- LOGIN
- MemberShip
- 2025-12-22 02:41:01
- Company
- SK Bioscience and Sanofi sign contract to co-develop
- by Cha, Jihyun Dec 26, 2024 05:51am
- SK Bioscience has extended the scope of vaccine development in collaboration with the global pharmaceutical company Sanofi. The company aims to advance the pneumococcal conjugate vaccine currently being developed to the next-generation vaccine. SK Bioscience and Sanofi announced on December 23 that they have signed an agreement to co-develop the next-generation pneumococcal conjugate vaccine for infants and young children. The new vaccine will provide a broad preventive effect compared to the currently commercialized products. This contract extends the scope of collaboration that both companies have previously signed for the development and commercialization of 'GBP410,' a 21-valent pneumococcal conjugate vaccine. Previously, SK Bioscience signed a co-development and sales agreement with Sanofi in 2014 for the next-generation pneumococcal conjugate vaccine. Based on the extended agreement, both companies plan to develop an innovative next-generation pneumococcal conjugate vaccine that is more advanced than 21-valent vaccines. Given new projects, SK Bioscience will receive a 50 million euros (about KRW 75.5 billion) upfront payment from Sanofi. The company will receive additional milestones per stage until the development is completed. Both companies will be equally responsible for development costs. Sanofi will be responsible for commercialization costs. Once commercialized, SK Bioscience will sell the vaccine in South Korea, and Sanofi will be responsible for global sales. Both companies will equally share revenues generated from sales at a predetermined rate. A conjugate form of pneumococcal vaccine is known to offer superior preventive effects among the developed pneumococcal vaccines. As of 2023, it takes 94% of the sales of the pneumococcal vaccine market worldwide. According to the global pharmaceutical statistics agency Evaluate Pharma, the pneumococcal vaccine market has achieved a compound annual growth rate (CAGR) of 4.7%. The market is projected to grow from KRW 11.9 trillion in 2024 to KRW 14.2 trillion by 2028. SK Bioscience plans to target the global pneumococcal vaccine market with advanced technology, aiming to secure a new growth engine for the future and establish itself as a leading global vaccine and biotech company. Photo of SK Bioscience In addition to developing next-generation vaccines, the clinical trial of GBP410 conducted by both companies is progressing smoothly. Last week, GBP410 entered a multinational Phase 3 clinical trial, with the first participant dosed. The Phase 3 trial involves over 7,700 infants, children, and adolescents aged six weeks to 17 years, comparing the immunogenicity·safety of up to four doses of GBP410 against an already approved pneumococcal vaccine. SK Bioscience and Sanofi confirmed the efficacy and safety of GBP410 in a Phase 2 clinical trial conducted last June. The comparative study involved 140 children aged 12 to 15 months and 712 infants aged 42 to 89 days, evaluating GBP410 against a control vaccine (Prevenar 13) for primary and booster vaccinations. The results demonstrated that the immunogenicity of GBP410 was comparable to that of the control vaccine. Regarding safety, no vaccine-related serious adverse events were reported in the GBP410 group. Even when co-administered with other recommended vaccines for infants and children, such as vaccines for tetanus, diphtheria, pertussis, polio, and Haemophilus influenza type B, GBP410 exhibited equivalent immunogenicity and safety to the control vaccine. GBP410 was the first to include a serotype over a 20-valent among the vaccine candidates that entered a phase 3 trial targeting infants. SK Bioscience anticipates that GBP410 will reduce invasive pneumococcal disease (IPD) in infants and young children. "The contract extension between SK Bioscience and Sanofi was based on trust between two companies, given the potential of a 21-valent vaccine and positive market outlook," Jaeyong Ahn, CEO of SK Bioscience. "As Korea's key company in the vaccine and biotech industry, SK Bioscience will secure vaccine market share and strive to launch a blockbuster vaccine successfully."
- Company
- Daiichi Sankyo to build manufacturing facilities in China
- by Kim, Jin-Gu Dec 26, 2024 05:50am
- Daiichi Sankyo will build a manufacturing facility in China for its ADC (antibody-drug conjugate) anticancer drug Enhertu. The plant, which will be built in Shanghai, is scheduled to be completed in 2030, and its products will be supplied to China. According to KoreaBIO, Daiichi Sankyo recently announced plans to build a manufacturing facility for Enhertu in Shanghai, China. A total of USD 152 million (approximately KRW 220 billion) will be invested in the construction of the facility. Completion is expected in 2030. Daiichi Sankyo explained that the products produced here will be supplied in China. The new construction plan is attributed to the fact that Enhertu was listed for health insurance reimbursement. China's National Healthcare Security Administration (NHSA) recently released the National Reimbursement Drug List for Basic Medical Insurance, Work-Related Injury Insurance and Maternity Insurance (NRDL). The list includes 91 new drugs, including Enhertu, that are covered by health insurance. The list includes 26 anti-cancer drugs, 15 drugs for chronic diseases such as diabetes, 13 drugs for rare diseases, 7 anti-infectives, 4 drugs for mental illness, 11 herbal medicines, and 21 other drugs. Their health insurance coverage will take effect next January. Pharmaceutical companies have been negotiating their drugs’ prices to be included in China's health insurance program. Of the 91 items to be reimbursed next year, 89 have undergone such price negotiations. The average reduction in drug prices is 63%. China's health authorities estimate that CNY 50 billion (about USD 10 trillion) in patient cost savings will be realized next year through reimbursement alone. However, the exact percentage reduction for Enhertu has not been disclosed. Enhertu was initially approved in China in 2023 for HER2-positive breast cancer. It has since been expanded to include HER2 low-expressing breast cancer, HER2-positive gastric or gastroesophageal junction cancer, and HER2-mutant NSCLC. “The ADC manufacturing facility for Enhertu is expected to be operational in 2030,” said Daiichi Sankyo. ”This investment marks the first instance of establishing an ADC manufacturing facility in China, and the products produced here will be supplied to China.”
- Company
- K-Bios seek drugs to be used in combination with ADCs
- by Son, Hyung Min Dec 26, 2024 05:50am
- The domestic pharmaceutical and bio-industry are changing clinical trial protocols and confirming the possibility of their use in combination therapy with antibody-drug conjugates (ADCs). In particular, a growing number of companies are trying to use their drugs in combination with Enterhu, which has shown an effect across solid cancers For example, GI Innovation and AbClon are aiming to maximize the effectiveness of their existing immuno-oncology and targeted anti-cancer drugs by using their drug in combination with Enhertu. In particular, they expect to show benefits in terms of side effects by combining a reduced dose of Enhertu with their respective new drug candidates that are under development. Change in clinical trial protocol…expects to double their drug’s effect by adding Enhertu Daiichi Sankyo and AstraZeneca According to industry sources on the 24th, GK Innovation and AbClon have been evaluating the possibility of combining their respective drug candidates with Enhertu. Enhertu is a new antibody-drug conjugate anticancer drug that was codeveloped by Daiichi Sankyo and AstraZeneca. It is a next-generation ADC that combines a monoclonal antibody with the same structure as trastuzumab, which binds to a specific target receptor overexpressed on the surface of cancer cells, and a topoisomerase I inhibitor payload with a tumor-selective cleavable linker, which is a novel and highly potent mechanism of action. ADCs are anticancer drugs manufactured by linking an antibody that binds to a specific target antigen on the surface of cancer cells with a drug that has cell-killing (cytotoxic) properties. ADCs act selectively on cancer cells, by using the selectivity of antibodies to their targets and the killing activity of drugs to increase therapeutic efficacy while minimizing side effects. While the first-generation ADC, Roche’s Kadcyla, was only approved for breast cancer, second-generation ADCs such as Enhertu have been succeeding in securing a variety of indications. Currently, Enhertu is approved for HER2-positive gastric cancer, breast cancer, and non-small cell lung cancer. The domestic pharmaceutical and biotech industry has also taken note of the effectiveness of Enhertu and is trying to change their clinical trials to attempt its use as a combination therapy. GI Innovation recently changed a Phase I/II clinical trial for its immuno-oncology drug candidate 'GI-102' in the U.S. to a study to confirm its efficacy in combination with Enhertu. GI-102’s pipeline targets tumors and immune cells by targeting CD80 and interleukin (IL)-2 and has been engineered to have lower alpha receptor binding compared to GI-101A. High alpha receptor binding is known to increase regulatory T cells, which reduces the anti-cancer effects. GI-102 is being developed as both intravenous (IV) and subcutaneous (SC) formulations. GI-102 has also shown promise as monotherapy in trials. Recently, the company's Phase I/IIa data showed an objective response rate (ORR) of 43% when GI-102 was administered to patients with melanoma. In addition, lymphocyte proliferation was enhanced by GI-102 treatment, with no serious drug toxicity observed. Therefore, GI Innovation expects that the combination of GI-102 and Enhertu will bring greater effect. The company believes that the combination of GI-102 with a reduced dose of Enhertu can reduce side effects such as interstitial lung disease (ILD) that occur with Enhertu alone. AbClon recently announced that a new IND for its lead drug candidate, AC1-01, has been approved in China. AC-101 is an antibody-drug developed by AbClon that targets HER2 mutations. Its technology was licensed out to Henlius in China in 2016. The new trial will test the effectiveness of AC-101 in combination with Herceptin or Enhertu, both of which are used in breast cancer. With this change, AbClon plans to test its potential in gastric cancer and other solid tumors. Previously, AC-101’s efficacy was validated in combination with Herceptin. In patients with HER2-positive gastric cancer, the ORR, which signifies the reduction in tumor size measured at 72 weeks post-dose, was 41.2% in the low-dose arm, 16.7% in the high-dose arm, and 5.6% in the control arm. Based on such results, the company expects the addition of Enhertu to extend the benefits of AC-101 across HER2-positive solid tumors. Voronoi is also open to the possibility of combining its drug with Enhertu. The company is developing VRN10, a HER2-positive targeted therapy. VRN10 entered Phase I clinical trials last month. The Phase I trial of VRN10 is being conducted at 5 sites in Korea and Australia in approximately 70 patients with solid tumors, including HER2-positive breast cancer. In preclinical studies, VRN10 was found to be highly active against Enhertu-resistant cells. Voronoi believes that its selectivity for the HER2 biomarker may improve side effects such as diarrhea and dermatitis, and its brain penetration is superior to existing therapies. Voronoi expects the combination of VRN10 and the HER2 ADC to bring synergy.
- Company
- Losartan prescription market has grown 15% in 3 years
- by Chon, Seung-Hyun Dec 26, 2024 05:50am
- The prescription market for the antihypertensive drug losartan has shown an upward trend. Its market plummeted in 2021 following the detection of excess impurities in all losartan products but has since recovered obviously. Prescriptions of both single and combination losartan drugs have risen over 10% from three years ago. Analysts say the recurring impurity issues have diluted fears of impurities in drugs. According to the drug research institution UBIST, outpatient prescriptions for losartan-containing drugs totaled KRW 70.3 billion in the third quarter, up 4.7% year-on-year. Compared to KRW 64.3 billion in the third quarter of 2022, prescriptions have increased 9.4% in two years. Quarterly prescriptions of losartan drugs in Korea (Purple: losartan combination therapy, blue: losartan monotherapy) This is the first time in 3 years since the fourth quarter of 2021 that quarterly prescriptions for losartan drugs exceeded KRW 70 billion. The prescription market for losartan formulations has been on the rise since 2021, despite a significant decline in prescriptions following the exposure of its impurity issue. In September 2021, 183 lot numbers of 73 products across 3 ingredients - losartan, valsartan, and irbesartan - were recalled for excess impurities. Later in 2021, impurity issues arose in the entire losartan formulation. In 2021, 295 batches of losartan formulations from 98 companies were voluntarily recalled for exceeding or potentially exceeding the standard for ‘losartan azide impurities.’ Of the 306 items from 99 companies on the market, 96.4% were included in the recall. The prescription market for losartan-containing drugs was worth KRW 81 billion in the third quarter of 2021, down 24.6% from KRW 61.1 billion in the second quarter alone. During the same period, sales of losartan monotherapies decreased 33.9% from KRW 24.5 billion to KRW 17.8 billion, and losartan combination drugs decreased 19.9% from KRW 51.9 billion to KRW 44.7 billion. The drop was due to the exposure of impurity issues across all losartan formulations, which led to prescription changes to other drugs in the same angiotensin II receptor blocker (ARB) class. At the end of 2021, 94 of the 295 total losartan products from 34 different manufacturers were available, effectively avoiding the total sales halt of losartan drugs. The prescription market appears to have recovered as many of the losartan formulations have resolved their impurity issues and returned to the market. In the second quarter of 2022, the prescription market for losartan drugs rebounded to KRW 62.7 billion, a 2.6% increase YoY, and the upward trend has continued since. In the third quarter, the total prescription market for losartan formulations expanded by 15.1% compared to the first quarter of 2021. Both losartan monotherapy and combination drugs have seen recent gains. Outpatient prescriptions for losartan monotherapy totaled KRW 20.7 billion in the third quarter, up 16.3% from Q1 2022. This is the first time in three years since the fourth quarter of 2021 that the quarterly prescription volume for losartan monotherapy exceeded KRW 20 billion. Combination losartan prescriptions grew 14.6% from KRW 43.3 billion in Q1 2022 to KRW 49.6 billion in the third quarter of this year. The recurrence of impurity issues across ARBs, starting with valsartan in 2018, has diluted the fears of such impurities in the prescription market. In 2018, the Ministry of Food and Drugs suspended sales of 175 products containing valsartan, an ARB antihypertensive drug. In 2021, impurity issues arose in losartan, valsartan, and irbesartan. Among the ARB class antihypertensives, telmisartan, candesartan, fimasartan, and olmesartan were not affected. Even if impurities above the approved threshold are detected, unless a sales ban or large-scale recall is conducted, some analysts argue that the detection itself is unlikely to affect the prescription market because there is no clear evidence of human harm.
- Company
- Soyun Oh appointed to head Organon Malaysia
- by Whang, byung-woo Dec 24, 2024 06:22am
- Soyun Oh, Sales and Customers Lead, Organon Korea Organon Korea announced today that Soyun Oh, who currently heads the company’s Sales and Customer Department, has been appointed Country Lead for Organon Malaysia, effective January 1, 2025. With more than 26 years of experience in the industry, Oh has been with Organon since its inception in Korea and has successfully led the company's growth as its Sales and Customer Lead. As Sales and Customer Lead, Oh strengthened leadership in the chronic disease space, rapidly expanded the company's presence in women's health, stabilized the company's operations, and established a foundation for the company’s growth. Throughout her career, Oh has maximized revenue, expanded the portfolio, and driven multiple commercial successes through management strategies for key products including Atozet, Propecia, Singulair, Vytorin, and Cozaar. She has also demonstrated exceptional leadership and management skills in leading a sales organization that consists of over 150 people, contributing to the growth and development of the organization. In her new role as Country Lead of Organon Malaysia, Oh will leverage her wealth of experience and leadership capabilities to drive the company to new heights with the Malaysian team. Prior to Organon Korea, Mr. Oh held various leadership roles at MSD Korea, including Director of Primary Care (PC) and Director of Diversified Brands (DV) (Respiratory, Dermatology, Urology, etc.), with an initiative to drive the development and growth of the industry.
- Company
- SK Bioscience and Sanofi reinforce vaccine R&D partnership
- by Dec 24, 2024 06:22am
- SK Bioscience has expanded the scope of its vaccine development partnership with global pharmaceutical giant Sanofi. The goal is to develop a next-generation vaccine that is more advanced than the existing jointly developed pneumococcal protein-conjugate vaccine. The expanded agreement is more than 10 times larger in total value than the original agreement signed by the two companies a decade ago. When including the technology export agreement that SK Bioscience signed with Sanofi in 2018, SK Bioscience will receive more than KRW 160 billion from Sanofi. On the 23rd, SK Bioscience and Sanofi announced that they have entered into an agreement to jointly develop a next-generation pneumococcal protein-conjugate vaccine for infants, children, and adults that will provide broader protection than commercially available products. The agreement expands the scope of the companies' existing collaboration to develop and commercialize GBP410, a 21-valent pneumococcal protein conjugate vaccine candidate. In 2014, SK Bioscience signed an agreement with Sanofi to co-develop and commercialize a next-generation pneumococcal vaccine. Under the expanded agreement, the two companies plan to develop an innovative next-generation pneumococcal vaccine that is more advanced than the 21-valent vaccine. Under the new project, SK Bioscience will receive an upfront payment of EUR 50 million from Sanofi. Additional milestone payments will be made upon achievement of milestones until development is completed. The total value of the agreement is EUR 350 million (approximately USD 528.7 billion). R&D costs for the vaccine will be shared equally by the two companies. All costs related to commercialization will be borne by Sanofi. Upon commercialization, SK Bioscience will be responsible for sales of the vaccine in Korea, and Sanofi will be responsible for global sales. Revenue will be shared in a defined ratio based on product sales. Previously, SK Bioscience and Sanofi signed an agreement in 2014 to co-develop and market a next-generation pneumococcal vaccine with an upfront payment of USD 23 million (approximately KRW 25.6 billion). The total worth of the agreement, including upfront technology fees and milestones, was $45 million. The worth of the expanded agreement is more than 10 times larger in total value than the previous agreement signed by the two companies 10 years ago. In terms of down payment, this agreement is approximately 3 times larger than the previous agreement. This is the 10th year of R&D collaboration between the 2 companies and further strengthens their partnership. SK Bioscience-Sanofi R&D agreement (Source: FSS, SK Bioscience) In 2018, SK Bioscience also exported its cell culture-based high-efficiency influenza (flu) vaccine production platform to Sanofi. The contract is worth USD 155 million, including a USD 15 million upfront payment and a USD 20 million milestone payment upon completion of the technology transfer. The flu vaccine production platform agreement was terminated at the end of 2021 with Sanofi returning the rights. However, SK Bioscience has no obligation to return the upfront payment of USD 35 million (approximately KRW 40 billion). SK Bioscience had received all the payments for the GBP410 agreement signed in 2014. Including the payments from the Sanofi technology export agreement and this expansion agreement, SK Bioscience's total payments from Sanofi amount to KRW 165.5 billion. Last year, SK Bioscience and Sanofi also jointly invested in the expansion of manufacturing facilities for the commercialization of GBP410. In October 2023, SK Bioscience decided to invest KRW 81.5 billion to expand its vaccine production facility in Korea, the Andong L House. The investment amount, which will be decided by SK Bioscience's board of directors, will be combined with Sanofi's co-investment to build a new production facility of approximately 4,200㎡(1,300 pyung) at Andong L House. The expanded production facility will be utilized for the production of GBP410, which is being co-developed by the two companies. The facility is expected to be completed by May next year. SK바이오사이언스 안동L하우스 전경. (자료: SK바이오사이언스 GBP410 is currently in a Phase III clinical trial. GBP410 entered a multi-country Phase III clinical trial last week and started administration first subject. The GBP410 multinational Phase III study will compare the immunogenicity and safety of GBP410 to licensed pneumococcal vaccines after up to 4 doses in more than 7,700 infants, children, and adolescents aged 6 weeks to 17 years. SK Bioscience and Sanofi confirmed the efficacy and safety of GBP410 in a Phase II clinical trial in June last year. The study, which included an initial and booster dose of GBP410 and a control vaccine (Prevenar 13) in 140 children aged 12 to 15 months and 712 infants and toddlers aged 42 to 89 days, confirmed that the immunogenicity of GBP410 and the control vaccine was equivalent. In terms of safety, no serious vaccine-related adverse events were reported in the GBP410 arm. Equivalent immunogenicity and safety to the control vaccine were also demonstrated when coadministered with other recommended vaccines for infants and children, including tetanus, diphtheria, pertussis, polio, and Haemophilus influenza type B vaccines. GBP410 is the first vaccine candidate to enter Phase III clinical trials in infants and children to include more than 20 serotypes. With this, SK Bioscience believes GBP410 will contribute significantly to reducing the frequency of invasive pneumococcal disease (IPD) in infants and young children. “The agreement expansion between SK and Sanofi is based on the high success potential of the 21-valent vaccine, positive market outlook, and mutual trust,” said Jae-Yong Ahn, President and CEO of SK Bioscience. ”As a Korean vaccine and bio leader, we will do our best to secure vaccine sovereignty and the successful launch of a blockbuster vaccine.
- Company
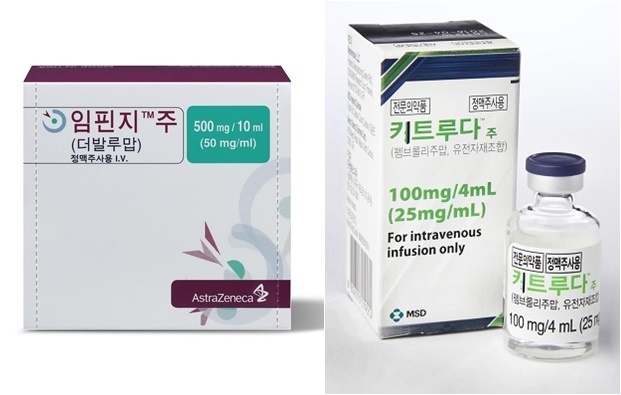
- Keytruda faces challenge from 'Steep Slope' CDRC
- by Moon, sung-ho Dec 24, 2024 06:21am
- The Cancer Disease Review Committee (CDRC) of the Health Insurance Review and Assessment Service (HIRA) is the first and most challenging hurdle in reviewing the insurance reimbursement of new anticancer drugs. A required step toward obtaining reimbursement listing, the committee has been nicknamed a "steep slope," giving many anticancer drugs a hard time. The industry faced significant challenges this year as well. New anticancer drugs from major global pharmaceuticals have been submitted for CDRC review but failed. Yet, some products passed the CDRC and successfully obtained reimbursement or expansion. Medical Time reported this year's review outcomes and next year's key news based on HIRA's CDRC documents and reporting from each pharmaceutical company. It was reported that the HIRA held nine CDRC meetings this year and discussed about the necessity of establishing reimbursement criteria for new anticancer drugs. After the meetings, the CDRC approved around 20 drugs that require establishing or expanding reimbursement criteria. If we were to pick a single treatment that gained attention from the pharmaceutical company and clinical practices, it would be immune checkpoint inhibitors. One of those immune checkpoint inhibitors is AstraZeneca's Imfinzi (durvalumab) and MSD Korea's Keytruda (pembrolizumab). These drugs have been submitted to establish reimbursement criteria for respective cancer types and have been followed to see if they will pass the CDRC review. At this year's CDRC meeting, Imfinzi passed, whereas Keytruda failed. Keytruda will need to submit again for next year's insurance reimbursement approval. In the case of Imfinzi, the drug was approved for requiring reimbursement criteria expansion for the treatment of bile duct cancer at the eighth CDRC meeting held in November. Additionally, Imfinzi's approval also led to the approval of Imjudo (tremelimumab), another treatment for bile duct cancer. Imfinzi will likely be considered for reimbursement for treating bile duct cancer and liver cancer at the Drug Reimbursement Evaluation Committee (DREC) review scheduled for next year. Despite its multiple attempts to obtain reimbursement expansion since last year, Keytruda has not succeeded. In the case of Keytruda, Keytruda has been submitted for reimbursement of almost 17 types of cancer. However, its attempt is stuck at the CDRC due to potentially involving substantial national health insurance finance. As of December 2024, Keytruda was approved for 33 indications in 17 cancer types. It has been submitted to the CDRC for insurance reimbursement of 17 indications. The company has applied for reimbursement of 13 indications. Then, it has added four additional indications, including ▲Gastric cancer with MSI-H ▲Bile duct cancer with MSI-H ▲HER2-positive gastric cancer ▲HER2-negative gastric cancer. In October, MSD Korea submitted a new proposal for financial contributions to expand reimbursement criteria for 17 indications, including gastric cancer. The company has made significant efforts this year to establish these reimbursement criteria. However, the company received a decision of 'reconsideration' for gastric cancer at the year's last CDRC meeting despite suggesting additional financial contributions. As a result, none of the 17 indications passed the CDRC hurdle this year. It has been reported that the members of CDRC were not satisfied with the additional financial sharing proposal presented by MSD Korea. The results indicate that there were more opposing opinions than supportive ones. "Although both are immune checkpoint inhibitors, the situations of Imfinzi and Keytruda are different. Imfinzi focuses on biliary tract cancer and liver cancer, while Keytruda is pushing for reimbursement expansion for 17 cancer types," a university hospital oncology professor and committee member stated. "As a result, Imfinzi's company has proposed a satisfactory financial contribution proposal, but it is challenging for Keytruda's company to present a corresponding plan due to its wide range of indications." "In other words, Imfinzi is focused on biliary tract cancer and liver cancer, where reimbursement expansion is urgently needed, and it has accepted significant financial contribution," professor added. "However, applying this standard to Keytruda, which has 17 indications, won't be easy. The last CDRC meeting only reviewed Keytruda for gastric cancer, and it seems there were more negative opinions about the financial contribution proposal." Additionally, the focus of CDRC discussions in the second half of this year has been on new drugs for blood cancers. This change is related to the recent introduction of bispecific antibody-based therapies for blood cancer in the Korean market, with their active pursuit for committee approval starting at the end of this year. Bispecific antibody drugs indicated for treating blood cancers include ▲Roche's Lunsumio (mosunetuzumab), Columvi (glofitamab) ▲Janssen's Rybrevant (amivantamab), Tecvayli (teclistamab), Talvey (talquetamab) ▲AbbVie's Epkinly (epcoritamab) ▲Pfizer's Elrexfio (elranatamab). Seven drugs received approval in South Korea. Among these, Roche's Columvi, AbbVie's Epkinly, and Janssen's Tecvayli have been submitted for the DREC review. Columvi and Epkinly are treatments for Diffuse Large B-Cell Lymphoma (DLBCL), a type of blood cancer. Tecvayli is a treatment for multiple myeloma. These drugs have been submitted to the DREC review but failed. They all received 'unestablished reimbursement criteria' decision and were not given 'reconsideration.' Consequently, the companies accepted the review result as equivalent to failure. In the case of Columvi, Roche Korea pushed to pass the CDRC this year but failed to establish reimbursement criteria at the CDRC meetings held in July and December. Even the patient organizations have joined the pursuit of requesting the establishment of reimbursement criteria but failed, delaying another attempt next year. Epkinly, which has the same indication as Columvi, was reviewed during this year's final CDRC meeting. However, the decision not to establish reimbursement criteria indicated a challenging reevaluation process ahead. This is the current landscape of bispecific antibody-based therapies held by global pharmaceutical companies. These therapies will become a key focus in next year's CDRC discussions. The discussions on blood cancer treatments may become a key issue depending on the results of next year's meetings. There are increasing demands from clinical fields for the reimbursement of these therapies. Additionally, as discussions surrounding reimbursement for blood cancer treatments intensify, there is an increasing demand for establishing a dedicated discussion body led by the Korean Society of Hematology to address these issues with the HIRA. In response to the growing prevalence of high-cost blood cancer treatments, HIRA expanded the CDRC this year, adding two hematology experts to the panel, now comprising nine members. This move is a response to the rapid introduction of innovative blood cancer therapies by global pharmaceutical companies and the increasing demand for their reimbursement. By incorporating more expert opinions, HIRA aims to enhance discussions surrounding the reimbursement of blood cancer therapies, ensuring that the concerns of clinicians specializing in hematology are better represented. However, clinical practices responsible for treating blood cancer are unsatisfied with the HIRA's decisions. "Two additional blood cancer experts were indeed appointed during the reorganization of the 10th CDRC members. Seven blood cancer experts are on the committee, including one from HIRA, while the remaining six are from university hospitals," Seok Jin Kim, Chair of the Korean Society of Hematology and a hematology-oncology specialist at Samsung Medical Center, stated. Professor Kim pointed out that "Over the past two years, 36 new blood cancer therapies have undergone review, compared to 58 new solid tumor therapies discussed during the same period. Out of the 43 members of the CDRC, only 5.5 members can be considered experts in blood cancer. This composition may not be adequate for evaluating blood cancer cases properly."
- Company
- Oral contraceptive market expands
- by Whang, byung-woo Dec 23, 2024 04:19pm
- As the oral contraceptive market expands, Yaz seems to maintain its market share despite the introduction of generics. According to a report by global market research firm Research and Markets, the global oral contraceptive market is expected to grow from approximately KRW 33.8 trillion (USD 23.6 billion) in 2023 to around KRW 67.3 trillion (USD 47.1 billion) by 2028. Product photo of Yaz.The South Korean market also shows a consistent growth rate, similar to the global trend, with a market size of approximately KRW 42 billion in 2023, encompassing both prescription drugs and over-the-counter drugs. Contraceptives, which contain hormones such as estrogen and progesterone, are classified into four generations. The first-generation drugs have been withdrawn from the market due to side effects. Second- and third-generation oral contraceptives are available as over-the-counter medications and are distributed through pharmacies. The third-generation contraceptives minimize the side effects of the second-generation, such as acne, hirsutism, and weight gain. Bayer's Yaz (drospirenone/ethinyl estradiol) has the largest market share. In 2023 (based on IQVIA), the sales of Yaz amounted to KRW 17.4 billion, holding 41% of the market share and remaining as the No.1 drug in the market. After obtaining domestic approval in 2008, Yaz has been increasingly prescribed over the past 15 years. Contrary to expectations for heightened competition following the patent expiration of Yaz in 2020, it is meaningful that Yaz has maintained its status as the market leader. 5-year sales trend of Bayer The five-year sales of Yaz amounted to ▲KRW 14.4 billion in 2019 ▲KRW 15.9 billion in 2020 ▲peaked at KRW 19.8 billion in 2021, and slightly decreased to KRW 17.5 billion in 2022. Although the sales declined due to the introduction of generics, the original product seems to maintain its impact in the market. Currently, generic versions of Yaz are available as ▲Hyundai Pharm's Yaroz ▲GL Pharma's Plan-A ▲Alvogen Korea's Gvez ▲TheU Pharmaceuticals' Yamiz ▲Kwang-dong Pharm's Esleesi. Besides Yaroz generating KRW 2.1 billion last year, the rest of the generics have not had a significant impact in the market. The analysis is Yaz's impact stems from being the fourth-generation original oral contraceptive as well as having indications for various menstruation-related conditions, such as ▲dysmenorrhea ▲premenstrual dysphoric disorder, and ▲moderate acne treatment. 2023 sales report of Yaz and Yaz generics: from the top, Yaz, Yaroz, Plan-A, Gvez (unit: KRW 100 million, source: IQVIA). The number of patients with menstruation-related conditions in South Korea has been steadily increasing over the past five years. Dysmenorrhea showed an increase of approximately 85% (162,020 in 2017→299,115 in 2022), while premenstrual dysphoric disorder (PMDD) increased by approximately 34% (11,442 in 2017→15,296 in 2022). Regarding this, Bayer emphasizes that they have obtained results of Yaz from real-world studies involving 410 patients with dysmenorrhea. This study demonstrated that short-term and long-term administration of Yaz immediately relieved pain and symptoms and recovered endometrial thickness to normal. Additionally, in a real-world study conducted at 68 domestic hospitals involving 770 healthy women aged 18–50 who visited gynecologists for contraception regardless of premenstrual dysphoric disorder status, 92.3% of participants reported an improvement in premenstrual dysphoric disorder symptoms after six cycles of treatment with Yaz. Expert analysis indicates that real-world data (RWD) from various studies have impacted the prescription of the drug, considering that the contraception and menstruation-related diseases require long-term administration. "Menstruation diseases, such as dysmenorrhea, significantly impact women's lives, and the disease prevalence rate is increasing, requiring more attention to treatments," Dr. Yun Bo Hyun, Professor of Severance Hospital's Department of Obstetrics and Gynecology, said. "The disease requires long-term and frequent treatments. When choosing a treatment option, patients' conditions must be considered for potential incompatibility in combining oral contraceptives." Dr. Yun added, "Yaz has 15 years of prescription history in South Korea, and it is a treatment option with confirmed benefits of improving symptoms related to menstrual diseases and established safety based on numerous studies."
- Company
- Multidisciplinary approach required for urothelial carcinoma
- by Whang, byung-woo Dec 23, 2024 05:50am
- New drugs, such as immunotherapy, have been introduced as the first-line treatment for urothelial carcinoma, which was primarily treated with platinum-based chemotherapies. The treatment landscape for the disease is constantly evolving. As more treatment options became available, doctors are now discussing the optimal treatment for each disease stage to establish the standard therapy. Discussions about being made about curative intent, in other words remission, in the long term. Dr. Enrique Grande, professor at MD Anderson Cancer Center Madrid, and Dr. In-ho Kim, professor at Seoul St. Mary's Hospital, have discussed the treatment landscape and unmet needs of urothelial carcinoma. (From left) Dr. In-ho Kim, professor at Seoul St. Mary's Hospital and Dr. Enrique Grande, professor at MD Anderson Cancer Center Madrid Urothelial carcinoma (UC) is a type of cancer that begins in the epithelial cells lining the urinary tract and accounts for approximately 90% of all bladder cancer diagnoses, making it the most common form of bladder cancer. Unlike other cancer types, such as lung and breast cancer, where the introduction of new drugs has rapidly transformed first-line standard treatments, UC has historically been viewed as a challenging area for anti-cancer drug development, with substantial unmet needs for first-line treatment options. "The treatment landscape has been changing in the past few years. The treatments are now targeting various types of patients, including metastatic UC patients and management of surgical cycles for patients with muscle-invasive bladder cancer (MIBC)," Dr. Grande said. "The maintenance treatment Bavencio plays a significant role in the UC treatment landscape in South Korea," Dr. Kim said. "The enfortumab vedotin+pembrolizumab combination therapy and nivolumab+gemcitabine+cisplatin combination therapy have been approved as first-line treatments in South Korea, offering various treatment options for patients with UC." Additionally, significant advancements in the field have been made, including the approval of therapies such as enfortumab vedotin monotherapy and erdafitinib monotherapy for second-line or later treatments in South Korea. The first-line maintenance treatment Bavencio continues to generate sales…"Expected to play a significant role in South Korea" How are these various options utilized in clinical practice? Dr. Grande emphasizes that patients prioritize achieving treatment effects and improving their quality of life. "Patients with UC are typically heavy smokers in their 60s to 70s with accompanying chronic diseases, and the cancer is often detected before extensive metastasis has occurred," Dr. Grande said. "Treatment begins with chemotherapy to achieve therapeutic efficacy while maintaining quality of life, followed by first-line maintenance therapy options like Bavencio." Dr. Grande stressed the importance of using safe, low-toxicity treatments over long-term use, allowing patients to maintain their quality of life while receiving treatment safely. While the number of available treatment options in South Korea has increased, Bavencio as a first-line maintenance therapy still plays a critical role when considering the reimbursement in practices. Dr. Enrique Grande, professor at MD Anderson Cancer Center Madrid"Many options have been introduced to the treatment landscape for UC. However, in South Korea, Bavencio maintenance therapy continues to play a crucial role and is expected to remain a key option for a significant period," Dr. Kim said. "Bavencio is currently the only reimbursed first-line maintenance therapy option for UC in South Korea." In fact, Bavencio's data has been accumulating. Bavencio's company presented the SPADE study at ESMO Asia earlier this December, reaffirming the drug's efficacy and safety as a first-line maintenance therapy for patients with locally advanced or metastatic UC. The SPADE study is the first prospective study to evaluate the efficacy of Bavencio as a first-line maintenance therapy in the Asia-Pacific (APAC) region. Interim analysis results showed that at the 12-month final follow-up, 61 patients (67.0%) who received first-line chemotherapy treatment proceeded to Bavencio first-line maintenance therapy. Additionally, 72% of patients who received Bavencio maintenance therapy subsequently received second-line treatments. Additionally, the Bavencio study demonstrated safety through global clinical trials, showing a low incidence of adverse events during the treatment period and extending the quality-adjusted time without symptoms or toxicity (Quality-TWIST) to more than double compared to best supportive care (BSC). "A comparison between Bavencio combination therapy and optimal supportive care revealed no significant difference in the quality of life between the BSC and Bavencio combination therapy groups. These outcomes show an excellent tolerability profile for Bavencio maintenance therapy," Dr. Grande said. "Although the results were from a subgroup analysis, Bavencio as a first-line maintenance therapy used for one or two years has demonstrated significant clinical survival benefits," Dr. Grande remarked. "The proportion of patients who die within a short period is less than 10–20%, making the continuation of Bavencio as a first-line maintenance therapy after chemotherapy the optimal scenario." "Various changes to treatment options for UC…curative intent approach must be discussed" A common concern among UC treatment experts is identifying the characteristics of patient groups capable of achieving long-term survival. Dr. Kim highlighted the importance of considering both an individual’s health status and their socioeconomic environment. Dr. Kim stated that simplifying treatment sequences could improve patient tolerability rather than repeatedly administering multiple therapies over an extended period. Dr. In-ho Kim, professor at Seoul St. MaryDr. Kim said, "Palliative treatments aimed at prolonging life are important, and there are instances where patients achieve a cure while receiving maintenance therapy with treatments such as Bavencio." Adding, "I believe that for urothelial carcinoma to ultimately achieve the goal of curing cancer, the field of curative treatment must advance further." "In the current treatment landscape for UC, various novel therapies and combination regimens with different mechanisms are being introduced. However, we need more time to observe the real-world clinical effects of these newly introduced treatment options," Dr. Kim said. Additionally, Dr. Grande highlighted the importance of biomarker studies and multidisciplinary approaches to seek optimal treatment options. "We hope to establish a biomarker that can be utilized across treatment planning to surgical cycles and be used towards practices. There are many factors to consider when treating patients. A multidisciplinary approach involving urology, medical oncology, radiology, and nuclear medicine is essential," Dr. Grande remarked.
- Company
- Takeda's Firazyr reimbursable up to four doses per Rx
- by Whang, byung-woo Dec 23, 2024 05:49am
- Product photo of Firazyr Takeda Pharmaceuticals Korea announced on December 19 that the National Health Insurance reimbursement coverage for Firazyr (ingredient: icatibant acetate), a treatment for acute attacks of hereditary angioedema (HAE), has been expanded to up to four doses per prescription starting this December. According to partial revisions to the 'Coverage and Scope of Benefits (Pharmaceuticals),' as notified by the Ministry of Health and Welfare (MOHW), reimbursement coverage for Firazyr has been expanded from two to up to four doses per prescription. According to the revised criteria, patients with a history of at least three self-administrations who, in the past three months, have experienced ▲Either one or more acute attacks per month or ▲Required additional doses for an acute attack, are now eligible for reimbursement for up to four doses per prescription. Firazyr was approved in South Korea in June 2014 and has been reimbursed for adult patients since September 2018. In July 2019, the eligible age group was expanded to include pediatric patients aged two years and older, establishing a basis for quick response during emergencies across various age groups. Since March 2021, reimbursement for up to two doses per prescription has been implemented, allowing patients to better prepare for additional acute attacks. This recent reimbursement expansion reflects the need to address the limitations of previous reimbursement of up to two doses per prescription and improve treatment accessibility for HAE patients in Korea, considering the unpredictable onset and severity of acute swelling episodes. HAE patients often hesitated to use medication during initial acute swelling symptoms due to concerns about depleting their remaining treatment supply, resulting in repeated delays in addressing acute episodes. This status conflicted with international treatment guidelines, which recommend 'prompt treatment at the onset of acute swelling symptoms.' Both patients and healthcare professionals have consistently emphasized the need for improvements in the timeliness and safety of HAE disease management. In response, the Ministry of Health and Welfare and the Health Insurance Review and Assessment Service reviewed textbooks, clinical guidelines, and expert opinions comprehensively and decided to expand reimbursement for Firazyr to cover up to four doses per prescription for patients experiencing frequent acute swelling episodes or requiring additional doses. This measure has been implemented three years and nine months after the reimbursement of up to two doses per prescription. Analysis suggests that it is part of Takeda Pharmaceutical Korea's effort to provide a stable treatment environment for patients with hereditary HAE and solve unmet healthcare needs. "Expanded scope of reimbursement for Firazyr will strengthen treatment access to patients with HAE in South Korea. It is an important improvement implementing the recommendations by the international treatment guidelines," Kim Na-kyung, Takeda Korea Rare Disease Business Unit Head, said. "We are pleased to provide a stable treatment environment to patients who experience anxiety and risks associated with acute attacks due to HAE."