbursement costs if the drugs fail to prove clinical efficacy.
This means that only itopride fully failed the reimbursement reevaluations, and all products containing itopride will be removed from the reimbursement list.
Itopride products from JW Life Science, JW Pharmaceutical, Kuhnil Biopharm, KyungDong Pharm, Kwangdong Pharmaceutical, Kukje Pharm, Nexpharm Korea, Novem Healthcare, NEWGEN Pharma, Daewon Biotech, Daewon Pharmaceutical, Dongsung Pharm, Dongwha Pharmaceutical, Mother’s Pharmaceutical, Medix Pharm, Bukwang Pharmaceutical, Samsung Pharm, Celltrion Pharm, Sinil Pahramcetucial, Shinpoong Pharmaceutical, iCure Pharmacueticals, Ahngook New Pharm, Ahngook Pharmaceutical, Alvogen Korea, SPC, Neo Bio Korea Pharm, Youngil Pharm, Young Poong Pharmaceutical, Yuyu Pharma, Yuhan Corp, Eden Pharma, Reyon Pharmaceutical, Intro Biopharma, Il-Yang Pharmaceutical, Ilwha, Jeil Pharmaceutical, Chong Kun Dang, Jinyang Pharmaceutical, Cosmax Pharma, PharmaKing, Poonglim Pharmatech, Hana Pharm, BMI Korea, Abbott Korea, Union Korea Pharm, Korus Korea, Korea Pharma, Pharmbio Korea, PMG Korea, Hutecs Korea Pharmaceutical, Han Wha Pharma, Whan In Pharm, Huons Meditech, Huons Life Sciences, will be withdrawn from the reimbursement market.
Itopride is used to treat digestive symptoms caused by functional dyspepsia.
According to the drug research institution UBIST, it generated KRW 23.6 billion in outpatient prescriptions last year.
Itopride's prescription volume has decreased 18.7% in 5 years from KRW 29.1 billion in 2019 but has maintained prescription sales of over KRW 20 billion annually.
From the pharmaceutical companies’ perspective, the reimbursement cut for itopride means a loss of more than KRW 20 billion a year.
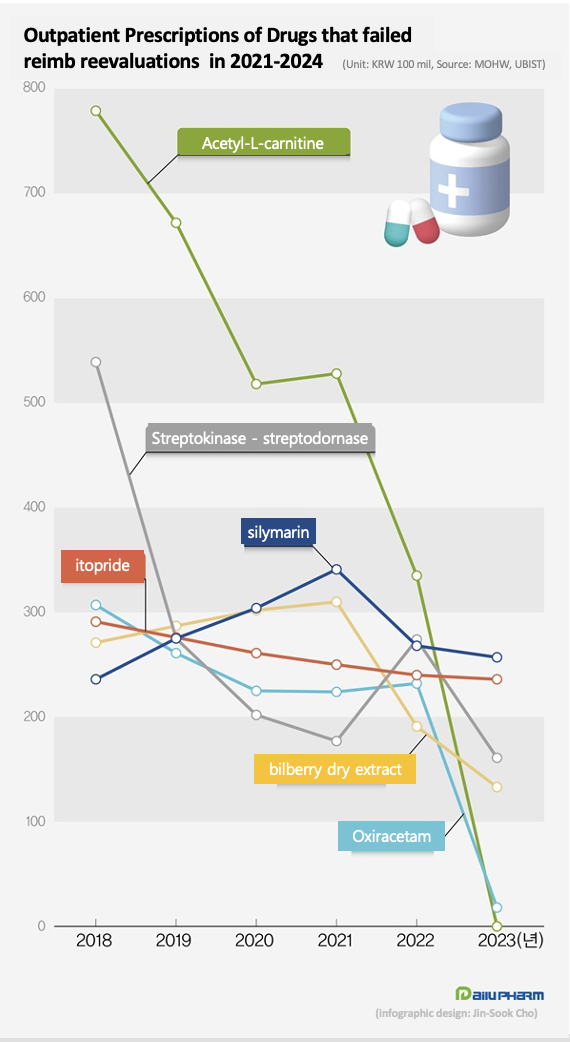
Among them, silymarin and bilberry dry extract were concluded to be inadequate for reimbursement and were removed from the health insurance reimbursement list.
For both silymarin and bilberry dry extract, the prescription market has not been eliminated as some products that have filed administrative lawsuits have retained their reimbursed status.
However, the prescription market has shrunk significantly due to the mass breakaway of products that accepted the reimbursement deletion.
Silymarin's prescription market size grew 44.6% over 3 years, from KRW 23.6 billion in 2019 to KRW 34.1 billion in 2022, showing a significant increase in demand in the prescription market.
Silymarin is an OTC drug used for toxic liver disease, hepatocyte protection, chronic hepatitis, and cirrhosis.
However, after failing reimbursement reevaluations, sales fell to KRW 26.7 billion in 2022, down 21.5% from the previous year, and then to KRW 25.7 billion last year, down 24.8% from 2 years ago.
Last year, the prescription amount of bilberry dry extract was KRW 13.3 billion, down 30.3% from the previous year.
Bilberry dry extract is a drug used to improve retinal degeneration and vascular disorders of the eye caused by diabetes.
The prescription market for bilberry dry extract was worth KRW 31 billion in 2021, but after the government deleted its reimbursement status, sales dropped 38.5% to KRW 19.1 billion in 2022 and shrank further last year.
Prescriptions for bilberry dry extract last year shrank by 57.1% compared to two years ago.
Streptokinase - streptodornase (Strepto preparations), oxiracetam, and acetyl L-carnitine, which were eligible for reimbursement but failed to pass the MFDS’s clinical reassessment, have seen their prescription market decline to the point of extinction.
Last year, the prescription market for strepto preparations was KRW 16.1 billion, down 41.0% from the previous year.
In 2022, the MFDS determined the strepto preparations lacked clinical efficacy during reimbursement reevaluations.
However, given the ongoing clinical reassessment, conditional reimbursement was offered to defer the reimbursement reevaluation results only for items that agreed to refund the reimbursed costs based on the outcome of the clinical reevaluation.
Strepto preparations are used for the “relief of acute inflammatory edema caused by ankle surgery or trauma to the ankle” and “difficulty in bile drainage accompanying respiratory diseases.” In 2017, the MFDS ordered a clinical reevaluation of strepto preparations after controversy arose over their efficacy.
However, the clinical reevaluation failed to prove their efficacy, and the drug was excluded from the health insurance reimbursement list in December last year, the the indications were also deleted.
Oxiracetam and acetyl L-carnitine were selected for reimbursement reevaluations last year but failed clinical reevaluations and were unable to proceed to reimbursement reevaluations.
In January, the prescription and dispensing of oxiracetam was discontinued after the clinical reevaluation failed to verify the drug’s efficacy.
Oxiracetam was approved for the treatment of cognitive impairment caused by Alzheimer's disease, multiple sclerosis, and temperamental brain syndrome due to brain dysfunction.
Oxiracetam generated KRW 23.2 billion in prescription sales in 2022, but its clinical reevaluation failure wiped out the prescription market.
Acetyl-L-carnitine was subject to reimbursement reevaluations in 2023 but was not evaluated due to its failure to pass clinical reevaluations.
Acetyl-L-carnitine is licensed for use in “primary degenerative diseases” or “degenerative diseases secondary to cerebrovascular diseases.” In 2013, the MFDS ordered a clinical reevaluation of acetyl-L-carnitine preparations.
However, in 2022, the indications were removed due to the drug’s failure to demonstrate efficacy in both indications.
Acetyl-L-carnitine had a prescription market worth KRW 77.9 billion in 2019, but its removal from both the approval and reimbursement list led to losses for pharmaceutical companies.
The 6 ingredients that were removed from the reimbursement list among those subject to reimbursement reevaluations over the past 4 years since 2021 had a combined prescription volume of KRW 242.3 billion in 2019.
Pharmaceutical companies have realized an annual loss of KRW 242.4 billion in prescription sales due to the removal of the 6 ingredients.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










