- LOGIN
- MemberShip
- 2025-12-18 18:40:20
- Fexuprazan taking over K-CAB market? Study unveiled
- by choi, sun | translator Byun Kyung A | 2020-05-11 06:15:13
As Daewoong Pharmaceutical unveiled Phase III clinical data of its next-generation gastroesophageal reflux disease agent in development, fexuprazan, the Korean pharmaceutical industry is keeping a close eye on the prospective competition between the existing proton pump inhibitors (PPI) tegoprazan (Brand name: K-CAB) and the novel agent.
Reversibly blocking the proton pump, a potassium-competitive acid blocker’s (P-CAB) efficacy compared to PPI has been confirmed through the clinical trial conducted in Korea, but the market competition would heavily rely on pricing, indication and improved efficacy.
▲Improved efficacy against PPI esomeprazole confirmed On May 2, the Korean pharmaceutical company presented the Phase III clinical data of fexuprazan at Digestive Disease Week (DDW) 2020 as an e-Poster.
Daewoong Pharmaceutical’s novel agent treating gastroesophageal reflux disease (GERD), fexuprazan is a P-CAB that reversibly blocks the proton pump secreting gastric acid from the stomach walls.
Whereas P-CAB agent is considered a new generation of medicine that covers most of the limitations PPI faces.
The Phase III clinical trial was conducted in patients with erosive esophagitis at 25 hospitals in Korea, and it compared efficacy in 40 mg of PPI esomeprazole (n=111) and 40 mg of P-CAB fexuprazan (n=107).
Until week 4, fexuprazan and esomeprazole respectively demonstrated endoscopic mucosal healing rate at 90.3 percent and 88.5 percent, but they reached 99.1 percent at week 8.
Specifically, P-CAB showed comparatively faster and better heartburn relief.
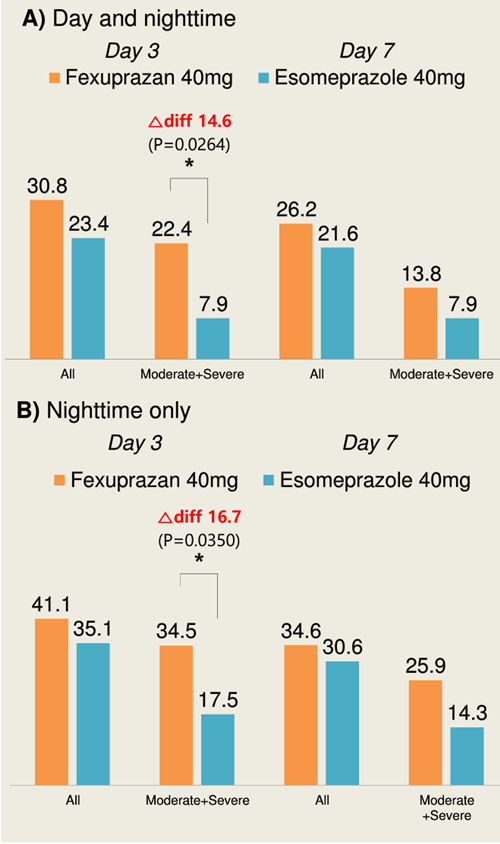
30.8 percent of fexuprazan group and 23.4 percent of esomeprazole group had their day and nighttime symptoms relieved at day 3.
Comparing only patients with moderate to severe symptoms, 22.4 percent of fexuprazan group experienced symptom relief, whereas only 7.9 percent of esomeprazole group did.
At day 7, the heartburn symptom relief rates were recorded at 26.2 percent and 21.6 percent in fexuprazan and esomeprazole users, respectively.
Comparing again the patients with moderate to severe symptoms, fexuprazan demonstrated better rate at 13.8 percent against 7.9 percent.
The results were similar when comparing symptom relief during nighttime.
Reportedly, PPI was unable to maintain the effect during nighttime.
The nighttime heartburn relief rate of fexuprazan and esomeprazole at day 3 each marked 41.1 percent and 35.1 percent, but in patients with moderate to severe symptoms, the rate was at 34.5 percent and 17.5 percent, respectively.
The atypical symptom relief rates in patients with GERD were at 81.2 percent and 68.6 percent in fexuprazan and esomeprazole users, respectively, at Day 3.
And the rate remained around the same at week 8 at 80.6 percent and 69.3 percent, respectively.
Adverse reactions reported from both groups were about the same.
In the future, the novel agent would be inevitably compared to the ‘Old Drug,’ esomeprazole.
▲Competitive against the market-dominating tegoprazan?
In 2018, CJ Healthcare has received the government’s approval on the 30th Korean-made novel P-CAB agent ‘K-CAB (tegoprazan).’ In Japan, vonoprazan is released in the market, but K-CAB is the only P-CAB available in the Korean market.
As a follow-on drug, fexuprazan would attempt to take over the market from tegoprazan, unavoidably.
In last March, tegoprazan has been indicated to treat helicobacter pylori infection and also it has ongoing clinical trials regarding maintenance therapy after treating GERD and preventive therapy against nonsteroidal anti-inflammatory drug-induced duodenal ulcer to expand indications.
Then, how about the differences in their efficacy?
In March last year, a SCI-level medical journal Alimentary Pharmacology & Therapeutics (AP&T) published Phase III clinical data of tegoprazan.
Same with fexuprazan, the study compared tegoprazan’s efficacy and safety in patients with erosive esophagitis against esomeprazole’s.
The eight-week multicenter Phase III trial on tegoprazan conducted in Korea tested 302 patients with erosive esophagitis by administering 50 mg (n=100) and 100 mg (n=102) of tegoprazan and 40 mg (n=100) of esomeprazole.
At week 8, the mucosal healing rate of three patient groups all reached 98.9 percent.
As for fexuprazane, the rate was at 99.1 percent.
The heartburn rate in tegoprazan 50 mg group started from 1.76 and was increased to 0.53 and 0.56 at week 4 and week 8, and in 100 mg group the rate fell from 1.86 to 0.62 and 0.62 at the same period.
The rate in esomeprazole group was dropped from 1.84 to 0.48 and 0.47 at week 4 and week 8.
The prevalence of adverse reaction in 50 mg and 100 mg of tegoprazan users reached 28.3 percent and 23.5 percent, respectively.
The rate was similar in 40 mg of esomeprazole users with 30.3 percent.
Professor Kim Gwang Ha of Pusan National University Department of Internal Medicine, who participated in both tegoprazan and fexuprazan studies, explained “The clinical trial on fexuprazan confirmed significantly improved efficacy in the novel agent against esomeprazole with patients having moderate to severe symptoms,” and “when it gets released in the market, it could be more expensive than PPIs but the benefit could outweigh the high pricing.” He added, “Based on the acting time and effect of inhibiting proton pump faster and better than PPI demonstrated in the clinical trial, the novel agent would fulfill the medical unmet needs the existing PPIs lacked,” and “patients who failed to relieve the symptoms with PPI would benefit from P-CAB.” “However, the healthcare providers should be aware that not all P-CABs have same effect and safety profile,” so “their marketability and competitiveness should be more accurately assessed with further head-to-head studies between different P-CABs,” the professor noted.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










