- LOGIN
- MemberShip
- 2025-12-22 09:29:03
- SK Bioscience and Sanofi reinforce vaccine R&D partnership
- by | translator Alice Kang | 2024-12-24 06:22:01

The goal is to develop a next-generation vaccine that is more advanced than the existing jointly developed pneumococcal protein-conjugate vaccine.
The expanded agreement is more than 10 times larger in total value than the original agreement signed by the two companies a decade ago.
When including the technology export agreement that SK Bioscience signed with Sanofi in 2018, SK Bioscience will receive more than KRW 160 billion from Sanofi.
On the 23rd, SK Bioscience and Sanofi announced that they have entered into an agreement to jointly develop a next-generation pneumococcal protein-conjugate vaccine for infants, children, and adults that will provide broader protection than commercially available products.
The agreement expands the scope of the companies' existing collaboration to develop and commercialize GBP410, a 21-valent pneumococcal protein conjugate vaccine candidate.
In 2014, SK Bioscience signed an agreement with Sanofi to co-develop and commercialize a next-generation pneumococcal vaccine.
Under the expanded agreement, the two companies plan to develop an innovative next-generation pneumococcal vaccine that is more advanced than the 21-valent vaccine.
Under the new project, SK Bioscience will receive an upfront payment of EUR 50 million from Sanofi.
Additional milestone payments will be made upon achievement of milestones until development is completed.
The total value of the agreement is EUR 350 million (approximately USD 528.7 billion).
R&D costs for the vaccine will be shared equally by the two companies.
All costs related to commercialization will be borne by Sanofi.
Upon commercialization, SK Bioscience will be responsible for sales of the vaccine in Korea, and Sanofi will be responsible for global sales.
Revenue will be shared in a defined ratio based on product sales.
Previously, SK Bioscience and Sanofi signed an agreement in 2014 to co-develop and market a next-generation pneumococcal vaccine with an upfront payment of USD 23 million (approximately KRW 25.6 billion).
The total worth of the agreement, including upfront technology fees and milestones, was $45 million.
The worth of the expanded agreement is more than 10 times larger in total value than the previous agreement signed by the two companies 10 years ago.
In terms of down payment, this agreement is approximately 3 times larger than the previous agreement.
This is the 10th year of R&D collaboration between the 2 companies and further strengthens their partnership.
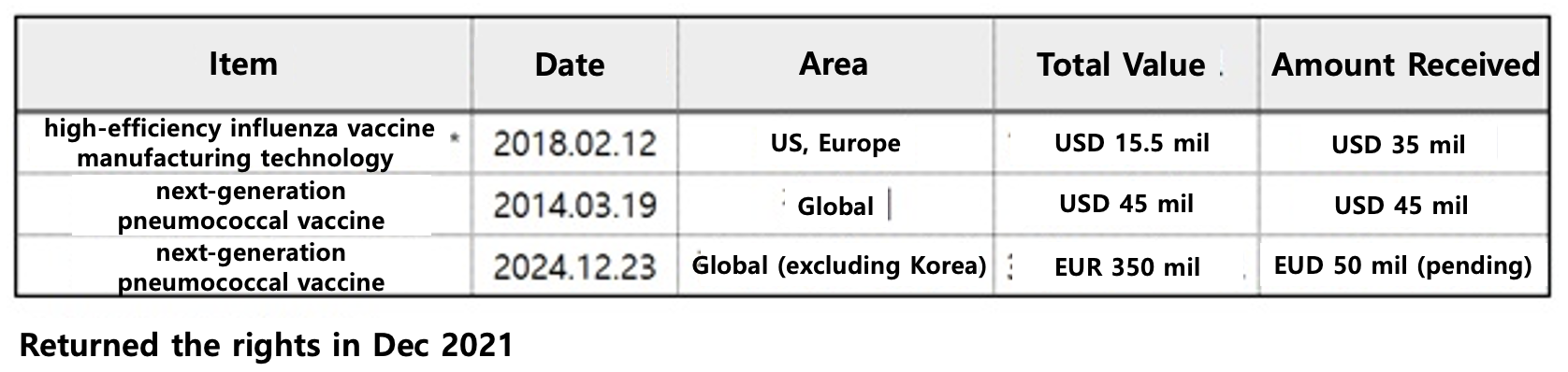
The contract is worth USD 155 million, including a USD 15 million upfront payment and a USD 20 million milestone payment upon completion of the technology transfer.
The flu vaccine production platform agreement was terminated at the end of 2021 with Sanofi returning the rights.
However, SK Bioscience has no obligation to return the upfront payment of USD 35 million (approximately KRW 40 billion).
SK Bioscience had received all the payments for the GBP410 agreement signed in 2014.
Including the payments from the Sanofi technology export agreement and this expansion agreement, SK Bioscience's total payments from Sanofi amount to KRW 165.5 billion.
Last year, SK Bioscience and Sanofi also jointly invested in the expansion of manufacturing facilities for the commercialization of GBP410.
In October 2023, SK Bioscience decided to invest KRW 81.5 billion to expand its vaccine production facility in Korea, the Andong L House.
The investment amount, which will be decided by SK Bioscience's board of directors, will be combined with Sanofi's co-investment to build a new production facility of approximately 4,200㎡(1,300 pyung) at Andong L House.
The expanded production facility will be utilized for the production of GBP410, which is being co-developed by the two companies.
The facility is expected to be completed by May next year.

(자료: SK바이오사이언스 GBP410 is currently in a Phase III clinical trial.
GBP410 entered a multi-country Phase III clinical trial last week and started administration first subject.
The GBP410 multinational Phase III study will compare the immunogenicity and safety of GBP410 to licensed pneumococcal vaccines after up to 4 doses in more than 7,700 infants, children, and adolescents aged 6 weeks to 17 years.
SK Bioscience and Sanofi confirmed the efficacy and safety of GBP410 in a Phase II clinical trial in June last year.
The study, which included an initial and booster dose of GBP410 and a control vaccine (Prevenar 13) in 140 children aged 12 to 15 months and 712 infants and toddlers aged 42 to 89 days, confirmed that the immunogenicity of GBP410 and the control vaccine was equivalent.
In terms of safety, no serious vaccine-related adverse events were reported in the GBP410 arm.
Equivalent immunogenicity and safety to the control vaccine were also demonstrated when coadministered with other recommended vaccines for infants and children, including tetanus, diphtheria, pertussis, polio, and Haemophilus influenza type B vaccines.
GBP410 is the first vaccine candidate to enter Phase III clinical trials in infants and children to include more than 20 serotypes.
With this, SK Bioscience believes GBP410 will contribute significantly to reducing the frequency of invasive pneumococcal disease (IPD) in infants and young children.
“The agreement expansion between SK and Sanofi is based on the high success potential of the 21-valent vaccine, positive market outlook, and mutual trust,” said Jae-Yong Ahn, President and CEO of SK Bioscience.
”As a Korean vaccine and bio leader, we will do our best to secure vaccine sovereignty and the successful launch of a blockbuster vaccine.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










