- LOGIN
- MemberShip
- 2025-12-24 07:27:45
- “Tagrisso shows effect as 1st-line in the real world"
- by | translator Alice Kang | 2022-12-12 05:48:14
AstraZeneca’s 3rd generation EGFR mutation-positive non-small cell lung cancer (NSCLC) treatment ‘Tagrisso (osimertinib)’ has shown consistent effects with the clinical trial results in practice.
In the real world, Tagrisso demonstrated excellent treatment effects even in patients with brain metastasis, poor systemic condition, or patients with rare mutations.
The real-world study results from Germany and Japan that contained the results above were presented at the ESMO Asia 2022 Congress that has been held in Singapore on the 2nd.
The large-scale, real-world studies evaluated the use of Tagrisso as a first-line treatment in the field on 600 patients in Japan and 200 patients in Germany.
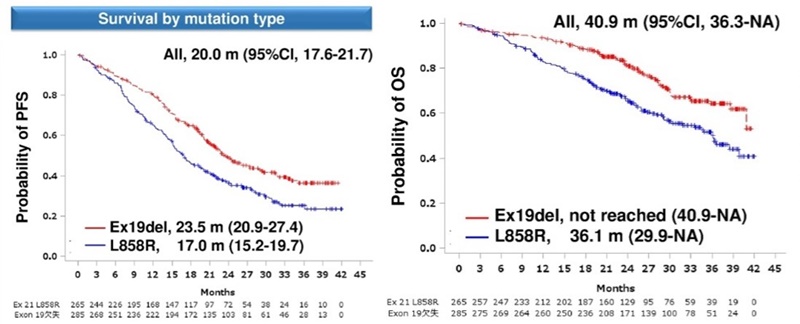
By mutation, Tagrisso’s PFS in patients with exon19 deletions reached 23.5 months.
In those with L858R mutations, the PFS was 17.0 months.
In terms of OS, the OS was 36.1 months in the L858R mutation group, and the median OS was not reached in the exon19 deletion group.
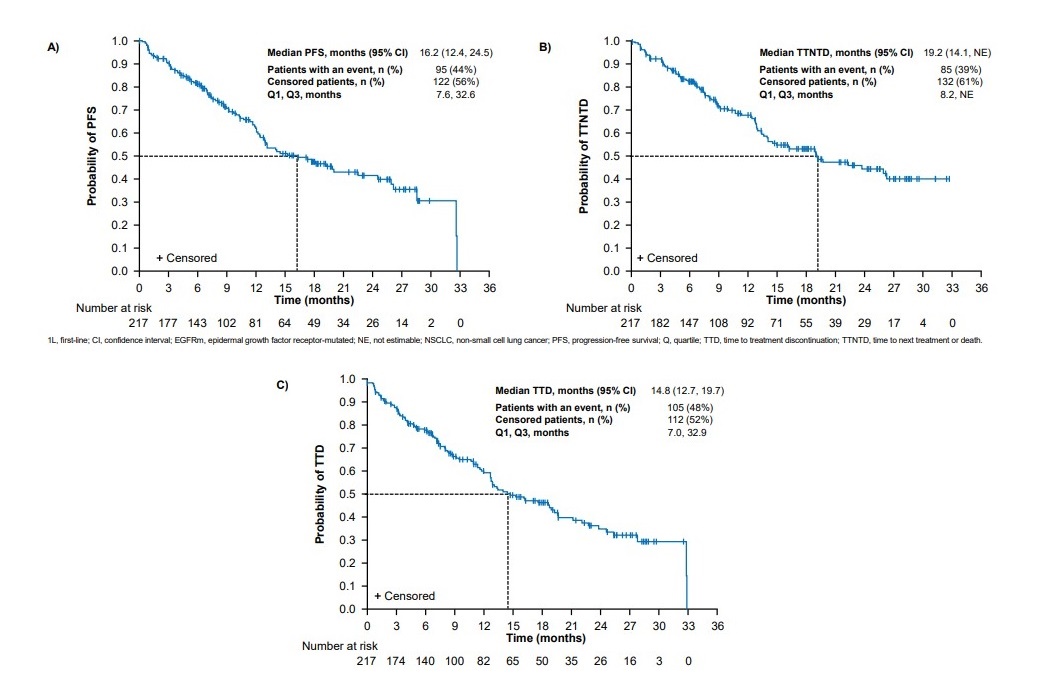
Results showed that Tagrisso’s mPFS was 16.2 months, TTNTD 19.2 months, and TTD 14.8 months.
In other words, Tagrisso demonstrated an excellent effect not only in patients with brain metastasis that are commonly found in NSCLC but also in patients with rare EGFR mutations.
Especially, the rate of patients with brain metastasis accounted for 38% of all patients enrolled in the German real-world study, and 11% were patients with rare mutations that had been excluded from clinical trials.
Also, 14% were patients with poor prognosis, the ECOG PS2-3 NSCLC patients.
During an interview with Dailyphram, Professor Frank Griesinger of Hemato-Oncology from Germany at Pius-Hospital Medical Campus, University of Oldenburg, said, “Unlike how tests are conducted every 6-8 weeks to evaluate PFS in clinical trials, the PFS in real-world studies tends to be shorter due to longer testing intervals in the field.
Tagrisso showed a consistent effect in the real-world study despite the fact that the study enrolled a higher proportion of patients with brain metastasis and rare mutations or poor general conditions, and their age was around 4 years older.

He explained that sequential therapy with Tagrisso where other 1st or 2nd-generation treatments are first used followed by 3rd generation drugs may deprive patients of their opportunity to use Tagrisso.
Professor Griesinger said, “If you look at the treatment journey of those that undergo sequential treatment, only 70 to 80% of those with advanced disease can receive mutation testing as patients who have already developed resistance often do not have enough tissue left to perform a biopsy.
Blood biopsy is somewhat less accurate.
Besides, only 38% of Asians are found to receive biomarker testing after developing resistance.
Considering how only around half of the few that are tested are T790M-positive, not many patients can use Tagrisso as later line therapies.” He added, “One mistake most people make in the course of making treatment decisions is that the OS data of Tagrisso is good as sequential therapy.
But the results have to be better as the data comes from the selected 30% of patients who are eligible to use Tagrisso in the first place.
We should not overlook the other 70% of the patients that cannot use Tagrisso in that environment” The OS from the Asian subgroup analysis results for Tagrisso’s Phase III trial has raised controversy.
Although Tagrisso showed statistically significant improvement in the entire patient group, the risk ratio (HR) was 0.995 when separately analyzing the Asian patients in the population.
An HR of 0.995 means that the difference between Tagrisso and the control group is 0.005, it could be interpreted that there is virtually no difference between Tagrisso and the control group.
Regarding Tagrisso’s effect on Asians, Professor Griesinger said “Some parts of the Asian sub-analysis results have been difficult to interpret, but I don't think it's is an issue because the total OS shown demonstrates improvement in the entire population.
Tagrisso effect was also consistent in the Japanese real-world study.” Professor Griesinger emphasized, “Based on my practice and research experience Tagrisso is a worthy first-line standard therapy.
If the patient has EGFR mutation, there is no reason not to use Tagrisso.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









