- LOGIN
- MemberShip
- 2025-12-24 05:38:50
- Company
- Severe asthma treatment Fasenra seeks reimb in KOR
- by Eo, Yun-Ho Dec 19, 2022 04:34am
- Another severe asthma treatment is attempting insurance reimbursement in Korea. According to industry sources, AstraZeneca is undergoing the reimbursement process for its monoclonal antibody for asthma, Fasenra (benralizumab). Other biological drugs including GSK’s ‘Nucala (mepolizumab),’ and Teva-Handok’s ‘Cinqair (reslizumab)’ have also been reattempting reimbursement in Korea as well. Fasenra was approved in Korea in 2019 as an add-on maintenance treatment in adults with severe eosinophilic asthma inadequately controlled with existing treatment options. The drug is administered once every 4 weeks for the first 3 months, then every 8 weeks thereafter. One of the main purposes of asthma management is to reduce the risk of asthma exacerbations. Fasenra directly binds to the alpha subunit of the interleukin-5 receptor (IL-5Rα) to induce apoptosis. The drug demonstrated efficacy in reducing asthma exacerbation and improving lung function. In the global SIROCCO trial that was conducted to evaluate the effect of Fasenra in treating asthma exacerbations in 1,205 asthma patients including 122 Korean patients, the annual rate of clinically significant asthma exacerbations was reduced by 45% in patients who were treated with Fasenra once every 4 weeks, and by 51% in patients who were treated with Fasenra once every 8 weeks. Also, in the CALIMA trial, Fasenra demonstrated a significant reduction in asthma exacerbations compared to the placebo. In the trial, the annual rate of asthma exacerbations was reduced by 36% in patients who were treated with Fasenra once every 4 weeks, and by 28% in patients who were treated with Fasenra once every 8 weeks compared with placebo. In both trials, a change from baseline in mean FEV1 (forced expiratory volume in 1 second) was observed using Fasenra, and a consistent improvement compared with placebo. The long-term efficacy and safety of Fasenra in severe eosinophilic asthma were evaluated through the BORA trial, a long-term extension trial on 1,926 patients that participated in the SIROCCO and CALIMA trials. In terms of safety, Fasenra showed no significant difference compared to the placebo, and 72% of the patients that were administered Fasenra did not experience asthma exacerbations and were able to maintain their FEV1. Surprisingly enough, asthma is associated with a high mortality rate. The mortality rate of hospitalized patients due to asthma exacerbation is nearly 1/3, and the expenses spent by patients that require emergency treatment or hospitalization due to asthma exacerbation account for more than 80% of the total asthma-related cost, representing a high burden of social cost. Eosinophilic inflammations are found in 50% of all asthma patients and may lead to reduced lung function or asthma exacerbations. In particular, eosinophilic asthma patients that show increased blood eosinophil levels despite appropriate ICS therapy are inadequately controlled with existing treatment therapy including ICS and LABA therapy. Therefore, the quality of life of these patients is threatened by the pain caused by the symptoms and the frequent exacerbation of the disease. Meanwhile, AstraZeneca sought to expand its indication to uncontrolled Fasenra to chronic rhinosinusitis with nasal polyp (CRSwNP), but FDA turned down the final approval and requested additional data.
- Company
- Asthma treatment Cinqair attempts reimb again in Korea
- by Eo, Yun-Ho Dec 15, 2022 05:55am
- The asthma treatment Cinqair for asthma is again attempting reimbursement listing in Korea. According to industry sources, Teva-Handok has recently submitted an application for the reimbursement listing of its interleukin-5 antagonist monoclonal antibody Cinqair (reslizumab). Following Cinqair’s steps, other biological drugs for asthma including GSK Korea’s ‘Nucala (mepolizumab) are also moving to start their reimbursement listing processes as well. As an interleukin-5 antagonist, Cinqair reduces levels of blood eosinophils, a type of white blood cell that is involved in the development of asthma exacerbation. The drug had first been first approved in Korea in 2017 as an add-on maintenance treatment for adult patients with an eosinophilic phenotype of asthma (those who have a blood eosinophil count of at least 400/㎕ before treatment) that were not adequately controlled with existing treatments. The company had applied for its reimbursement being launched without reimbursement in 2018 but failed. Teva-Handok has been jointly marketing Cinqair with Handok Pharmaceutical since its release. Meanwhile, Cinqair’s efficacy had been demonstrated through five placebo-controlled clinical studies that evaluated the safety and efficacy of Cinqair 3mg/kg in 1,028 adult and adolescent asthma patients that were uncontrolled with currently available therapies. In three Phase III clinical trial programs that were conducted on asthma patients with high blood eosinophil counts, Cinqair reduced the frequency of asthma exacerbations by up to 59% and significantly improved lung function, symptoms, and the asthma-related quality of life. Also, Cinqair received attention for releasing the post-hoc analysis results of asthma patients that require Step 4 and Step 5 treatment among all patients that participated in the Phase III trials. Cinqair reduced the clinical degree of asthma exacerbations in patients classified as Step 4 or 5 under the Global Initiative for Asthma guidelines by 53% and 72%, respectively, and increased the level FEV1 (forced expiratory volume in 1 second) by 103ml in Step 4 patients and by 237ml in Step 5 patients, demonstrating that the benefit was found to be greater in Step 5 patients.
- Company
- Verzenio expects to address the unmet demand
- by Eo, Yun-Ho Dec 15, 2022 05:55am
- Professor Son Joo-hyuk Expectations are high for securing early breast cancer indications for Verzenio, a breast cancer treatment with CDK4/600 inhibition mechanism. Lilly Korea held a press conference on the 14th to commemorate the expansion of permission for early breast cancer indications at high risk of recurrence of the anticancer drug Verzenio. Verzenio obtained approval from the Ministry of Food and Drug Safety on the 18th of last month as a treatment used in combination with hormone receptor-positive, human epithelial cell growth factor 2 negative (HR+/HER2-), early breast cancer adult patients at high risk of recurrence of lymph node-positive, and endocrine therapy. Verzenio's approval is meaningful in that the first CDK 4&6 inhibitor for early breast cancer patients at high risk of HR+/HER2-lymph node-positive recurrence was introduced in Korea. Sohn Joo-hyuk, a professor of oncology at Severance Hospital, shared the medical unfulfilled needs of HR+/HER2-lymph node-positive recurrence early breast cancer patients and the clinical value of Verzenio confirmed through the monarch clinical study, which was the background of approval of MFDS. In the first topic of the presentation, "HR+/HER2-High-Risk Early Breast Cancer Patients' Medical Unfulfilled Demand," Professor Sohn said, "Most of the female cancers in Korea are diagnosed early due to screening activation, especially in the standard treatment of HR+/HER2- patients, to prevent recurrence after surgery. He added, "The prognosis of HR+/HER2-early breast cancer is generally known to be good, but high-risk patients are highly likely to recur, making it difficult to expect long-term survival." According to domestic statistics, the survival rate of general early breast cancer patients is more than 90%, which is higher than that of other diseases. However, patients with recurrence risk factors such as lymph node-positive, high tumor grade, large tumor size, and fast cell proliferation are known to have a higher risk of remote recurrence and death than general patients. Studies have shown that if the tumor size exceeds 5cm, the 5-year survival rate of breast cancer patients decreases from 57% (without lymph node metastasis) to 21% (with lymph node metastasis). Professor Son said, "The recurrence of early breast cancer patients after the first treatment is usually one to two years, and more effective postoperative auxiliary treatment is needed to reduce the risk of recurrence and death." However, since the introduction of aromatase inhibitors in the early 2000s, the absence of new treatment options for HR+/HER2-early breast cancer patients has led to a medical non-fulfillment demand." Verzenio's monarch clinical trial is the only study that has confirmed successful results in about 20 years as a combination of endocrine therapy as an adjunct treatment for HR+/HER2-early breast cancer. In monarch cohort 1, which served as the basis for this indication expansion permit, Verzenio+ endocrine therapy confirmed remote recurrence risk reduction results through Distance Relapse-Free Survival (DRF) indicators as well as Invisible Disease-Free Survival (IDFS) indicators compared to endocrine therapy alone.
- Company
- Samsung Epis's Soliris biosimilar bioequivalent to original
- by Dec 14, 2022 05:56am
- Study results that show Samsung Bioepis’s Soliris biosimilar is bioequivalent to the original drug have been presented. On the 13th, the company announced that its biosimilar, SB12 has demonstrated equal effect to its original drug Soliris at the American Society of Hematology (ASH) Annual Meeting that is being held in New Orleans. Soliris is a rare disease treatment used to treat incurable rare diseases like paroxysmal nocturnal hemoglobinuria (PNH) that was developed by the US pharmaceutical company Alexion. SB12 is a biopharmaceutical drug developed using the same active ingredient contained in Soliris, eculizumab. Samsung Bioepis presented statistical analysis results in the form of a poster to add credibility to the clinical results through additional analysis of its Phase III clinical trial. The company had previously demonstrated the bioequivalence of SB12 to Soliris in terms of clinical efficacy and safety through a Phase III trial that had been conducted from 2019 to last year. The primary efficacy endpoint of the SB12 Phase III trial was the level of lactate dehydrogenase (LDH) at Week 26 and the time-adjusted area of under the effect curve (AUEC) of LDH from Week 14 to Week 26 and from Week 40 to Week 52. The red blood cell of patients are destroyed and LDH is released into the blood when a patient develops PNH. LDH is one of the biomarkers that can diagnose this. Elevated LDH levels are usually associated with liver disease and increased fatigue, etc. The trial looked at the time-adjusted area under the effect curve (AUEC) of LDH from Week 14 to Week 26 and from Week 40 to Week 52 to observe whether these levels were similar to the original. Results showed that the LDH level lay within the pre-defined equivalence margin, indicating that the SB12 was bioequivalent to the original drug. Samsung Bioepis conducted a further sensitivity analysis to add reliability to the accuracy of the results of the Phase III trial. Statistical analysis derived the same conclusions as the primary efficacy endpoint results that were observed in the Phase III trial. An official from Samsung Bioepis said, “SB12 contains the core purpose of biosimilars that seek to expand patient access to expensive medicines. We will continue to make efforts to provide more treatment opportunities to patients suffering from rare diseases.”
- Company
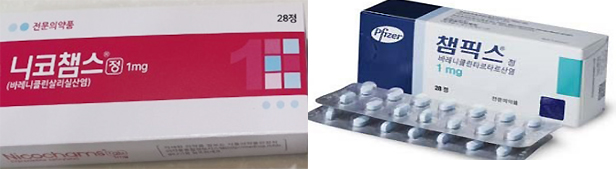
- Champix supply interruption
- by Kim, Jin-Gu Dec 14, 2022 05:56am
- Jeil Pharmaceutical's Nico Chams has dominated the market for anti-smoking treatment aids with Varenicline ingredients. Analysts say that while sales of major products, including Pfizer Champix, were suspended due to the detection of impurities in NNV components in September last year, it succeeded in digging into the gap and raising its influence to the level of market monopoly. ◆ First Pharmaceutical's Nico Chams share is 1% to 87% According to IQVIA, a pharmaceutical market research firm on the 13th, Jeil Pharmaceutical's Nico Chams' cumulative sales in the third quarter were 7.3 billion won. It increased more than nine times from 800 million won accumulated in the third quarter of last year. Sales have risen vertically since a series of impurities were detected in the anti-smoking treatment aid containing Varenicline in September last year. At that time, the Ministry of Food and Drug Safety announced the results of the NNV detection test on Varenicline-based drugs and decided to suspend sales only for products exceeding 185 ng/day. Products with a detection amount of 733 ng/day or more were ordered to be recovered. Among the products that were distributed in Korea, sales of seven products that CTC Bio had commissioned and one product that Pfizer Pharmaceutical Korea imported have been suspended. Nineteen manufacturing number items from three companies produced by CTC Bio have been ordered to be recovered. Pfizer Pharmaceutical Korea voluntarily recalled it. In the case of products produced by Jeil, impurities were detected for 16.70 to 43.28 ng/day, and they survived the recovery as well as the suspension of sales. Nico Chams' share also increased significantly around this point Until the second quarter of 2021, just before the suspension of sales, Nico Chams' market share was only 0.7%. However, the market share jumped to 24% in the third quarter of 2021, right after the suspension of sales. It then increased to 68% in the fourth quarter, and further expanded to 87% in the third quarter of this year. Based on sales in the third quarter, Nico Chams is actually over-exclusive with 2.4 billion won, and the rest is less than 200 million won. ◆Varenicline Market 34% in a Year↓ The lack of smoking cessation business overlaps with the prolonged suspension of sales. With the withdrawal of major products, the size of the anti-smoking treatment aid market for Varenicline ingredients decreased by 34% from 13 billion won in the third quarter of last year to 8.6 billion won in a year. This market has been on the decline for the past four consecutive years. As the government launched an anti-smoking treatment support project in 2015, the market expanded to 65 billion won in 2017 but has since shrunk to 16.2 billion won last year as it has steadily declined. This is the result of a combination of a reduction in drug prices and a decrease in the number of participants in the anti-smoking treatment project. On top of that, it is expected to decrease further this year due to the prolonged suspension of sales of impurity drugs that occurred in the third quarter of last year. Champix, which previously led the market, has still been suspended from sales and distribution as the impurity issue has not been resolved. Champix's sales plunged from 4.6 billion won in the second quarter of last year to 600 million won in the third quarter. Since then, sales of less than 10 million won have been recorded every quarter. Pfizer Pharmaceutical Korea said, "We are waiting for the resumption of supply at the global headquarters," adding, "We do not know the plans for the resumption of sales at this time."
- Company
- 2022 Pharmaceutical Industry Advertising Target Call Members
- by Dec 14, 2022 05:56am
- Dailypharm held the 2022 Pharmaceutical Bio Industry Advertising PR Awards on the 7th. Representative Lee Jung-seok (6th from left) and the winnersDaewon Pharmaceutical's "Coldewon" and Sanofi's "Honey Jam Market for Those Who Can't Sleep" won the grand prize in the advertising and PR categories at the 2022 Korea Pharmaceutical Bio-Industry Advertising and PR Awards. Chong Kun Dang Benfobell won the grand prize in the "Special Award for Pharmacist Selection" category, which was decided by the votes of pharmacists. Dailypharm (CEO Lee Jung-seok) held an award ceremony for the 2022 Korea Pharmaceutical Bio-Industry Advertising and PR Awards at the Diamond Hall of InterContinental Seoul COEX Hotel on the 7th. More than 200 advertising and public relations officials from domestic and foreign pharmaceutical bio companies, including Won Hee-mok and Lee Young-shin, vice chairman of KRPIA, attended the event. At the ceremony, which marks its 10th anniversary this year, a total of 72 works were submitted in five categories, including TV, printing and radio, the Internet, and PR. More than 200 advertising and PR workers from the pharmaceutical bio-industry attended the event The awards were divided into advertising and PR categories. The advertising sector was divided into two categories: TV, Internet, and radio. The grand prize in each field and three excellence awards were selected along with the grand prize. In the PR category, the grand prize, one grand prize, and three excellence awards were honored. A special award was also given to 1,300 pharmacists who voted directly. Daewon Pharmaceutical's Coldewon won the grand prize in this year's advertising category. The award-winning work was awarded a prize of 5 million won along with the trophy. Yoo Seong-kwon, director of Daewon Pharmaceutical, said, "This year seems to have been a year when Coldewon received a lot of attention and took a leap forward. I am grateful that I received that energy and received a good award, he said. "I will try to stand here next year with good advertisements for new items." In the TV category, Shinshin Pharmaceutical's Arex was selected as the best prize, and a prize of 3 million won was given along with the trophy. ▲ Sanofi Allegra, ▲ Ildong Pharmaceutical's Aronamin C Plus Tab, and ▲ Limited's Mag-B Speed Excellence Award. The excellence award was awarded a trophy and a prize of 2 million won. In the Internet/radio category, HKinno.N's "Condition" won the grand prize. ▲Houns Radio Corporate Advertising ▲ Johnson & Johnson Nicorette ▲ JW Pharmaceutical 'Himom bands' were selected for the Excellence Awards. The award-winning film was given a trophy and prize money. In the PR sector, there were many entries that threw meaningful messages, including social contribution activities. This year's PR category was won by Sanofi's campaign to improve awareness of atopic dermatitis diseases, Honey Jam Market for those who can't sleep. The award-winning work was awarded a prize of 5 million won along with the trophy. Kim Hyun-jung, director of Sanofi Specialty Care, said, "We have been campaigning to improve awareness of atopic dermatitis diseases for four years since 2019. This year, the "Honey Jam Market for People Who Can't Sleep" was held to form a consensus on the physical and mental pain of patients. It is an honor that the patient's sincerity has paid off with a good award. "I will continue to make efforts to hold various campaigns for patients," he said. Yoo Seong-kwon, director of Daewon Pharmaceutical (left), and Lee Jung-seok, CEO of Daily Farm, who won the Grand Prize in advertising Dongkuk Pharmaceutical's "pupil earthquake" campaign was selected as the best prize in the PR category. ▲Ginexin, the god of memory (SK Chemical) ▲Growup Bio-Up Campaign (Amgen Korea) ▲YYPharm, including blood donation of love, won the Excellence Award. In the special prize category selected by online voting of 1,300 pharmacists, Chong Kun Dang was selected as the Benfobell target and won a trophy and 5 million won in prize money. Lee Sang-soo, deputy director of Chong Kun Dang, said, "Since the launch of Benfobell, it has been loved by pharmacists so far. It has a special meaning because pharmacists voted for it themselves. I think it's thanks to your consistent communication with us. "I'll be Benfobell who works harder." Donghwa Pharmaceutical Itchi won the grand prize in the special award for pharmacist selection. ▲Master Pharmaceutical Mayqueen ▲ Dong-A Pharmaceutical Panpyrin and others received the Excellence Award. Ahn Dae-cheon, former chairman of the Korea Advertising Association (Professor of Inha University), was the judge, while Chung Jae-hoon, a professor at Chonbuk National University, and Lee Jae-guk, executive director of the Korea Pharmaceutical Bio Association, participated as professional judges. Ahn, chairman of the judging committee, said, "The review of the advertising sector was based on whether the message was well matched and clearly delivered, secondly on the novelty of the idea, and thirdly on the reliability of the information. The PR division considered whether it could contribute to enhancing its image from a long-term perspective and whether messages and activities could elicit timely and consumer sympathy, he said. "We praise your efforts to develop advertising and promotion in the pharmaceutical bio industry." The Korea Pharmaceutical Bio-Industry Advertising and PR Awards began in 2013 with the aim of encouraging advertising promoters who give new values to the pharmaceutical industry and drugs and encouraging pharmacists, who are primarily advertising consumers, to produce advertisements that form a consensus. Lee Jung-seok, CEO of dailypharm, said in a greeting, "Many excellent works have been submitted this year, so I would like to thank pharmaceutical bio officials. I would like to congratulate you on your award-winning work. "We will make it a more fruitful and beneficial event next year," he said. Sanofi Specialty Care Director Kim Hyun-jung and CEO Lee Jung-seok, who won the PR category grand prize Chairman Won Hee-mok then said, "In the age of aging, the category of health management and prevention along with treatment has expanded, and the proportion of OTC drugs is increasing. At the same time, as the subject of the information is digitally transferred to consumers, changes are taking place in general drug advertisements, he said. "I hope it will be a place to find a new paradigm of general drug advertisements." "Thank you to Daily Farm for planning the advertising and PR targets," he said. Vice Chairman Lee Young-shin said, "With the development of artificial intelligence and digital healthcare, it has become an era of understanding the development of science and technology in the global beyond Korea. Advertising and promotion of drugs is no exception, he said. "I would like to thank advertising and public relations officials and media officials who have recognized new changes and delivered more accurate information in the regulated healthcare industry and contributed to improving the quality of life of the people." "KRPIA will also make efforts to grow with the domestic pharmaceutical bio industry."
- Company
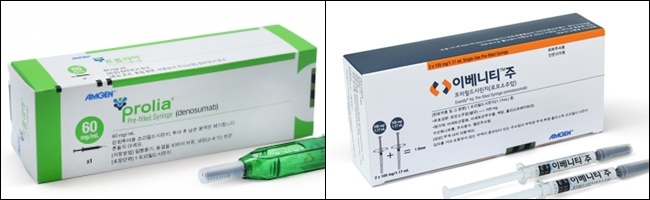
- Prolia's annual sales are expected to be 100 billion won
- by Kim, Jin-Gu Dec 14, 2022 05:56am
- Prolia & Evenity Amgen's Prolia, which dominated the osteoporosis treatment market, is further strengthening its dominance system. Sales are expected to surpass 100 billion won by the end of this year. Evenity, a generic for Prolia, is also rapidly increasing its sales. It has grown into the second-largest product in the osteoporosis treatment market in less than two years since the salary was applied in December 2020. According to IQVIA, a pharmaceutical market research firm, Prolia's cumulative sales in the third quarter of this year were 83.8 billion won. It increased by 28.6% compared to the same period last year-on-year. Prolia is a mechanism drug that inhibits bone absorption. It has become the first standard treatment for osteoporosis due to its superior effectiveness and convenience of administration compared to BP, which has been widely used in the past. It is difficult to take BP for patients, such as taking a sufficient amount of water on an empty stomach 1 to 2 hours before a meal and not lying down for at least 30 minutes after taking it. It was difficult for patients to continue to take medicine because side effects such as gastrointestinal disorders may occur when taking it for a long time. Prolia only needs to be administered once every six months. Even after 10 years of long-term treatment, the effect of continuous bone density improvement and consistent safety profile were confirmed. Prolia's beneifit was applied only to the secondary treatment in 2017. From April 2019, the range of benefits has been expanded to primary treatments. Sales rose vertically shortly after the benefit increase. Prolia's sales, which stood at only 14.3 billion won in 2018, more than tripled in a year to 47.3 billion won in 2019. It has been steadily increasing to 75.1 billion won in 2020 and 92.1 billion won in 2021. This year, sales of more than 80 billion won in the third quarter are expected to surpass 100 billion won at the end of the year. During this period, Prolia's price was reduced by 17.6% on three occasions. The drug price, which was 215,678 won at the time of initial registration, fell to 190,000 won in April 2019, 177,650 won in December 2020 and 168,800 won in July this year. As usage soared, drug prices were lowered by PVA. Considering the reduction in drug prices, it is possible to estimate that Prolia's use at the prescription site has increased significantly than sales growth. It is analyzed that the expansion of benefits and the strengthening of sales power also contributed to Prolia's growth. Amgen has been selling Prolia with Chong Kun Dang since September 2017. Amgen Korea is in charge of sales and marketing of Prolia at hospitals and Chong Kun Dang at clinics. Evenity's rise, released as Prolia's generic, is steeper. The cumulative sales in the third quarter of this year were 12.2 billio wonn, up 47.4% from the cumulative 8.3 billion won in the third quarter of last year. Evenity is a bone formation agent with a dual effect of promoting bone formation and inhibiting bone absorption.Amgen is devising Evenity's positioning strategy as sequential administration from Evenity to Prolia . Evenity, which was granted in June 2019, was registered in December 2020. Even before the registration, the drug received great attention at the prescription site. It generated 3.8 billion won in sales in 2020. It has been growing more rapidly since the benefit registration. In 2021, sales more than tripled in a year to 12.3 billion won. Until the third quarter of this year, sales were almost the same as last year. It is expected to record sales of more than 15 billion won at the end of the year. As Amgen's two products made strides, sales of existing BP formulations were low. Eli Lilly Forsteo's cumulative sales in the third quarter of this year were 9.7 billion won. It remains at the same level as last year. The cumulative sales of Hanmi Pharmaceutical's RaboneD in the third quarter decreased by 1.5% from 7.4 billion won last year to 7.3 billion won this year. The rest of the products have a bigger drop in sales. Daewoong Zoledronic Acid saw its sales fall 33.6% from 7.8 billion won to 5.2 billion won during the period. Jeil's Bonviva and Bonviva Plus fell 21.3% from 8.1 billion won to 6.4 billion won. Since October, Jeil has taken over the domestic rights of Bonviva and Bonviva Plus from Handok. Handok has been in charge of Bonviva's domestic sales and marketing for the past seven years.
- Company
- Baxter ranks first in the 70 billion hemostatic market
- by Nho, Byung Chul Dec 13, 2022 06:07am
- In the 70 billion won-shaped hemostatic market, Baxter Floseal is likely to surpass 30 billion won in sales this year, and is expected to rank first for the third consecutive year. According to the drug distribution performance data, Baxter Flossal's sales reached 25.3 billion won and Hyundai Pharmaceutical's Tachosil's sales reached 12.5 billion won until the third quarter of this year, ranking first and second, respectively. GC Pharma s Greenplast Q and Tisseel posted 8.8 billion won and 7.6 billion won in sales. The Beri Plast P Combi Set in Germany remained in the bottom ranks with 1.8 billion won in performance. Floseal's breakthrough has seen 23.2 billion won and 27.3 billion won (2021) from 2020, far exceeding Tachosil's sales of leading products, 17 billion won and 16 billion won. Except for Floseal and Greenplast Q, all three products of Tachosil, Tisseel, and Beri Plast P Combi Set have a right-downward sales curve in the box. Greenplast Q's performance in 2018, 2019, 2020, and 2021 was 9.8 billion, 10.9 billion, 11.5 billion, and 11.8 billion won. During the same period, the appearance of the Tisseel and Beri Plast P Combi Set is 10.3 billion, 10.6 billion, 10 billion, 10.1 billion, and 2 billion, 4.3 billion, 3 billion, and 2.4 billion, respectively. Floseal, which obtained approval from the Ministry of Food and Drug Safety in 2008, is a syringe kit-type product that is rarely listed as non-payment among major hemostatic products with 500 IU/mL of human thrombin and 704mg of purified gelatin. Tachosil is a white sponge-type hemostatic agent at room temperature below 25 °C. The health insurance drug price is listed as 71,752 won for 3cm*2.5cm, 374,597 won for 9.5cm*4.8cm, 193,697 won for 4.8cm*4.8cm, 211,642 won for 4.8cm*4.8cm. Greenplast Q in the form of PF syringe is a hemostatic agent mainly composed of 500 IU/mL of thrombin, 1000 kIU/mL of afrotin, and 95 mg/mL of fibrinogen. Like Tisseel - 20 °C is a biological product of frozen storage and distribution. The drug price is 85,028 won for 2mL and 167,314 won for 4mL. Tisseel, which was approved in 2004, has a pre-field syringe with 3 000kIU of Aporotinin, 5.88mg of calcium chloride hydrate, 500U/mL of human Thrombin, and 91mg of Fibrinogen, and the registration consists of 2mL, 119,508 won, 4mL, 191,268 won, 10mL, and 506,27 won. Beri Plast P Combi Se is a similar composition to Tisseel, and the packaging unit is in the form of freeze-dried vial + liquid vial, forming an insurance drug price of 95,590 won for 1mL and 191,234 won for 2mL
- Company
- Sales of Actinum series have been sluggish
- by Nho, Byung Chul Dec 13, 2022 06:07am
- The Actinum series, once known as Takeda Pharmaceutical's signature mixed vitamin, is experiencing its biggest sales slump in more than seven years since its launch. According to drug distribution performance data, the performance of the Actinium series last year was 2.3 billion won, down 74% from 8.9 billion won in 2018. Between 2018 and 2021, Actinium EX Gold's sales were 4 billion, 2.9 billion, 1.5 billion, and 990 million won, and Actinium EX Gold recorded 4.9 billion, 3.7 billion, 2.1 billion, and 1.2 billion won during the same period. In the first half of 2022, the appearance of the two products was 140 million won and 80 million won, respectively, the worst in the past five years. Actinum, whose main ingredient is Fursultiamine, is once expected to be a blockbuster OTC drug through two lineup of EX Gold and Plus, and is considered the main player in the so-called high-dosage vitamin drug. The inflection point of performance is the aftermath of the boycott of No Japan in 2019 and Takeda, which ended this yearIt is in line with the issue of the transfer rights. It is analyzed that the performance has drawn a gradual downward curve in the indiscriminate boycott of Japanese products, regardless of all industries such as pharmaceuticals, automobiles, and food and beverage. Takeda Pharmaceutical recently transferred its license to Celltrion Pharmaceutical for its OTC Whituben and Albotyl. Actinum is known to have handed over its copyright to Japanese company Alinamin Pharma in 2021. Actinium, which was launched in Korea in 2015, has been sold through cooperative wholesalers such as Geo-Young and Dongwon, and signed an exclusive domestic sales partnership contract with Dong-Wha from April 2019 to January this year. Currently, the authority to import and sell Actinium is in charge of ZP Therapeutics Korea, a commercial business corporation of Zuellig Pharma Korea. The rapid success factor of this product, which was renamed Arinamin in Japan and marketed as Actinum in Korea, lies in its product power. Actinium is a thin-layer sugar tablet and manufacturing method patent, which increases the convenience of taking vitamins with a small size of 9.2mm, and the content and ingredients are stable for four years with a stabilized vitamin product patent. According to Actinium clinical studies, about 80% of patients complaining of eye fatigue, shoulder stiffness, and back pain showed mild or higher symptoms, and at least 3.3 days to up to 5.9 days after starting to take each symptom. In recognition of these efficacy, Actinium EX Plus passed Seoul National University Hospital's Drug Committee (DC) three months after its launch in August 2015, and its product power was recognized. In particular, sufficient data such as clinical data must be available to pass OTC DC at Seoul National University Hospital. In fact, OTC landed at Seoul National University Hospital is only a small number of items such as Ildong's Aronamin and Pfizer's Centrum.
- Company
- “Tagrisso shows effect as 1st-line in the real world"
- by Dec 12, 2022 05:48am
- AstraZeneca’s 3rd generation EGFR mutation-positive non-small cell lung cancer (NSCLC) treatment ‘Tagrisso (osimertinib)’ has shown consistent effects with the clinical trial results in practice. In the real world, Tagrisso demonstrated excellent treatment effects even in patients with brain metastasis, poor systemic condition, or patients with rare mutations. The real-world study results from Germany and Japan that contained the results above were presented at the ESMO Asia 2022 Congress that has been held in Singapore on the 2nd. The large-scale, real-world studies evaluated the use of Tagrisso as a first-line treatment in the field on 600 patients in Japan and 200 patients in Germany. Japan large-scale real-world study results (Source: ESMO) In the Japanese trial that analyzed 583 patients, Tagrisso achieved a median progression-free survival (mPFS) of 20.0 months and median overall survival (mOS) of 4.09 months. By mutation, Tagrisso’s PFS in patients with exon19 deletions reached 23.5 months. In those with L858R mutations, the PFS was 17.0 months. In terms of OS, the OS was 36.1 months in the L858R mutation group, and the median OS was not reached in the exon19 deletion group. German large-scale real-world study results (Source: ESMO) In the German trial that analyzed 217 patients, the time to next treatment or death (TTNTD) and the time to discontinuation (TTD) were measured. Results showed that Tagrisso’s mPFS was 16.2 months, TTNTD 19.2 months, and TTD 14.8 months. In other words, Tagrisso demonstrated an excellent effect not only in patients with brain metastasis that are commonly found in NSCLC but also in patients with rare EGFR mutations. Especially, the rate of patients with brain metastasis accounted for 38% of all patients enrolled in the German real-world study, and 11% were patients with rare mutations that had been excluded from clinical trials. Also, 14% were patients with poor prognosis, the ECOG PS2-3 NSCLC patients. During an interview with Dailyphram, Professor Frank Griesinger of Hemato-Oncology from Germany at Pius-Hospital Medical Campus, University of Oldenburg, said, “Unlike how tests are conducted every 6-8 weeks to evaluate PFS in clinical trials, the PFS in real-world studies tends to be shorter due to longer testing intervals in the field. Tagrisso showed a consistent effect in the real-world study despite the fact that the study enrolled a higher proportion of patients with brain metastasis and rare mutations or poor general conditions, and their age was around 4 years older. Professor Frank Griesinger Professor Griesinger added that over 90% of EGFRm NSCLC patients in Germany choose to use Tagrisso in the first line. He explained that sequential therapy with Tagrisso where other 1st or 2nd-generation treatments are first used followed by 3rd generation drugs may deprive patients of their opportunity to use Tagrisso. Professor Griesinger said, “If you look at the treatment journey of those that undergo sequential treatment, only 70 to 80% of those with advanced disease can receive mutation testing as patients who have already developed resistance often do not have enough tissue left to perform a biopsy. Blood biopsy is somewhat less accurate. Besides, only 38% of Asians are found to receive biomarker testing after developing resistance. Considering how only around half of the few that are tested are T790M-positive, not many patients can use Tagrisso as later line therapies.” He added, “One mistake most people make in the course of making treatment decisions is that the OS data of Tagrisso is good as sequential therapy. But the results have to be better as the data comes from the selected 30% of patients who are eligible to use Tagrisso in the first place. We should not overlook the other 70% of the patients that cannot use Tagrisso in that environment” The OS from the Asian subgroup analysis results for Tagrisso’s Phase III trial has raised controversy. Although Tagrisso showed statistically significant improvement in the entire patient group, the risk ratio (HR) was 0.995 when separately analyzing the Asian patients in the population. An HR of 0.995 means that the difference between Tagrisso and the control group is 0.005, it could be interpreted that there is virtually no difference between Tagrisso and the control group. Regarding Tagrisso’s effect on Asians, Professor Griesinger said “Some parts of the Asian sub-analysis results have been difficult to interpret, but I don't think it's is an issue because the total OS shown demonstrates improvement in the entire population. Tagrisso effect was also consistent in the Japanese real-world study.” Professor Griesinger emphasized, “Based on my practice and research experience Tagrisso is a worthy first-line standard therapy. If the patient has EGFR mutation, there is no reason not to use Tagrisso.”