- LOGIN
- MemberShip
- 2025-12-22 12:52:36
- 'Choline alfoscerate' prescription market continues to grow
- by Chon, Seung-Hyun | translator Hong, Ji Yeon | 2024-11-13 05:54:17
The cognitive enhancer 'choline alfoscerate (choline products)' has expanded its presence in the prescription market.
Choline products' growth slowed earlier this year but rebounded in Q3, further expanding the market size.
Although a few companies withdrew from the market due to the risk of failing clinical re-evaluation, the prescription market continued sales boom.
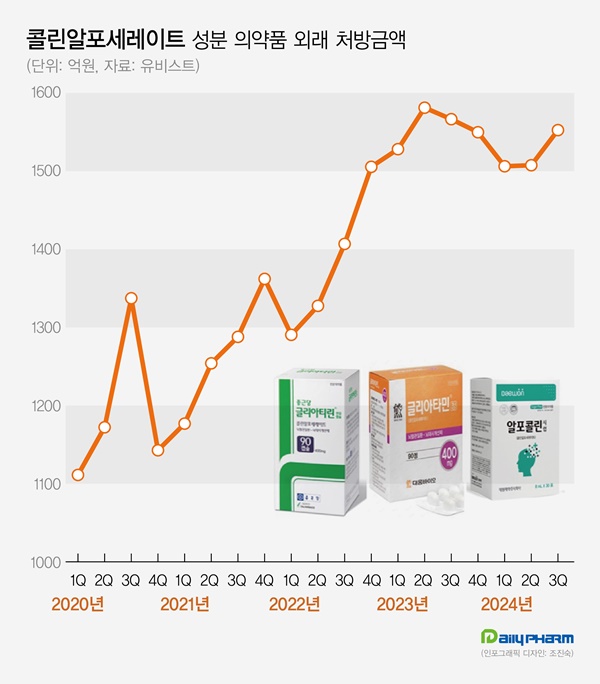
According to the pharmaceutical market research firm UBIST on November 11, the outpatient prescription market for choline products totaled KRW 155.3 billion in Q3.
It decreased by 0.9% compared to Q3 of last year but increased by 3.0% compared to the previous quarter.
Choline products' prescription sales recorded KRW 158.1 billion in Q2 of 2023.
Then they went on a downward slide for three consecutive quarters until Q1 this year.
In Q2, the sales slightly increased compared to the previous quarter.
In Q3, they showed a strong rebound.
Choline products' prescription size for Q3 was recorded as the third highest in history.
The slowing growth of choline products in the first half of this year is likely due to steep growth in the past few years.
The prescription market for choline products recorded KRW 308.8 billion in 2018.
Then, it continued to renew the best record every year.
Last year, the market amounted to KRW 622.6 billion, expanding more than twice in five years.
Quarterly prescription sales indicate a 46.4% increase over five years from KRW 106.1 billion in Q3 of 2019.
In June 2020, the Ministry of Food and Drug Safety (MFDS) requested companies with choline products to submit their clinical trial documents, and 57 pharmaceutical companies began clinical trials for reassessment.
Previously, three indications for choline products had been approved, including ▲secondary symptoms caused by cerebrovascular deficit or degenerative organic brain syndrome ▲emotional and behavioral changes ▲senile pseudodementia.
In the re-evaluation process, two out of three indications for choline products were deleted, excluding 'secondary symptoms caused by cerebrovascular deficit or degenerative organic brain syndrome.' Choline products are facing the possibility of reduced reimbursement in addition to the issue of their efficacy.
In August 2022, the MOHW issued revised regulations on 'The Criteria and Scope of National Health Insurance,' indicating that patients without prior dementia diagnosis will have a copayment increased from 30% to 80%.
After that, two groups of pharmaceutical companies, led by Daewoong Bio and Chong Kun Dang, filed an administrative suit to cancel the MOHW's notification.
However, they all lost in the first trial in 2022.
Chong Kun Dang also lost in the second trial in May.
However, as the suspension of execution filed by pharmaceutical companies has been accepted, reimbursement reductions are on hold.
Despite many products being withdrawn from the market after the clinical re-evaluation of choline products, the prescription market continued to grow.
According to the MFDS, choline products that received Korean approval total 278 items.
Among these, 134 items have been withdrawn from the market due to approval withdrawal or cancelation.
Previously, the MFDS ordered the clinical re-evaluation of choline products from 134 companies.
77 companies withdrew from undergoing re-evaluation, resulting in a significant number of withdrawals from the market.
More companies that commenced clinical re-evaluation of choline products are withdrawing.
In two months from September, Guju Pharmaceutical, Kyongbo Pharmaceutical, PharmGen Science, YooYoung Pharmaceutical, and Medix Pharm voluntarily withdrew choline product approval.
Increasing number of companies are exiting the market due to potential retrieval amounts that arise when they fail the re-evaluation for choline products.
In 2020, the MOHW issued a national health insurance contract to companies with choline products, entailing 'companies failing clinical trials must return the prescription sales.' Within eight months of the negotiation order, pharmaceutical companies agreed to the term that they would return 20% of the prescription sales from the time they received IND approval to the date of deletion when the indication for the product was deleted due to failed clinical re-evaluation.
The retrieval negotiations for choline products are determined by agreements between the MOHW and each pharmaceutical company, resulting in different contract details for each company.
While a 20% retrieval rate from prescription sales is commonly applied, the timing of the retrieval rate varies among companies.
Sources said that most companies have agreed to increase retrieval rate.
For instance, companies may have agreed to set a 10% retrieval rate for this year when they fail the clinical re-evaluation of choline products, then increase to 30% after five years.
As the prescription market for choline products continues to grow, companies that agreed to a gradual increase of retrieval rate would end up exponentially expanding retrieval amount due to the market growth.
Pharmaceutical companies may have to face increased retrieval amounts as the market for choline products continues to grow if they fail clinical re-evaluation.
For these reasons, sources said that more companies are considering exiting the market before completing the clinical re-evaluation.
However, analysis suggests that the entire market for choline products continues to grow as other products replace the withdrawn products.
Prescription sales by major products indicate that Daewoong Bio's Gliatamin recorded KRW 41.2 billion in Q3, a 4.4% reduction from the previous year.
Chong Kun Dang's Chongkundang Gliatirin generated prescription sales of KRW 31.1 billion in Q3, up 10.9% from last year.
Arlico's Choliatin recorded prescription sales of KRW 5.1 billion in Q3, a 28.7% reduction from the previous year.
Daewon Pharmaceutical's Alfocholine generated prescription sales of KRW 4.9 billion in Q3, down 10.3%.
Yuhan's Alfoatilin recorded KRW 3.7 billion, down 16.7% from the previous year.
Dongkoo Bio's Glifos' sales increased from KRW 2.7 billion in Q3 of last year to KRW 3.7 billion in a year, up 33.9%.
Mother's Pharm's Memoem recorded prescription sales at KRW 1.1 billion in Q3 of last year, then increased to KRW 3.3 billion in a year, an increase of over threefold.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










