- LOGIN
- MemberShip
- 2026-03-10 02:17:18
- Product
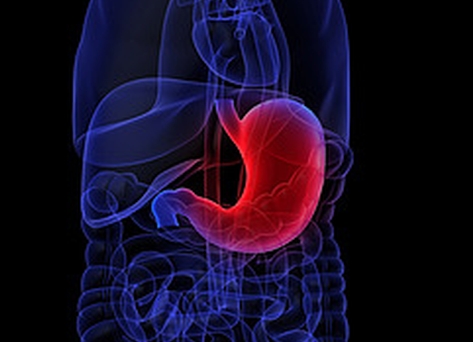
- Fexuprazan taking over K-CAB market? Study unveiled
- by choi, sun May 11, 2020 06:15am
- As Daewoong Pharmaceutical unveiled Phase III clinical data of its next-generation gastroesophageal reflux disease agent in development, fexuprazan, the Korean pharmaceutical industry is keeping a close eye on the prospective competition between the existing proton pump inhibitors (PPI) tegoprazan (Brand name: K-CAB) and the novel agent. Reversibly blocking the proton pump, a potassium-competitive acid blocker’s (P-CAB) efficacy compared to PPI has been confirmed through the clinical trial conducted in Korea, but the market competition would heavily rely on pricing, indication and improved efficacy. ▲Improved efficacy against PPI esomeprazole confirmed On May 2, the Korean pharmaceutical company presented the Phase III clinical data of fexuprazan at Digestive Disease Week (DDW) 2020 as an e-Poster. Daewoong Pharmaceutical’s novel agent treating gastroesophageal reflux disease (GERD), fexuprazan is a P-CAB that reversibly blocks the proton pump secreting gastric acid from the stomach walls. Clinical trial result on heartburn relief Frequently, a PPI is prescribed to treat patients with GERD, but its limitations like slow acting time, varying effects depending on prior food intake and individual CYP2C19 genotype, and drug-drug interaction have been reported. Whereas P-CAB agent is considered a new generation of medicine that covers most of the limitations PPI faces. The Phase III clinical trial was conducted in patients with erosive esophagitis at 25 hospitals in Korea, and it compared efficacy in 40 mg of PPI esomeprazole (n=111) and 40 mg of P-CAB fexuprazan (n=107). Until week 4, fexuprazan and esomeprazole respectively demonstrated endoscopic mucosal healing rate at 90.3 percent and 88.5 percent, but they reached 99.1 percent at week 8. Specifically, P-CAB showed comparatively faster and better heartburn relief. 30.8 percent of fexuprazan group and 23.4 percent of esomeprazole group had their day and nighttime symptoms relieved at day 3. Comparing only patients with moderate to severe symptoms, 22.4 percent of fexuprazan group experienced symptom relief, whereas only 7.9 percent of esomeprazole group did. At day 7, the heartburn symptom relief rates were recorded at 26.2 percent and 21.6 percent in fexuprazan and esomeprazole users, respectively. Comparing again the patients with moderate to severe symptoms, fexuprazan demonstrated better rate at 13.8 percent against 7.9 percent. The results were similar when comparing symptom relief during nighttime. Reportedly, PPI was unable to maintain the effect during nighttime. The nighttime heartburn relief rate of fexuprazan and esomeprazole at day 3 each marked 41.1 percent and 35.1 percent, but in patients with moderate to severe symptoms, the rate was at 34.5 percent and 17.5 percent, respectively. The atypical symptom relief rates in patients with GERD were at 81.2 percent and 68.6 percent in fexuprazan and esomeprazole users, respectively, at Day 3. And the rate remained around the same at week 8 at 80.6 percent and 69.3 percent, respectively. Adverse reactions reported from both groups were about the same. In the future, the novel agent would be inevitably compared to the ‘Old Drug,’ esomeprazole. ▲Competitive against the market-dominating tegoprazan? In 2018, CJ Healthcare has received the government’s approval on the 30th Korean-made novel P-CAB agent ‘K-CAB (tegoprazan).’ In Japan, vonoprazan is released in the market, but K-CAB is the only P-CAB available in the Korean market. As a follow-on drug, fexuprazan would attempt to take over the market from tegoprazan, unavoidably. In last March, tegoprazan has been indicated to treat helicobacter pylori infection and also it has ongoing clinical trials regarding maintenance therapy after treating GERD and preventive therapy against nonsteroidal anti-inflammatory drug-induced duodenal ulcer to expand indications. Fexuprazan would have to face tegoprazan, currently dominating the market not only with its effect, but as a first-in-class and its variety of indications. Then, how about the differences in their efficacy? In March last year, a SCI-level medical journal Alimentary Pharmacology & Therapeutics (AP&T) published Phase III clinical data of tegoprazan. Same with fexuprazan, the study compared tegoprazan’s efficacy and safety in patients with erosive esophagitis against esomeprazole’s. The eight-week multicenter Phase III trial on tegoprazan conducted in Korea tested 302 patients with erosive esophagitis by administering 50 mg (n=100) and 100 mg (n=102) of tegoprazan and 40 mg (n=100) of esomeprazole. At week 8, the mucosal healing rate of three patient groups all reached 98.9 percent. As for fexuprazane, the rate was at 99.1 percent. The heartburn rate in tegoprazan 50 mg group started from 1.76 and was increased to 0.53 and 0.56 at week 4 and week 8, and in 100 mg group the rate fell from 1.86 to 0.62 and 0.62 at the same period. The rate in esomeprazole group was dropped from 1.84 to 0.48 and 0.47 at week 4 and week 8. The prevalence of adverse reaction in 50 mg and 100 mg of tegoprazan users reached 28.3 percent and 23.5 percent, respectively. The rate was similar in 40 mg of esomeprazole users with 30.3 percent. Professor Kim Gwang Ha of Pusan National University Department of Internal Medicine, who participated in both tegoprazan and fexuprazan studies, explained “The clinical trial on fexuprazan confirmed significantly improved efficacy in the novel agent against esomeprazole with patients having moderate to severe symptoms,” and “when it gets released in the market, it could be more expensive than PPIs but the benefit could outweigh the high pricing.” He added, “Based on the acting time and effect of inhibiting proton pump faster and better than PPI demonstrated in the clinical trial, the novel agent would fulfill the medical unmet needs the existing PPIs lacked,” and “patients who failed to relieve the symptoms with PPI would benefit from P-CAB.” “However, the healthcare providers should be aware that not all P-CABs have same effect and safety profile,” so “their marketability and competitiveness should be more accurately assessed with further head-to-head studies between different P-CABs,” the professor noted.
- Product
- The KPA, criticized the MFDS/the Regulatory Reform Committee
- by Kang, Shin-Kook May 06, 2020 06:32am
- The Korean Pharmaceutical Association (KPA) strongly objected to the withdrawal of step-by-step abolition policy for generic co-biological equivalence testing. On the 28th, the Korean Pharmaceutical Association (Chairman Dae-up Kim) announced that the Regulatory Reform Committee recommended the withdrawal of the amendment to the 'Regulation for Pharmaceutical Approvals, Notifications and Reviews', which contains the phased abolition of the generic bioequivalence test, and the MFDS, which accepted this without policy alternatives, is pursuing lush policy. The KPA said, "Even if there are three or four alternative generics available in the pharmacy, the patients will have to experience the inconvenience of searching for drugs, and the cost of illegal rebate due to excessive competition is being passed on to the public." In addition, there is a high social cost for the retrieval of excess medicines and the retrieval of hazardous drugs, and the proportion of pharmaceutical expenses in health insurance finances is increasing day by day. In the current situation, due to the drug price system that guarantees the high price for most of the licensed drugs, there is no limit to the extent to which the number of generic items will increase. The MFDS and the Regulatory Reform Committee are playing into each other's hands. The KPA asked that the Regulatory Reform Committee should play a role in recognizing the problems more seriously and painfully in the pharmaceutical industry as well as in the fields, and it should play a role of deliberating and coordinating policies in the direction of restoring to the pharmaceutical industry and health care in addition to reviewing regulations. The necessity of improving drug management efficiency by improving the difficulty of generic drugs due to the NDMA impurities has been strongly raised, but the situation is further exacerbated by governments that have to implement policies that must prevent the indiscriminate approval of generic drugs. This is due to the MFDS' irresponsible policy promotion and recommendation by the Regulatory Reform Committee. The KPA said that it should reorganize the abnormal generic license system, which can even drop generic drugs that are in good quality and safety management at a low price. The KPA aurged the immediate enforcement of a policy that prohibits the use of different brand names of generic drugs and only permits the same ingredient names (generic names).
- Product
- Imported drugs are out of stock due to COVID-19 crisis
- by Kim, Min-Gun Apr 09, 2020 06:27am
- Difficulties in supplying medical products are also continuing in the aftermath of COVID-19, which hit Europe. According to the distribution industry on the 7th, domestic and foreign pharmaceutical companies, such as Kuhnil Pharmaceutical, Daewoong Pharmaceutical, JW Pharmaceutical, and Pfizer Korea, stopped supply due to delays in the production of some specialized pharmaceutical products. Recently, Amilo (100T/1000T), a diuretic sold by Kuhnil, was temporarily out of stock. This is because the factory in China, the main raw material for the production of Amiloride, was caught in the aftermath of COVID-19. Kuhnil announced that it will stop operating the plant by May 8th at distributors. Because of this, it will be temporarily out of stock for a month. Resupply is expected on May 11th. Fosrenol 500mg (45T), which JW Pharmaceutical has imported from the UK in the form of finished products, have been suspended from March. Fosrenol is used to treat hyperphosphatemia in patients with chronic renal failure who undergo hemodialysis or peritoneal dialysis. It seems that the spread of COVID-19 in the UK is seriously affecting domestic supply. In mid-March, when JW pharmaceutical announced that the supply would be temporarily suspended due to the manufacturer's circumstances, the British government banned and closed the business of public gathering places such as cafes, pubs and restaurants, except essential facilities such as pharmacies and supermarkets. As of today (7th), the total number of COVID-19 confirmed patients in the UK exceeded 50,000 people and 5373 patients died. British Prime Minister Boris Johnson infected with COVID-19, is undergoing a strong social distance campaign while receiving intensive treatment due to worsening symptoms. JW pharmaceutical announced that it can be replaced with Fosrenol powder 1g packets instead of Fosrenol tablets. COVID-19 is also causing problems in exporting medical products in Germany. Supply of Instanyl nasal spray 100mcg/1.8 mL, imported from Takeda, Germany by Daewoong Pharmaceutical was cut off. Due to the spread of COVID-19 in Europe, Daewoong said that the German government's export permission department in February was delayed for two months, causing a disruption in domestic supply. This month, Pfizer Korea Pharmaceutical's Solu-Medrol 125mg, produced in Belgium, a neighboring country of Germany, was also sold out for a long time. Pfizer Korea expects that normal supply will be possible by November. Pfizer Korea does not give details of the reasons for the out of stock, but the company only explained that it was a delay in the production schedule of Solu-Medrol. Currently, there are 20,000 COVID-19 confirmed patients in Belgium and 1632 deaths. The Belgian government has also been taking steps to close companies temporarily and measures to restrict national movement since mid-March. Meanwhile, Nitropress by Pfizer Korea, which is indicated for the immediate reduction of blood pressure of adult and pediatric patients in hypertensive crises, is also out of stock until May.
- Product
- Disaster subsidies must be paid to all citizens
- by Jung, Heung-Jun Apr 09, 2020 06:26am
- 9 out of 10 Dailypharm readers expressed their opinion that the government's emergency disaster aid under COVID-19 should be paid to the entire population, not just the bottom 70% of income. Dailypharm conducted an opinion poll on how to pay for emergency disaster aid through the online survey of Issue & Poll from the 1st to the 7th. Of the 262 Daily Farm readers working in the health and medical industry, 87.8% (230 people) said that all citizens should receive disaster relief funds. Only 12.2% (32 people) voted in favor of the government method of paying the bottom 70%. The readers in favor of the payment of the whole nation thought that it would be reasonable to give it to the whole nation even if the amount was lowered a little. On the other hand, those who favored the selective payment method for the lower 70% insisted that it would be better to pay only the lower 30% or even a little more to the lower income class.
- Product
- Boots, withdraws from the pharmacy market in 3 years
- by Kim JiEun Apr 08, 2020 06:21am
- 'Boots', which opened ambitiously in Korea, claiming to be a premium health & beauty store, eventually withdrew their business after failing to overcome the continuing sluggish profitability. According to the industry on the 6th, the company recently decided to withdraw its entire business and closed the recently opened stores. In 2017, E-Mart received a lot of attention in the H&B market as it landed in Korea by exclusively contracting with Walgreen Boots Alliance, the UK's No.1 H & B store company. In addition to opening and operating 33 stores only in the year of landing in Korea, it was also differentiated from existing H & B stores based on “Premium”. However, it was reported that e-mart decided to withdraw its business after failing to overcome the continued deficit and deteriorating profitability. It is also analyzed that the boots that failed to dominate the competition with other H & B stores in Korea are factors that failed. E-Mart closed 18 boots stores in the first half of last year, and it was confirmed that the six remaining stores were closed in turn following the closing of Starfield COEX and Sinchon stores earlier this year. With the withdrawal of the boots business, the pharmacies operated by each store were forced to close. It is known that there are about 6 pharmacies in Boots, 4 of which are now closed, and the other 2 are in business and are in negotiations with the head office. Boots Star Pharmacy in Starfield Hanam branch also closed on the 5th. At the time of opening the store, it contracted for a 5-year lease, but the business was withdrawn after 3 years of opening, so the branch could not fill the contract period and stopped operating the pharmacy. Yong-han Choi, Rph, said the branch had planned to withdraw from business since February, and that road shops were almost closed. Stores and pharmacies were closed on the 5th, when the discount event to remove inventories ended. Pharmacist Choi said it was unfortunate that the branch was the first pharmacy and could not finish the five-year contract period, and in fact it was closed by a compromise. He added that he plans to rent another store in Starfield Hanam, where he opened the pharmacy independently, and it is just like starting a new one.
- Product
- President Moon posted a memorial message on SNS
- by Kang, Shin-Kook Apr 07, 2020 06:40am
- On the 4th, President Moon Jae-in expressed his condolences to Ms. A, a medical doctor working in Gyeongsan, Gyeongbuk, infected with COVID-19. President Moon said through social networking services (SNS), "It was very sad that our infected medical staff was sacrificed for the first time while treating a COVID-19 infected patient." Mr. A, who ran a private clinicl, died while undergoing treatment after being diagnosed with COVID-19, showing symptoms of pneumonia after treating a COVID-19 confirmed patient in February. President Moon said he wishes the tranquil rest of the deceased, who was always strict to himself and kind to the patient, and said that the people will be of the same mind and give deep comfort to his family. In addition, President Moon welcomed the spring of April, saying that he would not be able to tell the sadness of the MD’s family who could not keep his self-isolated state even at the moment of leaving, but he sincerely pay tribute to the medical staff who don't take care of their bodies to overcome the unfinished COVID-19 disease. President Moon emphasized that aside from his clinic work, the enthusiasm to run for medical care is helping the community overcome COVID-19. and The medical staff is enough to win everyone's respect. " On the other hand, the KMA also held a time of silence for 1 minute at noon on the 4th to commemorate the deceased MD A.
- Product
- Does Tylenol prevent COVID-19 infection?
- by Kim JiEun Apr 03, 2020 06:34am
- Tylenol stockpiles are showing signs of prolongation which were initiated by the World Health Organization (WHO) According to pharmacies on the 1st, there are more customers who want to buy Tylenol, and many of them want to buy in bulk. Tylenol's full-scale hoarding began after WHO recently advised patients with suspicion of COVID-19 to use antipyretic analgesic “Acetaminophen” instead of anti-inflammatory analgesic “Ibuprofen”. Two days after the recommendation, the WHO withdrew its content on the grounds of lack of grounds. but pharmacists say that the purchase of nominations for acetaminophen-based tylenol is increasing. Since the first occurrence of COVID-19 confirmed patients, purchases of ready-to-use medicines increased and Tylenol stockpiling overlapped, making it difficult for pharmacists to secure inventory immediately. Currently, most of the Tylenol products are sold out in the online shopping malls used by pharmacists, and it is not easy to order separately through wholesalers. The number of patients seeking Tylenol suddenly increased, and a pharmacist in Seoul confirmed that there was a WHO recommendation. The pharmacist also said that some patients bought a lot of Tylenol at a time and took it regularly every day, even turning to fake news that Tyrenol has a COVID-19 preventive effect. A local pharmacist said, “Since the spread of COVID-19, there have been some shortage of OTC medications, as well as Tylenol. Tylenol has become more intense in recent years. Some hospitals even recommend or take prescriptions as a preventive measure. Pharmacists are concerned that such an overdose of acetaminophen-based tylenol may cause side effects such as gastrointestinal disorders. In addition, the hypothesis that some of the Acetaminophen that are currently being raised alleviate or prevent the early symptoms of COVID-19, an infectious disease, is an unconfirmed part and warns that it can be dangerous if patients believe it Another pharmacist in Seoul said that Acetaminophen may cause side effects such as gastrointestinal disorders when taken in excess, and may conceal fever when taken for a long time, so it is more likely to cause disease if patients do social activities for a long time while it is hidden. Reflecting this situation, the Ministry of Health and Welfare also advised frontline pharmacies to provide adequate medication guidance regarding the sale of antipyretics. The government recommends that people who have fever or respiratory symptoms (cough, sore throat, muscle pain, etc.) do not go to work and get enough rest through a strong social distance. When selling antipyretic drugs such as acetaminophen and ibuprofen, the government hopes to cooperate so that sufficient medication guidance such as efficacy, effectiveness, and precautions for use can be achieved.
- Product
- 7 out of 10 doctors, the government was wrong with COVID-19
- by Kang, Shin-Kook Apr 01, 2020 06:17am
- Seven out of ten doctors found that the government's response to the COVID-19 was wrong. The Korean Medical Association (Chairman Dae-Zip Choi) conducted a questionnaire survey on the COVID-19 events through the doctoral survey on the 20th to 24th, and published the results on the 30th. As a result of the survey, 39.1% (621 people) of the respondents answered that 'correct responses were not achieved at all' and 29.8% (473 people) answered that 'correspondence was somewhat insufficient' It was found that 68.9% of all respondents in the year were negatively evaluated. On the other hand, 16.6% (264 people) said that 'it worked to some extent' and 6.1% (97 people) said that they responded very well. In particular, the negative evaluation of doctors in the Daegu area, which suffered the most, was 83.2%, which is the only city in the country that has surpassed 80%. Regarding the 'restriction on the total entry of people via China', 84.1% (1337 people) of respondents said that 'there was a need to restrict the entry of people via China at the beginning of the situation'. Following this, 12.6% (200 people) said that 'there was no need to expand the limit of immigration via China' and 3.3% (52 people) said 'I don't know', and there were overwhelmingly negative opinions on the government response in the early stages of the situation. As for the KMA's response to the COVID-19 incident, the percentage of respondents who answered 'comparably appropriately responded' and 'very appropriately responded' was 44.6% (706 people) and 17.9% (284 people), respectively, accounting for 62.5% (990) People) as appropriate. On the other hand, 14.0% (221 people) had an opinion that 'the response was somewhat insufficient', and 7.6% (120 people) had an opinion that 'were wrong', and 21.6% answered negatively. Spokesperson Park Jong-hyuk of the KMA said that the survey was conducted to confirm the members' thoughts in response to the government's response to the COVID-19 incident, based on the results of the survey, it is used as a reference for preparing practical measures. Meanwhile, 1589 medical doctors nationwide participated in the survey, with Seoul accounting for the most with 33.9% (538 people), followed by Gyeonggi 17.4% (277 people), Daegu 8.3% (131 people), and Busan 8.2% (130 people).
- Product
- Korea’s first epidemiological report on COVID-19 deaths
- by Lee, in-bok Mar 31, 2020 06:29am
- A study analyzed deaths from COVID-19 in Korea and found male patients in 70s with chronic cardiac disorder like hypertension were critically affected. Apparently, most of them had died in average 10 days after the onset COVID-19 symptoms, and their average time to death was unaffected regardless of underlying illness. First epidemiological report by Korean Society of Infectious Diseases on death due to COVID-19 in Korea Korean Society of Infectious Diseases (KSID) has investigated 53 deaths, as of Mar. 15, from 7,513 patients infected by coronavirus in Korea and published the outcome on the Journal of Korean Medical Science on Mar. 30 (doi.org/10.3346/jkms.2020.35.e132). #Accessing data through Korean Centers for Disease Control and Prevention (KCDC), the researchers conducted a ten-day-long epidemiological investigation on 54 death caused by COVID-19. The investigation found, as of Mar. 15, total 5,833 cases resulted in death among 156,400 confirmed cases of COVID-19 around the world, which recorded 3.7 percent mortality rate. In Korea, the rate was at 0.7 percent with 54 deaths. On Feb. 19, the 104th confirmed case of COVID-19 in Korea has been deceased and was reported as the first COVID-19 caused death in Korea on the next day. And the 443rd case was the first death reported to have been confirmed positive posthumously on Feb. 21. Other than the two, the investigators highlighted the 1,443rd case reported on Feb. 27, as the patient was the first death reported during their self-isolation period. On Mar. 3, the 32nd case has reportedly been deceased without an underlying illness. The investigators also emphasized the descending trend of mortality rate while the number of confirmed cases has exponentially surged since the first death reported. They pointed out it indicates the management over patient with high severity is taking its place. In fact, on Feb. 20 when the first death was reported, the case fatality rate (CFR) was at 1.22 percent. But the CFR was dropped to 0.04 percent, as of Mar. 10. #And as of 12 a.m. Mar. 30, the COVID-19 CFR in Korea was at 0.7 percent. Categorized by each region, the number of deaths was highest in Daegu and Northern Gyeongsang Province, where 85 percent of confirmed cases were concentrated in. Daegu along had 38 patients died from COVID-19, but 13 have lost their lives in Northern Gyeongsang Province. However, the researchers pointed out the statistic figures are not an absolute indicator as a patient from Gangwon Province has died in a Northern Gyeongsang Province hospital, and patients from the Northern Gyeongsang Province have been treated at National Medical Center. Male patients in 70s with underlying health condition, three key words in COVID-19 death So who is most vulnerable to COVID-19? First, the investigators set down three key words—70s, male and underlying cardiovascular disorder. Analyzing critical cases resulted in deaths, the investigation found the median age at death was 75.5. And the mortality rate was higher in male patients at 61.1 percent than in female patients. The most prevalent underlying health condition was cardiovascular disease. The CFR analysis in the report supports the findings clearly. The CFR in male patients was at 1.16 percent with 33 deaths among 2,852 cases, but the rate was at 0.45 percent in female patients with only 21 deaths within 4,661 cases. The gap between sexes was even more significant in the older age group. The mortality rate in male patients 60 and over was at 4.73 percent with eight deaths in 592 cases, where as the rate in female patients in the same age group was at 1.88 percent with 19 deaths in 1,013 cases. # The report claimed the virus-induced death was also closely related to the age group. The CFR in age group of under 20 and 20s to 50s were at zero percent and 0.05 percent, respectively. But from the age group of 50 and up, the CFR was increased to 1.72 percent. Most of the deaths were reported with underlying health condition. Within 90.7 percent of deaths with underlying illnesses, 59.3 percent of them had cardiac disorder like hypertension, making it the most prevalent underlying disorder followed by diabetes and neurologic disease like dementia. The median time from onset symptoms to death of the particular patient group was ten days, regardless of their sex. And also the underlying illness has not affected the duration of survival time. The investigators explained, “Mortality rate is the most crucial indicator to consider when setting the prioritization of infectious disease control. Although it is too early to compare the tendencies with different countries, mortality rate ascending after identifying a mass infection was a meaningful finding.” “The most vital strategy is to maintain the capability of hospitals treating patients in initial stage to severe state by carefully and appropriately reviewing the disease,” and “As these key words from the investigation are the most significant key words as the mortality rate around the world is surging, an improved response with broader perspective based on them is needed.,” the investigators added.
- Product
- Who will succeed in COVID-19 treatment?
- by choi-sun Mar 25, 2020 06:01am
- As pharmaceutical companies around the world are plunging into the development of vaccines and treatments for COVID-19 treatment, attention is focused on who will succeed in commercialization. Among the treatments, Gilead Sciences entered Phase III of clinical trials, and in the field of vaccines, it entered Phase I by Moderna Therapeutics. However, it is not the first to release it because it may fail clinically. Among the other candidates that follow, the mechanism is slightly different, and it is also a concern that they may be able to get the title of a COVID-19 treatment or COVID-19 vaccine. ▲Treatment = Pre-Clinical ~ Phase III There are two ways to respond to the COVID-19. There is a 'therapeutic agent' that suppresses the proliferation of the virus after infection, and there is a 'vaccine' that focuses on preventing infection. COVID-19 is a type of RNA virus that shares its roots with SARS and MERS. A similar principle is that previously used Ebola treatments are used to treat COVID-19. #Remdesivir, an Ebola treatment that Gilead Science is developing, has a mechanism to inhibit RNA polymerase necessary for virus replication and proliferation. It is for this reason that it quickly entered phase III clinical trials. On the 26th of last month, Gilead started phase III of Remdesivir in the United States and China, followed by investigational new drug application on the 2nd of this month in Korea and completed clinical preparation. In fact, Seoul National University Hospital on the 9th concluded a clinical research agreement with the National Institutes of Health and started enrolling patients immediately from this day, so it is expected that the results will be confirmed in the first half of this year. The main goal is whether to lower the boby temperature and get cured within two weeks at the same time. However, it is still unknown whether Ebola virus clinical trials will lead to commercialized products in that it failed with a high mortality rate compared to competing drugs. The rest of the treatment remains in the Pre-Clinical, except that Remdesivir is in Phase III clinical trials. This means that it takes at least one or two years to confirm the reality. Johnson & Johnson is approaching COVID-19 as a treatment and vaccine development based on its past experience in developing Ebola and Zika viruses. Johnson & Johnson is developing a virus-inactivating vaccine at the preclinical stage, and is also working with the Biomedical Advanced Research and Development Authority (BARDA) to develop therapeutics for patients who are already infected. Through this, it is examined whether existing medicines, such as Remdesivir, an Ebola treatment, Kaletra, an AIDS treatment, and Avigan, a flu drug developed in Japan, are effective in suppressing COVID-19. US bio-company Regeneron Pharmaceuticals is focusing on the development of antibody therapeutics through genetic engineering. Although it is still in the preclinical stage, Regeneron plans to develop an antibody therapy against COVID-19 through mouse genetic manipulation technology capable of producing human antibodies. The candidate is expected to go into animal testing soon, and is preparing for human clinical trials by the third quarter of this year. In fact, during the Ebola incident in 2015, Regeneron had experience in developing a mixed-antibody drug that doubled the patient's survival rate. Vir Biotechnology is also developing antibody-based therapeutics. The difference from Regeneron is that instead of generating antibodies by genetic manipulation, antibodies that are naturally produced after infection with similar corona-like viruses such as SARS in the past are used. In fact, this method has been applied to MERS and SARS in the past. A method of collecting serum from a patient who formed a natural antibody and administering it to a serious patient was achieved. Vir Biotechnology is investigating whether the SARS virus is similar to COVID-19, so that antibodies from patients recovering from SARS can be isolated and applied to COVID-19. #Vir is in the early stages of development in cooperation with Chinese company WuXi Biologics, and it is not clear when the clinical trial is expected. ▲Vaccine = Pre-Clinical ~ Phase I Currently, phase I is at the forefront from vaccine development. Modena therafutics developed the vaccine candidate mRNA-1273 only 42 days after the nucleotide sequence of the COVID-19 was confirmed and jumped to the promising group. mRNA has a mechanism in the body that allows cells to form antibodies on their own. Modena is expected to begin research next month with healthy volunteers in cooperation with the National Institutes of Health (NIH). Once the safety of mRNA-1273 has been demonstrated, Phase II will be undertaken to confirm the effectiveness of the vaccine in hundreds. However, Modena, which is developing mRNA or mRNA designed for cells in the body to produce antibodies against viruses, has not been approved by the FDA as a related drug. Except for Modena, vaccines of various mechanisms are still in the Pre-Clinical. CureVac, like Modena, develops a vaccine that promotes the production of antibody proteins using synthetic mRNA. It is expected to be ready for clinical trials within a few months. China's Clover Biopharmaceuticals, was transferred its technology to a proprietary adjuvant compound that improves the effectiveness of the vaccine from GSK in February and started developing vaccines. The company is developing a vaccine against infection by injecting a protein that stimulates the immune response and activating body immunity, but the clinical trial plan has not yet been detailed. Inovio, the United States, has been developing DNA medicines for the past 40 years, and has received subsidies for the development of DNA-based vaccines from the Coalition for Epidemic Preparedness Innovations (CEPI). In partnership with Chinese manufacturer Beijing Advaccine Biotechnology, INO-4800 candidate vaccine is in Pre-Clinical, and clinical trials are expected in the second half of this year. Sanofi, which has successfully developed a yellow fever and diphtheria vaccine, is working with the BARDA to develop COVID-19 vaccine. Sanofi aims to create a chimeric DNA vaccine that activates the patient's immunity by mixing a portion of the DNA of COVID-19 with the genetic material of the harmless virus. The vaccine candidate is expected to be tested in the laboratory within 6 months, and the clinical trial is expected to be available within 1 to 18 months. Sanofi said the technology has been applied to SARS. However, Sanofi expects to take at least three years to get approval. Experts say it is too early to make a positive outlook, as various therapeutics and vaccines are still in the Pre-Clinical of exploring candidates and evaluating safety. Professor Ki-seok Jeong of the department of Pulmonology at Hallym University said, "I think it is difficult to cure the drug in the near future. However, in the case of Remdesivir, an ebola drug, it would be better if indications were added as much as it promotes clinical trials in Korea". "An RNA virus has many mutations, so it is not easy to develop a therapeutic drug and it is more difficult to develop a vaccine. Considering that multinational companies spend ₩1 trillion on new drug development, a government-level investment in development is necessary" he advised.