- LOGIN
- MemberShip
- 2025-12-20 12:40:27
- Company
- ‘TNF-α inhibitors, a cornerstone for rheumatoid arthritis'
- by Whang, byung-woo Jul 02, 2025 06:09am
- Rheumatoid arthritis is a chronic autoimmune condition that causes inflammation in the joints, leading to pain and deformity. Its prevalence in Korea is estimated to be approximately 0.5–1% Recently, with the development of new treatments and improved awareness of the disease, early diagnosis rates are on the rise. In fact, over the past 5 years, the number of rheumatoid arthritis patients diagnosed in Korea has increased by more than 30%, with the elderly aged 50 and older accounting for 79% of all patients, highlighting the aging population. Sang-Heon Lee, Professor of Rheumatology, Konkuk University Medical CenterAs a result, it has become important to go beyond simple treatment and focus on addressing elderly patients. Professor Sang-heon Lee of the Rheumatology Department at Konkuk University Hospital, an expert with the latest insights in the field, emphasized the importance of selecting the right treatment and management for the growing number of elderly patients with rheumatoid arthritis. According to Professor Lee, rheumatoid arthritis was once considered incurable, but with the advent of new treatments, perceptions have changed, increasing early diagnosis and treatment of the disease in general. Professor Lee said, “With the message that rheumatoid arthritis is a treatable disease spreading, early diagnosis rates are also increasing. In the past, we only checked the condition of the patient's joints with the naked eye, but the development of imaging diagnostic technologies such as joint ultrasound and MRI, rendered early diagnosis possible.” Another change is that, along with the overall aging population, the proportion of elderly patients with rheumatoid arthritis is also increasing. Professor Lee explained that elderly patients require a multifaceted approach to treatment, including management of complications, compared to younger patients. He emphasized, “Just as machines lose functionality over time, elderly patients often have multiple comorbidities due to the decline of various bodily functions. Complications such as cardiovascular disease, cerebrovascular disease, and diabetes are common, and immune function is also impaired, so caution is necessary when using immunotherapy agents.” 10 Years since the launch of Simponi IV Formulation, GO-FURTHER study results gain attention The emergence of TNF (tumor necrosis factor)-α inhibitors is an indispensable part of the paradigm shift in the treatment of rheumatoid arthritis. These drugs have been used in clinical practice for more than 25 years and are still considered effective in treating approximately 70% of all patients. Professor Lee stated, “The various treatment options developed since then have established themselves as alternatives for patients not adequately treated with TNF-α inhibitors. As such, TNF inhibitors remain the cornerstone of rheumatoid arthritis treatment. Even after patent expiry, biosimilars for TNF-α inhibitors are being launched most actively.” Among such TNF-α inhibitors, Simponi (golimumab) was introduced in Korea in 2012 as a subcutaneous (SC) injection formulation, and in May 2015, an intravenous (IV) injection formulation was launched, offering faster symptom improvement, longer dosing intervals, and easier monitoring by healthcare professionals, and establisehd a strong market presence. Professor Lee explained, “Simponi IV is a TNF-α inhibitor developed as a fully human monoclonal antibody, offering superior antibody purity and significantly reduced injection-related adverse reactions. While infliximab requires approximately two hours for administration, Simponi can be administered in about 30 minutes, and the dosing interval is every two months, significantly reducing the number of clinic visits.” Professor Lee is particularly interested in the advantages of Simponi for the increasing number of elderly patients with rheumatoid arthritis. A subanalysis of the GO-FURTHER study showed that Simponi IV showed more improvement in elderly patients aged 65 years or older. Specifically, in a subanalysis of the GO-FURTHER study that divided patients with active rheumatoid arthritis into two groups—those under 65 years of age and those 65 years of age and older—Simponi demonstrated higher initial symptom improvement compared to the placebo group in the elderly patient group during the first 6 months. At week 14, 61.0% of patients achieved ACR20, which was approximately six times higher than the placebo group (11.1%), and at Week 24, 69.5% of patients achieved ACR20, demonstrating significant clinical value of Simponi compared to the placebo group (33.3%). Professor Lee said, “Although the overall numerical differences were not significant in younger patients, there were more meaningful differences in response speed and response rates in high-risk patients. Elderly patients often have reduced physical function due to inflammation, so when they respond to treatment, the improvement is much greater. In this regard, Simponi has clinical advantages for elderly patients.” In terms of safety, Simponi IV did not show any new concerns in elderly patients. He said, “Generally, super-elderly patients over the age of 70 are at high risk of infection, so careful monitoring is necessary regardless of the drug used. The safety data for Simponi in elderly patients is generally consistent with previous reports, and injection-related side effects were rare and mostly mild.” “More treatment options emerge for rheumatoid arthritis... TNF-α inhibitors remain the cornerstone” From a broad perspective, the development of new drugs with various mechanisms of action for the treatment of rheumatoid arthritis has led to a wider range of options. In this regard, Professor Lee predicted that TNF-α inhibitors will remain competitive due to their accumulated treatment experience and safety data. Professor Lee stated, “At present, TNF-α inhibitors will continue to maintain their position as the cornerstone of rheumatoid arthritis treatment because of their over 25 years of accumulated clinical experience and identification of most major side effects, which allow physicians to prescribe them with confidence.” While new oral treatments like JAK inhibitors have emerged, concerns about long-term safety mean that TNF-α inhibitors remain the most trusted treatment option at this point. “JAK inhibitors are highly effective, but as they regulate intracellular signaling, there are concerns about long-term safety, such as cancer development. At least 5 to 10 years of additional data will be required to verify this.” In addition, Professor Lee emphasized the importance of personalized treatment for rheumatoid arthritis. He explained, “While most patients are initially prescribed TNF-α inhibitors as first-line therapy, they may not be suitable for all patients. In cases where there are contraindications for TNF-α inhibitors, such as a history of tuberculosis or heart failure, alternative medications with different mechanisms of action are considered.” Ultimately, the strategy of selecting the optimal medication based on the patient's individual condition and risk factors, and switching medications when necessary, is crucial. Finally, the professor noted, “While anyone can prescribe medications, managing complications or the progression of comorbidities associated with aging, as well as appropriately addressing side effects, requires the experience and knowledge of specialized medical professionals. Early treatment that strongly suppresses inflammation in the initial stages can prevent disease recurrence and enable long-term management. Therefore, it is crucial to extinguish the inflammatory ‘sparks’ in the early stages through prompt treatment.”
- Company
- K-CAB·Entresto awaiting the Supreme Court rulings
- by Kim, Jin-Gu Jul 02, 2025 06:09am
- Patent disputes involving blockbuster drugs, K-CAB (tegoprazan) and Entresto (valsartan/sacubitril), are reaching their final stages. Observations suggest that the Supreme Court's final ruling could come as early as the second half of this year. K-CAB's annual prescription sales amount to KRW 200 billion, while Entresto's are KRW 70 billion. In other words, the patent barriers for these blockbuster drugs could be lifted, depending on the Supreme Court's decision. K-CAB Crystal Form and Substance Patent Disputes Head to Supreme Court… Will KRW 200 Billion Patent Barrier Be Lifted? Product photo of K-CABAccording to the pharmaceutical industry on July 2, HK inno.N and generic companies are awaiting the Supreme Court's ruling in their disputes over K-CAB's substance patent and crystal form patent. For the crystal form patent, generic companies won both the first and second instances. Approximately 80 companies filed declaratory judgment actions to circumvent K-CAB's crystal form patent and won in the first instance. Generic companies continued their winning streak in the second trial following HK inno.N's appeal. HK inno.N, having lost in the second trial, chose to appeal to the Supreme Court. Cases were successively filed with the Supreme Court starting in March this year. In May, a presiding Supreme Court Justice and the presiding panel were assigned. The panel is currently reviewing the legal arguments for the appeal. Industry observers predict that a Supreme Court ruling could come as early as this year. In contrast, in the substance patent dispute, HK inno.N has consistently won. The Intellectual Property Court of Korea has issued successive rulings in favor of HK inno.N since May this year. Generic companies that lost in the second trials have submitted appeals to the Supreme Court. In some appeals, the Supreme Court's final judgment has already been rendered. In May, the Supreme Court dismissed the appeals filed by generic companies without deliberation, upholding the original judgment. Dismissal without deliberation is a system in which the Supreme Court rejects an appeal without conducting a full review of the merits if it determines that there are no specific grounds, such as legal violations, in the original judgment. However, an analysis suggests it is difficult to interpret this as HK inno.N having definitively secured victory in the substance patent dispute. This is because if generic companies present arguments different from those of LitePharmTech and HLB Pharmaceutical, there remains a possibility for the Supreme Court to initiate a full review of the merits. In such a case, some view that the final ruling could change. The timing of the early launch for generics will be determined by the Supreme Court's final ruling on both the crystal form patent and substance patent disputes. This means that the patent barrier for K-CAB, which has grown to an annual market size of KRW 200 billion, could be lifted as early as this year. K-CAB's substance patent is set to expire in August 2031, and its crystal form patent is scheduled to expire in March 2036. If the Supreme Court rules in favor of the generic companies in both the substance patent and crystal form patent disputes, generic companies could launch their products immediately. In contrast, if, as in the first and second instances, generic companies win the crystal form patent dispute and HK inno.N wins the substance patent dispute, the generic launch date would be August 2031. If the Supreme Court rules in favor of HK inno.N in both the crystal form patent and substance patent disputes, a generic launch would not be possible until 2036. Entresto Patent Dispute Nearing Conclusion with One Case Each Pending in 2nd·3rd Trials...Crystal Form Patent Appeal is Key Product photo of EntrestoThe Entresto patent dispute is also heading towards its final stages. Generic companies filed invalidation and declaratory judgment actions against six Entresto patents starting in January 2021. Generic companies won all cases in the first trial. After losing in the first trial, Novartis appealed three of the six patents. Novartis lost the appeal related to the use patent. Novartis subsequently appealed to the Supreme Court but received a dismissal without deliberation ruling in April last year. With this, generic companies have overcome the use patent, which expires in July 2027. Novartis also lost the appeal related to the crystal form patent and appealed to the Supreme Court. In April this year, the deliberation period expired. This means that, unlike the use patent dispute, a full review of the merits will proceed, and a ruling could potentially come within the year. The appeal for the salt·hydrate patent is still pending in the second trial. A hearing date has not yet been set. Industry observers predict that once a ruling is issued for the crystal form patent appeal, a ruling of similar conclusion will follow. If the crucial crystal form patent appeal ruling is handed down this year, the possibility of a generic early launch will further increase. Entresto's crystal form patent is set to expire in September 2040. The salt·hydrate patent is set to expire in November of next year. Suppose the Supreme Court rules in favor of the generic companies in the crystal form patent appeal, as in the first and second instances. In that case, the challenging companies can launch their products immediately. Although the salt·hydrate patent dispute is still ongoing, the product launch becomes possible based on the victory in the first trial. Once the salt/hydrate patent expires in November next year, related risks will also be resolved. Dukarb Patent Dispute Nearing Conclusion...Will Zemiglo Dispute End in 2nd Trial? Patent disputes surrounding Boryung's hypertension combination drug Dukarb (fimasartan/amlodipine) and LG Chem's diabetes treatment Zemiglo (gemigliptin) are also nearing their conclusions. In the case of Dukarb, a Supreme Court ruling was recently issued. The Supreme Court remanded the Dukarb patent invalidation lawsuit appeal on the 26th of last month. This overturned the second trial ruling and sided with the generic companies. Following the Supreme Court's decision, the Dukarb patent dispute will be re-litigated at the original court, the Intellectual Property Court of Korea. The pharmaceutical industry expects that since the Supreme Court found legal issues in the second trial's judgment, the Intellectual Property Court of Korea is highly likely to overturn its previous ruling and side with the generic companies. Once the Intellectual Property Court of Korea issues ruling, the Dukarb patent dispute, which has lasted over four years, will effectively conclude. If generic companies secure a final victory at the Intellectual Property Court of Korea, the launch of generics for Dukarb's core 30/5mg dosage product will become possible. Dukarb was launched in four dosages: ▲30/5mg ▲30/10mg ▲60/5mg ▲60/10mg. Among these, the 30/5mg dosage is reported to account for over 60% of Dukarb's total sales. Furthermore, the combination composition patent, which is at the heart of the Dukarb dispute, applies only to this dosage. Generic companies launched Dukarb generics after the expiration of the fimasartan single-agent (Kanarb) substance patent in February 2023. However, generics for the 30/5mg dosage could not be launched due to the combination composition patent. As a result, Dukarb generics have struggled to gain traction in the prescription market. In Q1 this year, the combined prescription sales of Dukarb generics amounted to only KRW 700 million. The Zemiglo patent dispute is nearing its second trial ruling. The Intellectual Property Court of Korea has scheduled the verdict for the Zemiglo patent invalidation lawsuit for the 21st of next month. Generic companies filed invalidation trials and declaratory judgment actions against the Zemiglo use patent starting in May 2023. Generic companies prevailed in the first instance. The Intellectual Property Trial and Appeal Board issued rulings in favor of the generic companies in April and September 2024, respectively. LG Chem appealed. Generic companies also won in the subsequent appeal. The Intellectual Property Court of Korea sided with the generic companies in the appeal against the declaratory judgment ruling. LG Chem decided not to appeal this ruling to the Supreme Court. The appeal against the patent invalidation ruling is scheduled for a verdict next month. Some in the industry predict that even if generic companies win in the second trial, LG Chem will likely forgo appealing to the Supreme Court. This is because LG Chem had already lost and chose not to appeal the previous judgment related to the declaratory order. This ruling could significantly accelerate the launch of Zemiglo generics. The Zemiglo use patent, challenged by generic companies, expires in October 2039. In addition to this patent, two other patents remain: a salt·hydrate patent that expires in October 2031 and a substance patent that expires in January 2030.
- Company
- Generics of Jardiance expected to compete for KRW 100B mkt
- by Kim, Jin-Gu Jul 02, 2025 06:08am
- Product photo of JardianceThe substance patent for Jardiance (empagliflozin), a diabetes treatment generating annual sales of approximately KRW 100 billion, is set to expire this October. Generic competition in the SGLT-2 inhibitor diabetes drug market is likely to heat up again. Approximately 50 companies have already secured approvals for related generic products. In November, the substance patent of Pfizer's oral autoimmune disease treatment, 'Xeljanz (tofacitinib),' will expire. Thiswill be followed by the first generic launch for a drug in the Janus Kinase (JAK) inhibitors category, with over 60 generics poised to enter a market valued at approximately KRW 15 billion annually. The priority sales period for 'Cetus (pranlukast)' generics, treatment for asthma and allergic rhinitis, will also end in October. Therefore, generic drugs without this priority sales marketing authorization are expected to enter the market. 'Generic Jardiance' expected to launch in October...variable is the 'unlisted patent' According to the Ministry of Food and Drug Safety (MFDS), on June 30, Boehringer Ingelheim's Jardiance substance patent is set to expire on October 23. The pharmaceutical industry anticipates a mass launch of generics around the time of the patent expiration. Generic companies have successfully circumvented the remaining listed patents in their patent disputes with Boehringer Ingelheim, excluding the substance patent. Currently, 49 companies have received product approvals for 333 empagliflozin-based single and fixed-dose combination products. Specifically, 48 generic companies have obtained approval for 100 Jardiance generic products, while 36 companies have approval for 213 Jardiance Duo (empagliflozin + metformin) generic products. For Esglito (empagliflozin + linagliptin) generics, five companies have obtained approval for 10 products. Products with active ingredients or combinations not found in the original drug are also ready for launch. Chong Kun Dang received approval for four follow-on products combining empagliflozin + sitagliptin, and and Daewon Pharm received approval for six follow-on products combining empagliflozin + sitagliptin + metformin. Generic competition is expected to heat up again in the diabetes drug market. Generic companies have signaled their entry into the empagliflozin market, which has grown to over KRW 100 billion annually. According to pharmaceutical market research firm UBIST, the combined prescription sales of Jardiance and Jardiance Duo last year reached KRW 108.2 billion, an 11% increase from KRW 97.5 billion in 2023. The withdrawal of Forxiga (dapagliflozin) from the market is analyzed to have significantly contributed to Jardiance's upward trend. AstraZeneca decided to withdraw Forxiga from the Korean market at the end of 2023. However, they retained Xigduo, a combination product containing metformin. Forxiga was supplied domestically until the middle of last year. Jardiance took over the KRW 50 billion market gap left by Forxiga. Jardiance's prescription sales increased by 22% over the year, from KRW 15 billion in Q4 2023 to KRW 18.2 billion in Q4 2024. In Q1 this year, it further increased to KRW 19 billion. Additionally, Jardiance Duo, the metformin combination product, shows a steady upward trend. In Q1 this year, it recorded KRW 10.8 billion in prescription sales, representing a 10% year-over-year (YoY) increase. Given that annual prescription sales of Jardiance and Jardiance Duo totaled KRW 100 billion, challenges from generics are expected to be intense. The variable lies in 'unlisted patents.' Boehringer Ingelheim Korea holds over nine patents related to Jardiance, Jardiance Duo, and Esglito that are registered with the Korean Intellectual Property Office (KIPO) but not listed in the MFDS patent registry. While their absence from the listed patents does not affect product approval, there is a risk of patent infringement upon launch. Boehringer Ingelheim Korea plans to enforce its patent rights vigorously. In the case of Trajenta's (linagliptin) substance patent expiration last year, Boehringer Ingelheim Korea proactively warned generic companies about patent infringement by sending certified mail. In response, generic companies are challenging the undisclosed Jardiance patents through avoidance or invalidation efforts. The industry anticipates that a significant number of generic companies will proceed with their generic launches after the substance patent expires. This is because many generic companies launched products with similar patent infringement risks when Trajenta's substance patent expired. On November 22, Pfizer's Xeljanz patent will expire. All eyes are on this because it is the patent expiration of one of the oral autoimmune disease treatments. Fifty-five companies have received product approvals for 63 generic items. These companies are set to challenge the KRW 14.4 billion Xeljanz market. Furthermore, competition with other original JAK inhibitor drugs, including Xeljanz, is also expected. JAK inhibitors, such as Xeljanz, Rinvoq (upadacitinib), Olumiant (baricitinib), and Jyseleca (filgotinib), have been launched in Korea. Prescription sales for these drugs last year totaled KRW 62.2 billion, a 56% increase YoY. Priority sales period for 'Cetus' with market worth KRW 46 billion annually, expires in October…more generics expected to enter Product photo of CetusThe priority sales period for generic Cetus, an asthma and allergic rhinitis treatment with annual sales of KRW 46 billion, will expire on October 1. This will allow the launch of generics that did not receive priority sales rights. Previously, generic companies filed a passive rights scope confirmation trial against Sam-A Pharm's Cetus formulation patent. Subsequently, in October last year, they received a favorable judgment from the Intellectual Trial and Appeal Board. This judgment was confirmed without an appeal from Sam-A Pharm. Based on their first-instance victory, the challenging companies have successively obtained generic approvals. Among these, Dasan Pharmaceutical's 'Prituss,' Dongkook Pharmaceutical's 'Pranpid,' Green Cross's 'Neofran,' and Daewoong Bio's 'Cituone' received priority sales rights. The priority sales period runs from November last year to October this year. Generic companies that did not receive priority sales rights during this period can launch their products after October. The launch of follow-on generics from Hanwha Pharmaceutical and Dongkoo Bio is expected. These companies have previously won patent disputes against Sam-A Pharm. Hanwha Pharmaceutical has even obtained generic product approval under the name 'Citurien'. Despite Cetus's annual prescription sales of KRW 46 billion, and the fact that earlier launched Cetus generics have not firmly established themselves in the asthma and allergic rhinitis treatment market, analysis suggests that follow-on drugs launching about a year later will still have significant competitiveness. In Q1 this year, Cetus generics recorded only KRW 600 million in prescription sales, representing about a 5% market share in the overall pranlukast market. In contrast, the original Cetus saw a slight increase to KRW 11.4 billion in Q1 this year compared to the same period last year.
- Company
- Asthma drug 'Trelegy 200 Ellipta Inhaler' becomes available
- by Eo, Yun-Ho Jul 01, 2025 06:03am
- Product photo of The asthma treatment 'Trelegy 200 Ellipta Inhaler' is now available for prescription at tertiary general hospitals. According to industry sources, Trelegy 200 Ellipta Inhaler (fluticasone furoate·umeclidinium·vilanterol) has passed the drug committees (DC) of Korea's 'Big 5' tertiary general hospitals, including Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary's Hospital, Asan Medical Center in Seoul, and Sinchon Severance Hospital. Trelegy 200 Ellipta Inhaler has been included in the insurance reimbursement list since March last year. It can be prescribed to patients with 'severe asthma that is not properly managed with combination therapy containing intermediate-dose or high-dose inhaler corticosteroid or long-acting beta2-agonists.' The efficacy of Trelegy 200 Ellipta was demonstrated through the Phase 3 CAPTAIN study, which compared Trelegy Ellipta and dual combination therapy FF/VI (fluticasone furoate/ vilanterol) in 2,436 adult asthma patients aged 18 and above whose asthma cannot be controlled despite treatment with dual combination therapy ICS/LABA. As the primary endpoint, each cohort’s Forced Expiratory Volume in 1 second (FEV1) changes at Week 24 were evaluated. The results demonstrated statistical significance as Trelegy Ellipta-treated cohort showed 110 mL more FEV1 compared with FF/VI-treated cohort. The safety profile of Trelegy Ellipta for asthma treatment in this study was consistent with the known profile of the individual drug components and their combinations. The most common side effects were nasopharyngitis v(13-15%), headache (5-9%), and upper respiratory tract infection (3-6%). Severe adverse responses occurred similar in all treatment groups. Dr. Jae-Won Jeong, a Professor at Inje University Ilsan Paik Hospital, explained, "Asthma is a chronic respiratory disease that requires lifetime management. It's important for patients who experience worsening of asthma to receive proper treatments." Additionally, Dr. Jeong said, "Major guidelines also recommend using triple combination therapy, with LAMA added to the ICS/LABA, as the optimal treatment option before the start of oral corticosteroid treatment for patients with severe asthma whose symptoms are not controlled with double therapy of ICS/LABA." Meanwhile, Trelegy 200 Ellipta is a once-daily single-inhaler triple combination therapy, approved in September 2022 as a maintenance therapy for Korean adult patients. It obtained approval in 10 countries, including the United States, Japan, Canada, and Taiwan, and is being prescribed globally. Additionally, Trelegy 100 Ellipta, approved for the first time in May 2018 in South Korea, is a once-daily single inhaler triple combination therapy for COPD and asthma. It was approved in June 2021 for national health insurance reimbursement as the COPD maintenance therapy. In March 2024, it expanded the scope of reimbursement for asthma indication.
- Company
- Dong-A ST-Ipsen to comarket prostate cancer drug Diphereline
- by Son, Hyung Min Jul 01, 2025 06:02am
- On the 30th, Dong-A ST signed a joint sales agreement with Ipsen Korea for the premature puberty and prostate cancer treatment ‘Diphereline.’ Dong-A ST (CEO Jae-Hoon Jeong) announced on the 30th that it has signed a joint sales agreement with Ipsen Korea (CEO Mi-sun Yang) for the premature puberty and prostate cancer treatment ‘Diphereline.’ Diphereline is a GnRH (gonadotropin-releasing hormone) agonist developed by the multinational biopharmaceutical company Ipsen, used to treat central precocious puberty and prostate cancer. The agreement ceremony was attended by Dong-A ST CEO Jae-hoon Jeong, Ipsen Korea CEO Mi-sun Yang, along with other officials from both companies, who reaffirmed their commitment to strengthening the partnership. Under the agreement, the two companies will jointly conduct domestic promotional and marketing activities for Diphereline starting July 1. Sales targeting general hospitals will be conducted jointly by both companies, while sales targeting clinics and private hospitals will be handled exclusively by Dong-A ST. Dong-A ST owns the growth hormone ‘Growtropin’ and urology drugs ‘Zydena’ and ‘Flivas,’ based on which the company accumulated extensive sales and marketing experience and expertise in the fields of pediatric endocrinology and urology. Ipsen Korea supplies various anticancer drugs in Korea, including ‘Diphereline,’ ‘Cabometyx’ for advanced renal cell carcinoma, hepatocellular carcinoma, and differentiated thyroid cancer, and ‘Somatuline’ for acromegaly and neuroendocrine tumors. Recently, the company has also entered the field of rare biliary diseases and plans to launch ‘Bylvay,’ a new drug for progressive familial biliary disease, in Korea. The two companies plan to actively expand Diphereline’s stance in the domestic market, forging synergy between the companies’ accumulated capabilities and expertise. Mi-sun Yang, CEO of Ipsen Korea, said, “Diphereline is the global standard therapy for children with precocious puberty and prostate cancer patients struggling with the boundaries between masculinity and cancer treatment. We plan to leverage Ipsen's scientific approach and Dong-A ST's expertise and network in the domestic market to bring Diphereline closer to patients in Korea.” Jae-hoon Jeong, CEO of Dong-A ST, remarked, “This collaboration with Ipsen Korea will serve as a crucial opportunity to expand the domestic supply of Diphereline and improve patient access. By combining the strengths and expertise of both companies, we aim to provide domestic patients with more effective treatment options and create synergies in the fields of precocious puberty and anticancer agents.”
- Company
- Metabolic disease drug development landscape evolves
- by Son, Hyung Min Jul 01, 2025 06:02am
- Innovative efforts in the development of new drugs for metabolic disorders such as obesity and diabetes are steadily gaining traction in Korea and abroad. The success of standalone GLP-1 therapies has driven the evolution toward multi-hormonal agents, and recently, major global pharmaceutical companies and biotech firms are accelerating new drug development by targeting not only GLP-1, GIP, and glucagon combinations but also new metabolic hormones such as FGF21 and IGF-1. In essence, a multi-target approach that goes beyond simple weight loss to regulate energy metabolism, insulin sensitivity, and liver fat improvement is becoming a reality. Development of multi-agonists starts with Mounjaro... Biomed announces Phase II trial According to industry sources on the 30th, the US pharmaceutical and biotech company Biomed Industries recently announced the results of its Phase II clinical trial for NA-931, an oral quadruple receptor agonist candidate for obesity and metabolic disorders. The results were presented at the American Diabetes Association's annual diabetic conference (ADA 2025) that was held in Chicago from the 21st to the 24th. NA-931 is a small-molecule drug that acts on four receptors: glucagon-like peptide (GLP-1), gastric inhibitory peptide (GIP), glucagon (GCG), and insulin-like growth hormone type 1 (IGF-1). Recently, research has also been conducted on quadruple receptor agonists that utilize additional metabolic-related hormones such as IGF-1, FGF21, and PYY, in addition to GLP-1, GIP, and GCG. FGF21 is a hormone produced in the liver that is involved in fat oxidation, insulin sensitivity, and temperature regulation. It is gaining attention as a next-generation target due to its ability to induce metabolic improvements similar to those observed with intermittent fasting. PYY is an appetite-suppressing hormone derived from the gut that can contribute to enhancing satiety by interacting with GLP-1. The Phase II clinical trial presented at the conference was a 13-week, randomized, double-blind, placebo-controlled, parallel-group study involving 125 adults with obesity (BMI ≥ 30 kg/m²) or overweight (BMI ≥ 27 kg/m²). The primary endpoints were the safety, tolerability, and weight loss efficacy of the investigational drug NA-931. Results showed that the group administered NA-931 at a dose of 150 mg once daily experienced an average weight reduction of 13.8% compared to baseline, with a statistically significant reduction of 12.4 percentage points compared to the placebo group. The incidence of treatment-emergent adverse events was also favorable. Gastrointestinal (GI) adverse events were mostly mild, with nausea and vomiting observed in 7.3% of participants and diarrhea in 6.3%. No muscle mass reduction was reported, and there was no clinically significant difference in the incidence of GI-related adverse events among subjects treated with NA-931 compared with placebo. The researchers explained, “NA-931 appears to induce weight loss while preserving muscle mass and has a lower incidence of side effects than existing drugs. It can be regarded as a viable alternative that can improve the shortcomings of existing GLP-1 class treatments.” Development of quadruple receptor agonists continue… to treat MASH in addition to obesity Clinical studies of quadruple receptor agonists targeting indications other than obesity are also actively underway. Among domestic companies, Wonjin Biotechnology has entered the clinical trial phase for a quadruple receptor agonist. Wonjin Biotechnology received approval in the US last year for the Investigational New Drug (IND) application for its candidate compound ‘OGB21502’ for the treatment of metabolic dysfunction-associated steatohepatitis (MASH) and fibrosis. OGB21502 is an innovative drug candidate that combines GLP-1, glucagon, FGF21, and an IL-1 receptor agonist (IL-1RA), an immune modulator, to simultaneously regulate metabolic disorders and chronic inflammation. OGB21502 induces appetite suppression and energy metabolism activation through GLP-1 and glucagon receptors while promoting lipid metabolism via FGF21 action. Additionally, by blocking IL-1 signaling involved in inflammation, the company expects it to demonstrate a multi-layered therapeutic effect by preventing inflammatory exacerbation of metabolic diseases. According to Wonjin Biotechnology, there is growing evidence that therapies blocking IL-1 signaling are effective in alleviating liver fibrosis. In fact, anakinra, a recombinant IL-1 receptor antagonist, has been shown in multiple studies to inhibit NLRP3 activation and reduce hepatic stellate cell (HSC) activity and blood-based fibrosis markers. In preclinical studies, OGB21502 demonstrated a reduction in lipid markers, including cholesterol and triglycerides, as well as the expression of fibrosis-related markers, compared to semaglutide and FGF21 analogs in obese mouse models. In a mouse model of non-alcoholic fatty liver disease (NAFLD), liver tissue staining analysis revealed that OGB21502 significantly reduced the non-alcoholic fatty liver disease activity score (NAS) and improved steatosis and lobular inflammation.
- Company
- AstraZeneca Korea launches ‘Lung Health Checkbus’ campaign
- by Whang, byung-woo Jul 01, 2025 06:00am
- Launch ceremony for AstraZeneca Korea AstraZeneca Korea announced on the 30th that it held a ceremony for the launch of its ‘Lung Health Checkbus’ campaign at the COEX Square in Seoul on the 27th. The campaign aims to help people detect lung nodules that they are not aware of at an early stage by operating buses equipped with AI-based chest X-ray imaging nationwide. AstraZeneca Korea, which has been making various efforts to create a world where lung cancer is no longer a cause of death, has partnered with the Korea National Tuberculosis Association and medical AI solution company Maihub to operate the ‘Lung Health Checkbus’ nationwide. Lung cancer is the leading cause of cancer-related deaths in Korea, according to 2023 data. Over the 5-year period from 2018 to 2022, the relative survival rate was 79.8% when the cancer was detected at an early stage, but the rate dropped sharply to 12.9% when the cancer had spread to distant parts of the body. However, it has been reported that more than 40% of patients are diagnosed with cancer at an advanced stage of metastasis, emphasizing the importance of regular screening. Low-dose chest CT is an effective screening method that can accurately detect lung cancer and reduce mortality, and AI-equipped chest X-rays are more effective than conventional X-rays in detecting lung nodules. According to a study comparing the lung nodule detection rates of AI-equipped chest X-rays and conventional X-rays at a single institution in Korea, the lung nodule detection rate in the AI group was more than twice that of the non-AI group. Se-Hwan Chon, General Manager of AstraZeneca Korea, said, “Lung cancer can affect anyone, so lung cancer screening is necessary even for non-smokers. In particular, lung cancer has a significantly higher survival rate when detected early, so it is important to detect it early through regular screening.” On the same day, AstraZeneca Korea signed a three-party memorandum of understanding (MOU) with the Korea National Tuberculosis Association and Maihub to ensure the successful operation of this campaign. Many citizens had chest X-rays taken at the lung health check bus set up on-site and received reports analyzed by artificial intelligence (AI) to check their lung health status for themselves. Min-Seok Shin, Chair of KNTA, said, “We find it meaningful that KNTA can expand its social responsibility beyond respiratory diseases to a broader range of diseases through the campaign. We plan to continue supporting customized health management programs to ensure that everyone, including medically vulnerable groups, can easily check their lung health. Hyuck Yang, CEO of Maihub, said, “This campaign is a meaningful example of public and private sectors coming together to create an AI-based chest X-ray examination environment that is easily accessible to everyone, going beyond simply providing technology. Maihub will contribute to the early detection of lung nodules through AI-based image interpretation solutions and strive to build a digital healthcare ecosystem.” Meanwhile, AstraZeneca Korea is a member of the Lung Ambition Alliance (LAA), a global non-profit collaboration organization, and is conducting various lung cancer awareness improvement activities to create a future where lung cancer is no longer a cause of death.
- Company
- New K-drugs for metabolic diseases make international debut
- by Son, Hyung Min Jun 30, 2025 06:07am
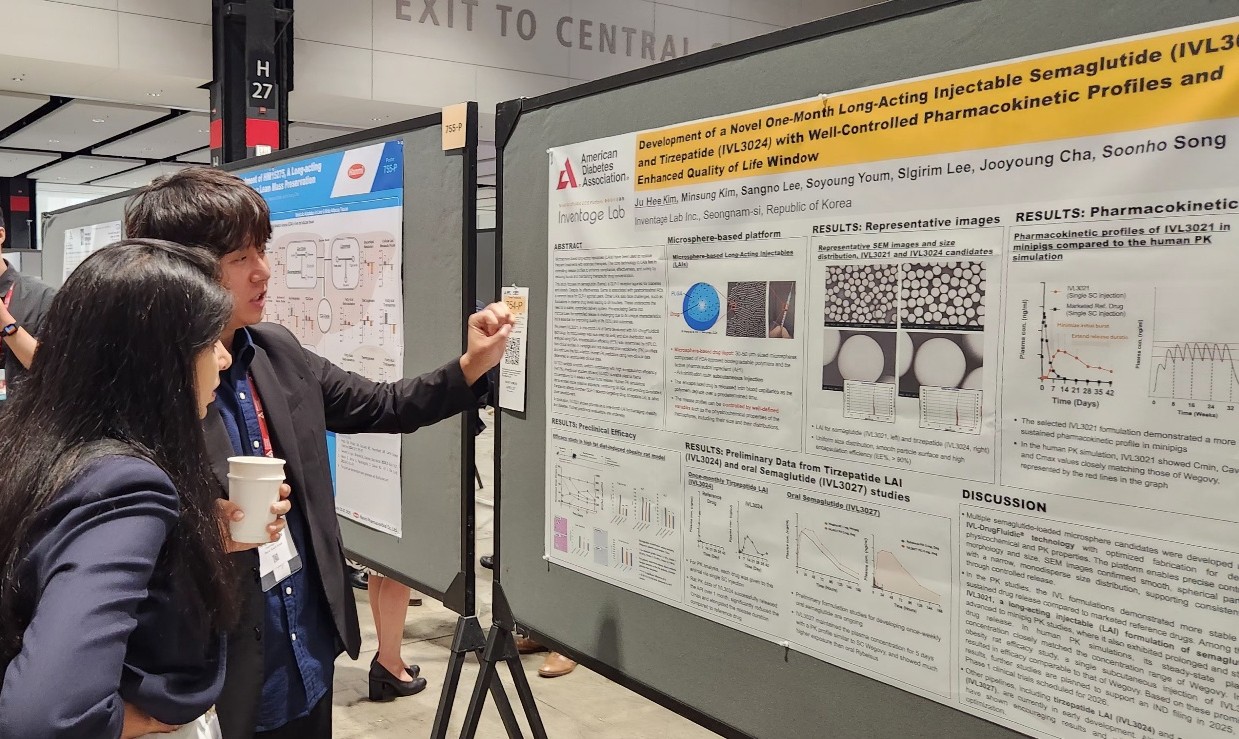
- Major Korean pharmaceutical and biotechnology companies have signaled their full-scale entry into global clinical trials, presenting new drug development results at overseas conferences. The companies presented their achievements in developing new drugs for various metabolic diseases, including obesity, type 2 diabetes, and metabolic dysfunction-associated steatohepatitis (MASH). Although most of the data disclosed is focused on early clinical or preclinical trials, the companies are attempting to diversify their mechanisms of action with triple agonists and oral small molecule formulations. Hanmi reveals triple agonist data... Oral and long-acting injectable formulations are also under development 30According to industry sources, the American Diabetes Association Diabetes Conference was held in Chicago, USA, from the 21st to the 24th. At the conference, various domestic companies, including Hanmi Pharmaceutical, Ildong Pharmaceutical, Dong-A ST, and Inventage Lab, revealed the clinical results of their novel drug candidates. Obesity drugs are rapidly emerging as a global R&D trend. With Novo Nordisk and Lilly's GLP-1-based obesity treatments becoming global blockbuster drugs, latecomers are also intent on developing their versions. Major domestic companies are conducting clinical studies on GLP-1 agonists in various areas, including obesity and MASH with drugs that have different methods of administration or focus on the quality of weight loss effects. Hanmi Pharmaceutical announced the results of its Phase I clinical trials and preclinical data for HM15275, a GLP-1 class triple agonist, and HM17321, a UCN2-based candidate substance, at the conference. First, Hanmi Pharmaceutical announced the results of the Phase I clinical trial for HM15275. HM15275 is a new obesity drug candidate that acts on glucagon-like peptide (GLP-1), gastric inhibitory polypeptide (GIP), and glucagon (GCG). No new drug with this mechanism targeting all three has been commercialized to date. The Phase I clinical trial was conducted on 74 healthy and obese adults. HM15275 was administered subcutaneously once a week for four weeks, followed by an evaluation of the candidate drug’s safety, tolerability, pharmacokinetics, and pharmacodynamics. The results confirmed HM15275’s tolerability and safety. Specifically, the average weight loss rate at day 29 in the highest dose group was 4.8%. At day 43, the maximum weight loss rate was 10.6%. Preclinical data for HM15275 demonstrated greater weight loss efficacy compared to semaglutide (brand name Wegovy) and tirzepatide (Zepbound), which are currently marketed as obesity treatments. In animal models, switching from tirzepatide to HM15275 resulted in additional weight loss effects. Hanmi Pharmaceutical also announced the results of preclinical studies on HM17321, a new drug candidate that simultaneously targets weight loss and muscle gain. HM17321 is a UCN-2 analogue that selectively targets the CRF2 (corticotropin-releasing factor 2) receptor rather than GLP-1 or other incretin receptors. It is being developed as an innovative first-in-class drug that selectively reduces fat while increasing muscle mass. HM17321 demonstrated weight loss effects and improved body composition in both mouse models and non-human primate models. Yunovia, a subsidiary of Ildong Pharmaceutical Group specializing in new drug research and development, is conducting Phase I clinical trials for ID110521156, a GLP-1 receptor agonist class new drug candidate targeting metabolic disorders such as diabetes and obesity. ID110521156 is a low-molecular-weight compound-based drug, and the company aims to develop it as an oral synthetic new drug for diabetes and obesity with distinct advantages such as superior productivity and excellent ease of use over existing representative treatments like peptide injections. Previously, Yunovia confirmed the efficacy of insulin secretion and blood glucose control through preclinical efficacy and toxicity evaluations. It also demonstrated superior safety compared to competing drugs in the same class and confirmed the drug's characteristics in a recently completed Phase 1 single-ascending dose (SAD) trial. According to the study poster presented at the conference, in the single-dose escalation trial, ID110521156 demonstrated good tolerability with fewer gastrointestinal side effects across the entire effective dose range, unlike existing GLP-1 class drugs. Inventage Lab also introduced preclinical data for its 1-month long-acting injectable formulations ‘IVL3021’ and ‘IVL3024’ based on semaglutide and tirzepatide, as well as its oral semaglutide formulation ‘IVL3027.’ After GLP-1 class obesity treatments such as Saxenda, Wegovy, and Zepbound emerged as global blockbuster drugs, the pharmaceutical industry has been actively pursuing formulation changes. The existing drug Saxenda requires a once-daily injection, while Wegovy and Zepbound require weekly injections. Oral formulations or long-acting injectables are expected to gain a competitive edge in terms of convenience of administration if commercialized. According to the company's preliminary preclinical results, IVL3021 showed stable drug release in the blood over a one-month period. Also, the long-acting injectable suppressed initial over-release and maintained stable drug release. IVL3027 demonstrated high bioavailability compared to existing oral formulations and sustained drug release over a one-week period. Poster presentation by Inventage Lab (Source=Inventage Lab). MASH clinical trial results also announced Dong-A ST and its subsidiary MetaVia announced the results of non-clinical studies on DA-1241, which is being developed as a treatment for MASH, and combination therapy that uses efruxifermin, a fibroblast growth factor (FGF21) analog. Metabolic dysfunction-related fatty liver disease was previously referred to as non-alcoholic steatohepatitis (NASH), but overseas academic societies such as the American Association for the Study of Liver Diseases have decided to change the name to metabolic dysfunction-associated steatohepatitis (MASH). To date, Madrigal's rezdiffra is the only new drug for MASH that has cleared regulatory hurdles. The U.S. Food and Drug Administration (FDA) approved rezdiffra in March last year for the treatment of adult patients with non-cirrhotic MASH in combination with diet and exercise. Rezdiffra is a selective thyroid hormone receptor (THR)-β agonist designed to target the core pathophysiological mechanisms of MASH within the liver. The pharmaceutical industry is also developing new drugs for MASH that target not only THR-β but also GLP-1 and FGF21, which influence lipid metabolism. DA-1241 is a synthetic new drug that activates GPR119. Preclinical results have confirmed that DA-1241 improves blood sugar and lipid levels and directly acts on the liver to improve inflammation and fibrosis, making it a promising candidate for MASH treatment. A Phase 2a clinical trial targeting patients with estimated MASH was completed in December last year. Efruxifermin is a recombinant protein designed based on FGF21 (Fibroblast Growth Factor 21), a hormone secreted by the liver. FGF21 is involved in energy consumption and the regulation of glucose and lipid metabolism in the body and is used as a target for the development of treatments for MASH, obesity, diabetes, and other conditions. According to the study results presented at the conference, in the DA-1241+efruxifermin combination therapy group, approximately 94% of subjects showed an improvement of 2 points or more in NAS (Non-Alcoholic Steatohepatitis Activity Score) compared to baseline. Additionally, the DA-1241+efruxifermin group showed a significant reduction in liver fibrosis area compared to the MASH control group that did not receive combination therapy, and in some individuals, a decrease in fibrosis stage was observed compared to pre-treatment levels. Dong-A ST is currently conducting clinical studies on the combination therapy of DA-1241 with semaglutide, a GLP-1 agonist, in addition to a Phase II clinical trial on DA-1241 as monotherapy.
- Company
- Adempas may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jun 30, 2025 06:06am
- Adempas, a new treatment for pulmonary arterial hypertension that has emerged after a long wait, is now available for prescription at general hospitals in Korea. According to industry sources, Bayer Korea's Adempas (riociguat) has been approved by the Drug Committee (DC) of tertiary hospitals in Korea, including Samsung Medical Center and Seoul National University Hospital. As it has been listed for reimbursement since this month (June), the number of medical institutions that can prescribe it is expected to continue to increase. Adempas was approved in Korea as an orphan drug in June 2014 and is available in 5 dosage forms. It is indicated for: ▲Improvement of exercise capacity in adult patients with chronic thromboembolic pulmonary hypertension (CTEPH, WHO Group 4) who are unable to undergo surgery or who have persistent or recurrent symptoms after surgery ▲Improvement of exercise capacity adult patients with pulmonary arterial hypertension (WHO Group 1) who are classified as having functional class II or III. In particular, it was known as the first new drug for CTEPH. CTEPH is caused by patients who develop chronic pulmonary embolism, which leads to fibrotic stenosis and occlusion, resulting in pathological vascular remodeling and increased resistance in the pulmonary artery. CTEPH is a chronic disease that causes progressive dyspnea and right heart dysfunction, which weakens the heart. Symptoms include dyspnea, fatigue, chest pain, dizziness, peripheral edema, cough, and hemoptysis, which reduces the patient’s quality of life. Ultimately, it can progress to heart, kidney, and liver failure, which can lead to death. Meanwhile, Adempas is a stimulator of soluble guanylate cyclase (sGC), an enzyme found in the heart and lungs, and its efficacy has been confirmed in two Phase III clinical trials in patients with chronic thromboembolic pulmonary hypertension. Results showed improvement in exercise capacity, which was the primary endpoint, and good tolerability. No unexpected adverse reactions were reported. In the CHEST-1 study, when comparing the 6-minute walking test (6MWT) results after 16 weeks with the baseline, results showed that the group of patients who received riociguat showed statistically significant improvement compared to the group of patients who received placebo. In the PATENT-1 study, the change in the 6MWT score after 12 weeks of treatment, showed statistically significant improvement in the riociguat arm compared to placebo, meeting the primary endpoint.
- Company
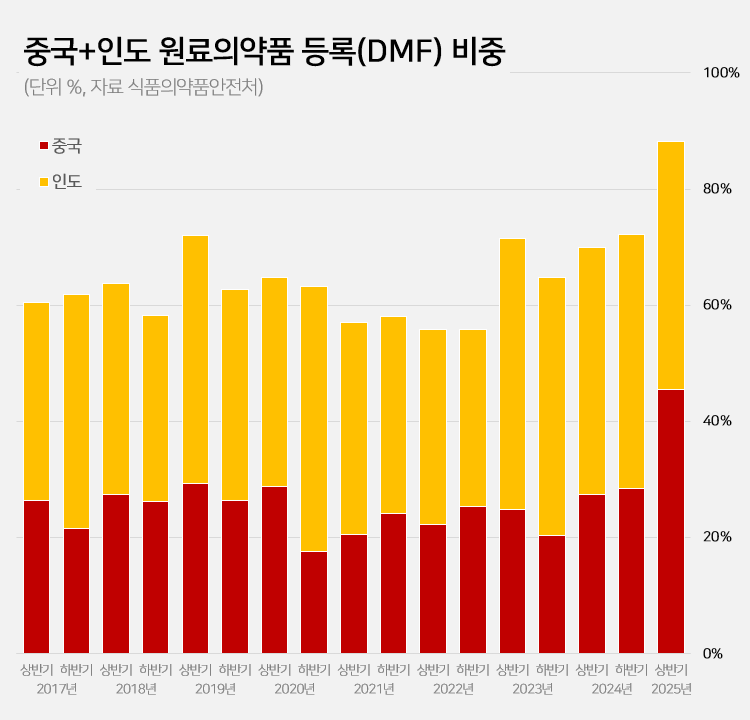
- 88% registered APIs imported from China or India
- by Kim, Jin-Gu Jun 27, 2025 06:04am
- Amid a surge in the number of drug master file registrations in the first half of this year, the share of raw materials from China and India rose to 88.2%. This is a sharp increase compared to the average of 62.1% share the two countries had during the past 5 years. This is attributed to the large number of previously delayed raw material drug registrations that had been made upon the relaxation of DMF regulations, as well as the domestic pharmaceutical and bio industry's increased use of raw materials from China and India to reduce costs. 606 DMF registration from China and India source materials made in the first half of this year... accounts for 88%, which is the highest-ever share According to the Ministry of Food and Drug Safety on the 26th, 687 DMF registrations were made in the first half of this year. Among them, 313 raw materials were from China and 293 were from India. The two countries combined accounted for 606 cases or 88.2% of the total DMF registrations. This is the highest proportion ever recorded for a half-year period. Until last year, the proportion of Chinese and Indian raw materials had never exceeded 75%. The average proportion of Chinese and Indian DMF imports over the past 5 years was 62.1%, which is more than 26 percentage points higher than in the first half of this year. The proportion of Chinese and Indian imports in the DMF has increased rapidly over the past 3 years. After steadily declining since the first half of 2019, the share of Chinese and Indian DMF rose to 55.8% in the first half of 2022 and then began to increase. It reached 69.9% in the first half of last year and 72.2% in the second half. In the first half of this year, it soared to nearly 90%. Pharmaceutical industry's dependence on Chinese and Indian raw materials deepens amid cost pressures The rapid increase in Chinese and Indian DMF is attributed to cost reduction pressures in the pharmaceutical and bio industry. China and India are representative “low-cost mass production bases” in the global raw material drug market. Following the global economic downturn after the pandemic, the pharmaceutical and bio industry in general faced a decline in profitability. As a result, attempts to reduce costs were made, which led to an increase in the use of Chinese and Indian DMF. An industry insider explained, “Domestic pharmaceutical and biotechnology companies are feeling a significant burden from manufacturing costs due to high exchange rates, rising labor costs, and declining profitability. Chinese and Indian raw materials are sometimes almost half the price of domestically produced materials, leading to increased use of imported materials.” Additionally, the relaxation of DMF regulations has further increased the use of Chinese and Indian raw materials. The government eased DMF requirements earlier this year by replacing on-site GMP inspections with the submission of GMP certificates and reducing the administrative processing period from 120 days to 20 days. As a result, imported raw materials for which registration had been delayed were registered en masse. In particular, it is analyzed that the abolition of on-site inspections has led to a significant increase in Chinese and Indian raw materials. In the past, inspections in these two countries were physically difficult, and administrative procedures complex, often causing delays in registration. This year, however, registration became possible with only a GMP certificate, significantly lowering barriers, and leading to a significant increase in the registration of Chinese and Indian raw materials. Domestic raw material share in DMF only 5%, raising concerns about increased dependence on Chinese and Indian products On the other hand, the share of domestically produced raw materials registered in the DMF has decreased significantly. In the first half of this year, the share of domestically produced raw materials registered was only 4.9% (34 cases). This is less than half of the 12.6% recorded in the second half of last year. The share of DMF registrations of raw materials from Europe and Asia also decreased sharply. The share of European raw materials decreased by more than 10 percentage points from 14.5% in the first half of last year to 4.4% in the first half of this year. The number also decreased from 37 to 30. The share of DMF registrations of raw materials from Asian countries other than China and India also decreased from 3.9% to 1.5%. Concerns have been raised that the dependence on raw materials from China and India may become excessively high. If this trend intensifies, it could pose a threat to the stability of domestic drug supply. In fact, during the early stages of the COVID-19 pandemic, export restrictions imposed by China and India directly impacted domestic drug production. A pharmaceutical industry insider stated, “If raw material production becomes overly concentrated in specific countries, it becomes vulnerable to external factors such as export restrictions, logistics disruptions, and sharp exchange rate fluctuations. In the long term, policies are needed to strengthen domestic raw material production capabilities and provide various incentives for the use of domestically produced raw materials.”