- LOGIN
- MemberShip
- 2025-12-20 17:31:06
- 88% registered APIs imported from China or India
- by Kim, Jin-Gu | translator Alice Kang | 2025-06-27 06:04:04
Amid a surge in the number of drug master file registrations in the first half of this year, the share of raw materials from China and India rose to 88.2%.
This is a sharp increase compared to the average of 62.1% share the two countries had during the past 5 years.
This is attributed to the large number of previously delayed raw material drug registrations that had been made upon the relaxation of DMF regulations, as well as the domestic pharmaceutical and bio industry's increased use of raw materials from China and India to reduce costs.
606 DMF registration from China and India source materials made in the first half of this year...
accounts for 88%, which is the highest-ever share According to the Ministry of Food and Drug Safety on the 26th, 687 DMF registrations were made in the first half of this year.
Among them, 313 raw materials were from China and 293 were from India.
The two countries combined accounted for 606 cases or 88.2% of the total DMF registrations.
This is the highest proportion ever recorded for a half-year period.
Until last year, the proportion of Chinese and Indian raw materials had never exceeded 75%.
The average proportion of Chinese and Indian DMF imports over the past 5 years was 62.1%, which is more than 26 percentage points higher than in the first half of this year.
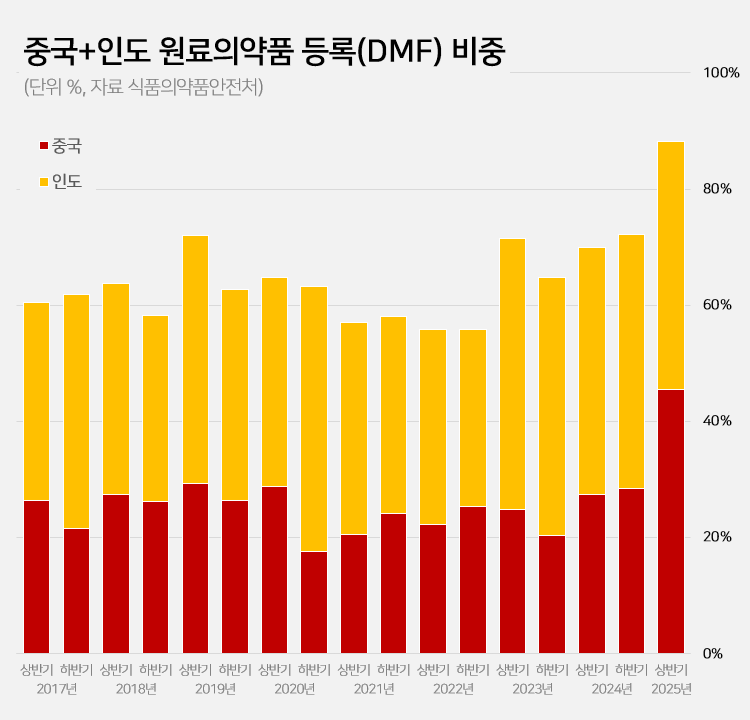
After steadily declining since the first half of 2019, the share of Chinese and Indian DMF rose to 55.8% in the first half of 2022 and then began to increase.
It reached 69.9% in the first half of last year and 72.2% in the second half.
In the first half of this year, it soared to nearly 90%.
Pharmaceutical industry's dependence on Chinese and Indian raw materials deepens amid cost pressures The rapid increase in Chinese and Indian DMF is attributed to cost reduction pressures in the pharmaceutical and bio industry.
China and India are representative “low-cost mass production bases” in the global raw material drug market.
Following the global economic downturn after the pandemic, the pharmaceutical and bio industry in general faced a decline in profitability.
As a result, attempts to reduce costs were made, which led to an increase in the use of Chinese and Indian DMF.
An industry insider explained, “Domestic pharmaceutical and biotechnology companies are feeling a significant burden from manufacturing costs due to high exchange rates, rising labor costs, and declining profitability.
Chinese and Indian raw materials are sometimes almost half the price of domestically produced materials, leading to increased use of imported materials.” Additionally, the relaxation of DMF regulations has further increased the use of Chinese and Indian raw materials.
The government eased DMF requirements earlier this year by replacing on-site GMP inspections with the submission of GMP certificates and reducing the administrative processing period from 120 days to 20 days.
As a result, imported raw materials for which registration had been delayed were registered en masse.
In particular, it is analyzed that the abolition of on-site inspections has led to a significant increase in Chinese and Indian raw materials.
In the past, inspections in these two countries were physically difficult, and administrative procedures complex, often causing delays in registration.
This year, however, registration became possible with only a GMP certificate, significantly lowering barriers, and leading to a significant increase in the registration of Chinese and Indian raw materials.
Domestic raw material share in DMF only 5%, raising concerns about increased dependence on Chinese and Indian products On the other hand, the share of domestically produced raw materials registered in the DMF has decreased significantly.
In the first half of this year, the share of domestically produced raw materials registered was only 4.9% (34 cases).
This is less than half of the 12.6% recorded in the second half of last year.
The share of DMF registrations of raw materials from Europe and Asia also decreased sharply.
The share of European raw materials decreased by more than 10 percentage points from 14.5% in the first half of last year to 4.4% in the first half of this year.
The number also decreased from 37 to 30.
The share of DMF registrations of raw materials from Asian countries other than China and India also decreased from 3.9% to 1.5%.
Concerns have been raised that the dependence on raw materials from China and India may become excessively high.
If this trend intensifies, it could pose a threat to the stability of domestic drug supply.
In fact, during the early stages of the COVID-19 pandemic, export restrictions imposed by China and India directly impacted domestic drug production.
A pharmaceutical industry insider stated, “If raw material production becomes overly concentrated in specific countries, it becomes vulnerable to external factors such as export restrictions, logistics disruptions, and sharp exchange rate fluctuations.
In the long term, policies are needed to strengthen domestic raw material production capabilities and provide various incentives for the use of domestically produced raw materials.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










