- LOGIN
- MemberShip
- 2026-03-10 02:17:20
- Product
- The Minister Lee, Enhanced post-de-factor management
- by Jung, Heung-Jun Nov 18, 2019 10:21pm
- Lee Eui-kyung, the Minister of MFDS has released a long-term follow-up plan to strengthen the post-de-factor safety management system. On the 15th, the Minister Lee attended Korean Academy of Social & Managed Care Pharmacy and presented the four directions of drug safety management in four categories: patient safety, accessibility, safety ecosystem, and globalization. In particular, the Minster Lee emphasized that she will lead to a paradigm shift centered on patients by strengthening post-de-factor management. She said, "In the meantime, in the event of Invossa’s case , it was most of the time that the license was revoked or changed. In the future, we will thoroughly control the side effects, operate the long-term follow-up management system, and carefully regulate the damage. I will lead the paradigm. " For 3,000 patients for injecting Invossa, she will select 20 medical institutions and conduct a long-term follow-up survey for 15 years. "We have registered for 80 to 90 percent of our patients." she said, "Patients have used 400 medical institutions, but the concern is about cancer. So long-term follow-up should be in a hospital with oncology." "We plan to track them all at large hospitals, and we will also carry out causality research of cancer." On this day, the “Long-term patient tracking survey system (Draft)”, which will be applied to future risk medicines, was announced. According to the plan, when the MFDA issues a follow-up order, companies make a plan and MFDA goes through approval process. Companies conduct follow-up investigations. Causality evaluations and follow-up actions are conducted with the MFDS. The Minister Lee said, "We still have to be specified liability compensation. The government thinks that product defects should be shouldered by pharmaceutical companies. But, pharmaceutical companies have different positions that they are not responsible for what they didn't know at the time. We are discussing the responsibility with the relevant committees” ◆Strengthen practical use of RWD (Real World Data), R &D investment extension. In addition, MFDS will strengthen the use of RWD (Real World Data) and RWE(Real World Evidence) next year to strengthen patient safety management It plans to establish big data utilization system such as clinical site RWD and RWE in clinical trial RCT. To this end, it will actively invest in R & D costs. She said, “We will make an official announcement in January about the plans for using big data. MFDS R & D expenses rose from ₩85 billion to ₩100 billion this year. But most of them are the cost of experimentation. I'm going to turn this into RWD study”. She also announced that she will soon disclose ways to improve safety for conditional phase3 permits. "There is a lot of controversy about whether a conditional phase3 permit will guarantee the patient first, or if he will give the opportunity after ensuring full safety, if there is no alternative." We will soon release to the media on how to make it work more securely. ” ◆As new drug development countries become more important, permit strengthening of MFDS expertise She said that as Korea is actively developing new drugs, licensing is becoming more important, so the MFDA will also strengthen its expertise. She said, “If the drugs were reviewed at least once because foreign new drugs were introduced into the country in the past, currently there is a need to be more burdensome in terms of initial authorization and to reinforce professionalism since new drugs are being developed in Korea. Only about 350 people are screened by the MFDS, but there are 6,000 Ph.D in US. The MFDS will focus on strengthening its expertise in the future.” She also said “MFDS or pharmaceutical companies can’t do it alone, Infrastructure maturity must increase” "The long-term policy is needed to fundamentally improve the problem," She said. It is true that there is a lack of policy research think tank, ” She also recognized the necessity of analyzing and evaluating the future system.
- Product
- 7 out of 10 doctors exposed to patient violence
- by Kang, Shin-Kook Nov 15, 2019 06:29am
- Apparently, seven out of ten doctors have faced verbal abuse or physical violence against them by a patient or their family. On Nov. 13, Korean Medical Association (KMA, President Choi Dae-zip) published a result of an urgent survey conducted on 2,034 member doctors of the organization. The survey result found among 1,455 doctors (71.5 percent), who have experienced verbal abuse or physical violence against them committed by a patient or their family in exam room in last three years, about 15 percent of them have been physically assaulted, and 10.4 percent ended up with a physical damage. KMA President Choi Dae-zip And more than a half of the surveyed doctors said they have experienced verbal abuse and physical violence against them about once or twice a year. 9.2 percent answered they have been in such situation at least once a month. Most of such aggression was fired up by medical exam results, and some other cases were sparked from complaints of long waiting time and expensive medical service charges. Moreover, 61 percent of the doctors answered a patient or their family came back to the abused doctor later for medical service. 1,254 doctors (61.7 percent) among them have also been forcefully demanded by a visitor to forge a medical report or falsely edit already issued medical record. “The association aims to protect member doctors by introducing a new bill that stipulates punishment on visitors demanding doctors to forge medical record. KMA is committed to have the lawmakers to promptly legislate bills to protect doctors in vulnerable position. The association has been constantly urging the law turning down an indictment for committed assault in healthcare institute when a victim drops the charges should be abolished, and also doctor’s rights to refuse patients should be legally justified. The latest survey has provided us enough concerning evidences,” said KMA official. Moreover, KMA officials pointed out “The survey result says only 6.9 percent of the doctors at the moment have a protected space or facility to avoid verbal abuse and physical violence in an exam room. It means most of the doctors are vulnerably exposed to the harmful threats. The government should pay more attention on creating a safer environment for healthcare institutes”.
- Product
- Lixiana joins NOAC market race late but takes the lead
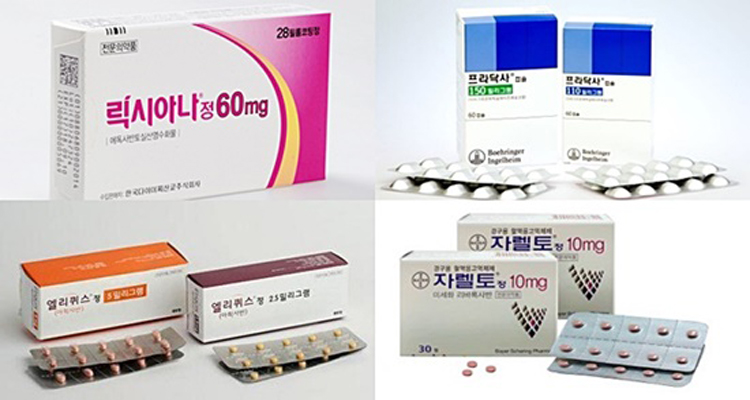
- by Byun Kyung A Oct 31, 2019 09:59am
- (Clockwise from upper left) Images of Lixiana, Pradaxa, Xarelto, and Eliquis A follow-on drug in the non-vitamin K antagonist oral anticoagulant (NOAC) market, Lixiana, continues to lead the market. The treatment took the lead from Xarelto in last January for the first time, and it has been the most prescribed drug in the market for nine consecutive months. Pfizer and Bristol-Myers Squibb’s Eliquis had to face a Korean-made generic as a competitor, but its sales performance was unaffected with its original price maintained. On Oct. 28, UBIST, a pharmaceutical market data research firm, reproted Lixiana (edoxaban) marked an accumulated prescription of KRW 40.1 billion in the third quarter. The figure skyrocketed by 68.5 percent from last year same period. Lixiana recorded prescription sale of 14.9 billion won in the third quarter only and marked its record-breaking quarterly performance. The treatment reached outpatient prescription sale of 4 billion won in last January and beat Xarelto’s (rivaroxaban) prescription sale of 3.8 billion won, for the first time. The top spot in the NOAC prescription market has been solidified for nine months straight. The total sale gap between Lixiana and Xarelto’s prescription marked 6.6 billion won and was widened in the third quarter, due Xarelto’s sale dropping 0.2 percent in the third quarter than the year before. The treatment was ranked on second place in the market with 33.5 billion won accumulated prescription sale. Outpatient prescription sales trend of four NOAC items by month (Unit: KRW1 million) Source: UBIST Both internal and external factors played a part in shuffling the NOAC market rank. Bayer’s German production plant issue prolonged the unstable supply management in Korean market, and also the revised pharmaceutical product labeling regulation required all dosage containers to be reprocessed and slowed down Xarelto’s come back. In the meantime, Daiichi Sankyo and Daewoong’s co-promotion revved up the prescription sales of a follow-on drug, Lixiana. Insider from Daiichi Sankyo evaluated, “The healthcare providers and patients were positive about Lixiana’s better drug compliance with once-daily regimen, and improved safety for patients backed by Phase 3. The long-lasting co-promotion partnership with Daewoong, started from Sevika HCT deal, also created a great synergy effect boosting the sales performance”. Monthly prescription sales trend for Eliquis and apixaban generics (Unit: KRW) Source: UBIST Eliquis cumulated 31.3 billion won prescription sales this year, showing 32.1 percent surge than the year before. As of the third quarter, difference in accumulated prescription sales between Xarelto and Eliquis is only about 2.1 billion won. Eliquis was the first NOAC original to compete against generics, but its sales were not influenced as it maintained its original price. After the Korean Supreme Court in last June invalidated drug patent protecting Eliquis, the drug now has Korean-made generics competitors from Chong Kun Dang, Yoo Young, Yuhan, Huons, and Hanmi. But as Ministry of Health and Welfare has decided to defer Eliquis’ price reduction until the final decision on the treatment’s substance patent case is to be made on coming Dec. 31. Currently, the drug still maintains its initial price of 1,185 won per tablet. On the other hand, Chong Kun Dang’s competing generic with the highest content apixaban is about half the price of Eliquis. Boehringer Ingel Heim’s Pradaxa (dabigatran) marked accumulated prescription sales of 12.4 billion won in the quarter. The company sought for a significant increase in market share with a co-promotion deal with Boryung, but the sales performance did not show much increase than last year with 12.3 billion won.
- Product
- Maviret takes up 83% of HCV treatment market share
- by Byun Kyung A Oct 31, 2019 09:41am
- Maviret product image Abbvie broke through the hepatitis C virus (HCV) treatment market and dominated 83 percent of oral outpatient prescription market only with its pan-genotype HCV treatment, Maviret, launched last year. Gilead once dominated the market with two items, but their prescription ratio shriveled down to 12 percent. On Oct. 30, a pharmaceutical market research agency, UBIST reported this year’s third quarter outpatient prescription sales of eight direct-acting antiviral (DAA) regimen HCV treatments recorded 13.2 billion won, soaring 41.4 percent from last year’s third quarter at 9.3 billion won. Abbvie’s Maviret prescription skyrocketed from the launch in last year September, and brought back up then descending total volume of HCV outpatient prescription market. The market had plummeted to 9.3 billion won in September last year, but turned around with Maviret’s launch. Outpatient prescription sales trend of major hepatitis C virus treatments (Unit: KRW 1 million) Source: UBIST This year’s third quarter outpatient prescription sales of Maviret reached 10.9 billion won. The sales easily doubled since last year’s fourth quarter with 4.2 billion won. As its accumulated prescription sales this year marked 31.1 billion won, Maviret is projected to make over 40 billion won by the end of the year. Taking up 82.9 percent of the eight DAA regimen treatments’ outpatient prescription sales, Maviret affirmed its unmatched position in the market. Maviret is the first treatment approved in Korea for chronic HCV infection in adults across all major genotypes. It is a pan-genotype ribavirin-free treatment that combines glecaprevir (100mg), an NS3/4A protease inhibitor, and pibrentasvir (40mg), an NS5A inhibitor, dosed once-daily as three oral tablets. Industry insiders view Maviret rapidly took over the market as it shortened drug treatment period by a month, compared to other DAA regimen. The treatment also mainly targets for patients without cirrhosis and who are new to treatment, the condition most of hepatitis C patients have. Recently, Maviret was able to improve consumption convenience as FDA approved of the drug shortening treatment period of chronic hepatitis C patients with cirrhosis and who are new to treatment, from 12 weeks to eight weeks. Sources also report Ministry of Food and Drug Safety’s approval on the expanded indication is imminent as related application has been submitted. However, due to the fact number of HCV patients is limited and the virus has fairly high full recovery rate, it is unclear if Maviret’s golden days would last long. In fact, Maviret’s prescription sales growth has turned from the third quarter. The total prescription soared every quarter since its launch and peaked in the second quarter, but it’s the figure dropped 9.1 percent in just about three months. Meanwhile, a former HCV treatment market leader, Gilead Sciences seems to have lost a big cut from its own market share. Sovaldi marked 18.1 billion won in last year’s first quarter, but its third quarter prescription sales in this year plunged to 4.68 billion won. Two top selling products of Gilead, Sovaldi and Harvoni, made total prescription of 1.5 billion won. The two drugs combined accumulated prescription sales of 5 billion won, merely taking up 11.7 percent of the whole hepatitis C treatment market. BMS’ Daklinza and Sunvepra combined marked outpatient prescription sales of 4.5 billion won in the third quarter, which is about one-tenth of what they had made last year same quarter. Launched around same season in the second quarter of 2017, MSD’s Zepatier and Abbvie’s Viekira and Exviera watched their sales continue dropping after the launch of Maviret and had short period of sales peak. In the third quarter, Zepatier marked prescription sales of 700 million won, whereas prescription sales for Viekira and Exviera were not even counted from last July. Abbvie insider commented, “Demand for Viekira and Exviera has decreased after Maviret was launched. There isn’t any issue with supply from the company”.
- Product
- Government likely to list Zejula in December
- by Byun Kyung A Oct 31, 2019 09:36am
- Sources predict Zejula, an ovarian cancer treatment, would get listed for healthcare reimbursement in coming December. According to an industry insider on Oct. 16, Takeda Pharmaceuticals Korea is waiting for Health Insurance Policy Deliberation Committee’s (HIPDC) deliberation, after recently reaching an agreement with National Health Insurance Service (NHIS) on drug pricing of Zejula (niraparib), a poly (ADP-ribose) polymerase (PARP) inhibitor. The niraparib treatment has been passed by Drug Reimbursement Evaluation Committee of Health Insurance Review and Assessment Service (HIRA) only after four months it was approved in last July. Zejula can be prescribed as a maintenance treatment for adult patients with recurrent epithelial ovarian cancer, including fallopian tube or primary peritoneal cancer, who are in a complete or partial response to platinum-based chemotherapy. However, the National Health Insurance (NHI) reimbursement would be first available for BRCA mutation patients only. Zejula was approved based on results of Phase 3 trials (ENGOT-OV16/NOVA). Progression-free survival (PFS) of patients taking Zejula, with or without BRCA mutation, showed a meaningful extension than the the control group. Moreover, the treatment proved its efficacy through PRIMA trial testing as a first-line treatment for ovarian cancer, regardless of BRCA mutation. In Korea, AstraZeneca’s Lynparza was listed for NHI reimbursement as a first PARP inhibitor. Lynparza was exempted from pharmacoeconomic analysis and was listed as reimbursed drug with expenditure cap risk sharing agreement (RSA) in October, 2017. However, the reimbursement benefit was suspended for some patients from last January as it was initially listed as post-chemotherapy maintenance therapy up to 15 months. From then on, AstraZeneca underwent reimbursement expansion talks with Korean government and lifted limitation on the fixed period of 15-month since last May.