- LOGIN
- MemberShip
- 2025-12-21 23:26:54
- Company
- 1st RSV preventive inj 'Beyfortus' can be prescribed
- by Eo, Yun-Ho Jan 06, 2025 05:56am
- Product photo of Beyfortus The first injectable antibody drug approved in South Korea to prevent respiratory syncytial virus (RSV), 'Beyfortus,' is now available for prescription at general hospitals. According to industry sources, Sanofi Korea's Beyfortus (nirsevimab) has passed the drug committees (DC) of medical centers, including Seoul National University Hospital, Cha University Gangnam Medical Center, Korea University Anam Hospital, Korea University Ansan Hospital, Cha University Bundang Medical Center, and Pusan National University Yangsan Hospital. Beyfortus was approved by the Ministry of Food and Drug Safety (MFDS) in May as an injectable antibody to prevent RSV. It can be administered to all newborns and infants during their first RSV season. Beyfortus can also be given to infants under 24 months who are at high risk for severe RSV disease during their second RSV season. The Phase 3 MELODY study, which was the basis of Beyfortus approval, showed that the lower respiratory infection by RSV was reduced by 74.5% in the Beyfortus-treated group. This study evaluated the effectiveness of Beyfortus in 3012 infants born after 35 weeks during their first RSV season. The effectiveness in preventing RSV infections requiring medical attention was assessed until 150 days after the Beyfortus administration. Based on the Beyfortus real-world evidence demonstrated in the interim results from the national immunization program in Galicia, Spain, infants six months or younger who were administered Beyfortus had an 82% reduction in hospitalization due to RSC compared to non-administered infants. "Individuals of all ages can be contracted with RSV, but 90% of infants under 2 years old become infected. When infected, a mild cold symptom can advance to lung infections, possibly leading to hospitalization," Ki Wook Yun, Professor of Seoul National University College of Medicine, explained. "When infants without completely developed bronchial tubes contract RSV, symptoms can be worse. It could result in socioeconomic loss in addition to affecting family members." Yun added, "Since taking care of one's hygiene was the only RSV prevention available, there were unmet needs for RSV prevention. With the availability of injectable antibody drug, active RSV prevention is expected to be possible."
- Company
- Balversa may be prescribed at general hospitals in Korea
- by Eo, Yun-Ho Jan 06, 2025 05:56am
- The new bladder cancer drug Balversa may now be prescribed in Korea’s Big 5 tertiary hospitals in Korea. According to industry sources, Janssen Korea's FGFR-inhibiting urothelial carcinoma (bladder cancer) drug Balversa (erdafitinib) recently passed the drug committees (DCs) of tertiary hospitals including Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary's Hospital, Asan Medical Center, and Sinchon Severance Hospital. In addition, the drug’s prescription codes have been generated at major medical institutions across the country, including Kangnam Sacred Heart Hospital, GangNeung Asan Hospital, Hallym University Kangnam Sacred Heart Hospital, National Cancer Center, Keimyung National University Hospital, Ulsan National University Hospital, Korea Cancer Center Hospital, and Chonnam National University Hwasun Hospital. Balversa was approved by the Ministry of Food and Drug Safety in January 2022, but it is still not reimbursed in Korea. But expectations have been rising since Janssen submitted a reimbursement application for Balversa at the end of last year. As such, it remains to be seen if Balversa will be reimbursed by the end of the year and become a viable treatment option in Korea. Specifically, the drug is indicated for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma (mUC) with FGFR2 or FGFR3 genetic alterations whose disease has progressed on or after at least one line of prior systemic therapy, which includes platinum-based chemotherapy, or whose disease has progressed within 12 months of neoadjuvant or adjuvant treatment with platinum-based chemotherapy. However, the approval of PD-1 and PD-L1-directed immuno-oncology agents in the first- and second-line settings that followed Balversa’s approval led to the need for Balversa to demonstrate efficacy in patients who previously received these agents. The situation was addressed with the publication of Balversa’s Phase III THOR trial study, which demonstrated a prolonged overall survival (OS) benefit with Balversa over chemotherapy in patients with metastatic urothelial carcinoma with FGFR3/2 gene alterations whose disease progressed after first-line treatment with immuno-oncology agents. In the study, Balversa prolonged overall survival (OS) compared with chemotherapy in patients with metastatic urothelial carcinoma. Results showed that over a median follow-up of 15.9 months, the mOS was 12.1 months in the Balversa arm, reducing the risk of death by 36% compared with the 7.8 months in the chemotherapy arm. Based on these findings, the U.S. Food and Drug Administration granted Balversa formal approval in January, but with a more restricted indication than originally approved. The European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) also recently recommended expanding Balversa’s indication. Janssen Korea has additionally submitted the results from the THOR study to Korea’s Ministry of Food and Drug Safety. Therefore, the company may start the reimbursement progress in earnest for Balversa in the second half of the year. Therefore, it remains to be seen whether Balversa will be able to go beyond landing in medical institutions and gain insurance reimbursement in Korea. Meanwhile, bladder cancer is one major cancer that has lacked a targeted therapy option. Balversa is the first targeted anti-cancer drug for bladder cancer with a novel mechanism of action that inhibits fibroblast growth factor receptor (FGFR). FGFR is a biomarker involved in cancer cell growth that is associated with various cancers. FGFR mutations are particularly common in bladder cancer, with 20 to 30% of patients carrying mutations.
- Company
- Lily's 'Jaypirca' launches in KOR
- by Whang, byung-woo Jan 03, 2025 06:28am
- Product photo of Jaypirca Lily Korea announced on January 2 that Jaypirca (ingredient name: pirtobrutinib), a treatment for mantle cell lymphoma (MCL), was launched in South Korea on December 26. To date, Jaypirca is the first reversible Bruton's tyrosine kinase (BTK) inhibitor of any kind. Jaypirca was approved by the Ministry of Food and Drug Saftey (MFDS) in August 2024 for its efficacy and effects as a 'monotherapy for adult patients with relapsed or refractory MCL previously received at least two treatments.' Before the approval of Jaypirca, there was no drug approved in South Korea for patients with relapsed or refractory MCL whose symptoms advanced after previously receiving a BTK inhibitor. Jaypirca is the first reversible BTK inhibitor to demonstrate clinical significance in patients with relapsed or refractory MCL who have undergone one or more BTK treatments. It selectively inhibits BTK with a 300-fold greater selectivity than any other kinases (98%) in a preclinical study. The basis for approval was the BRUIN Phase 1/2 clinical trials, which evaluated the effectiveness and safety of Jaypirca in patients with relapsed or refractory MCL who had previously received one or more BTK inhibitors. The primary analysis set (PAS), involving 90 patients who had previously been treated with one or more BTK inhibitors, had an overall response rate (ORR) of 56.7% and a duration of response (DoR) of 17.6 months. The most common adverse reactions after the Jaypirca treatment were fatigue (26.3%), decreased neutrophil count (22.8%), diarrhea (22.1%), and bruising (21%). The rate of discontinued treatment due to adverse reactions was 1.2%. The rate of reduced volume of treatment due to adverse reactions was 3.3%. Previously, Jaypirca was approved under the FDA's Accelerated Approval in January 2024 based on the response rate results. In South Korea, in June 2024, it was designated an orphan drug as the monotherapy for patients with relapsed or refractory MCL previously treated with BTK inhibitors. Last month, Jaypirca was the only drug considered for the reimbursement review by the Cancer Disease Review Committee (CDRC) of the Health Insurance Review and Assessment Service (HIRA) to receive a reimbursement criteria decision. The remaining steps are the Drug Reimbursement Evaluation Committee (DREC) review, drug price negotiations with the National Health Insurance Service (NHIS), and a review from the Health Insurance Policy Review Committee. "The launch of Jaypirca is expected to expand the treatment opportunity for patients with MCL who couldn't continue treatment because there has been no alternative medication after the disease progressed after previous therapies," Mira Kyon, Head of Lily Korea Oncology Division, stated. "Lily will continue to put efforts into providing better patient treatment opportunities and suggesting new paradigms to blood cancer therapies."
- Company
- Will Fabhalta settle as a PNH treatment option in Korea?
- by Eo, Yun-Ho Jan 03, 2025 06:27am
- Whether another PNH treatment option will be born is gaining attention in Korea. According to industry sources, Novartis Korea has submitted a reimbursement application for its oral paroxysmal nocturnal hemoglobinuria (PNH) treatment Fabhalta (iptacopan). PNH is a rare disease with an estimated incidence of 1.5 per million people worldwide. The treatment of this disease has historically relied on C5 inhibitors. Soliris (eculizumab) was first approved in Korea in 2010 and Ultomiris (ravulizumab) was approved in 2022 for PNH. Both are C5 inhibitors that inhibit the terminal C5 in the alternative pathway of the complement system involved in the body's immunity and are injected intravenously. However, in April last year, Empaveli (pegcetacoplan), a subcutaneous injectable that binds to C3 and C3b and inhibits the complement chain reaction, was approved, followed in August by Fabhalta, an oral factor inhibitor. Mechanistic limitations of C5 inhibitors, ‘extravascular hemolysis’ PNH begins with a genetic deficiency in red blood cells, which leads to intravascular hemolysis (IVH) and extravascular hemolysis (EVH). Such type of hemolysis can soon lead to thrombosis and bone marrow failure, which can be life-threatening. This is why it is important to control hemolysis to treat PNH, and while the current standard of care for PNH, C5 inhibitors, reduce the risk of thromboembolism by controlling intravascular hemolysis, they have limitations in limiting extravascular hemolysis. This is why the reimbursement of factor B inhibitor Fabhalta is gaining interest. Factor B is an alternative pathway upstream of C5, as well as C3 and C3b, and its inhibition can comprehensively control not only IVH but also EVH. In fact, Fabhalta has shown efficacy in treatment-naïve patients. In the APPOINT-PNH study on treatment-naïve PNH patients, 19 of 33 patients achieved hemoglobin levels of 12 g/dL or higher without red blood cell transfusions. In addition, 92% of patients had shown a clinically significant increase in hemoglobin levels of 2 g/dL or more, and 63% of patients sustained a hemoglobin level of 12 g/dL or higher without transfusion. Over the 24-week study period, hemoglobin levels continued to increase, with normalized hemoglobin levels reached at week 20 and sustained through week 24. In addition, 98% of patients overcame transfusion dependency. “When C5 inhibitors first appeared, experts said they brought a paradigm shift in the treatment of PNH,” said Dr. Junho Jang, Dr. Jun Ho Jang, Professor of Medicine, Department of Hematology-Oncology, Samsung Medical Center. “However, C5 inhibitors still have limitations in controlling extravasation hemolysis (EVH).” He added, “Fabhalta represents another paradigm shift in the treatment of PNH. Its mechanism of action, which inhibits factor B, involves factor B at the top of the alternative complement pathway, which can control both intravascular and extravascular hemolysis. It has shown encouraging results in clinical trials.”
- Company
- Yuhan’s anticancer drug Leclaza secures European approval
- by Cha, Jihyun Jan 02, 2025 06:10am
- Pic of Yuhan Corp Yuhan Corp’s new anti-cancer drug Leclaza (Lazertinib) has crossed the European threshold. The European Commission (EC) approved the drug a month after receiving a positive opinion from the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP). As a result, the company will receive a USD 30 million European launch milestone. According to industry sources on the 31st, the EC granted final marketing authorization to the combination of Yuhan Corp’s non-small cell lung cancer drug Leclaza and Johnson & Johnson's Rybrevant (amivantamab) as a first-line treatment for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with a confirmed epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation. The CHMP had previously issued a positive opinion recommending approval of the combination on March 14th. The final approval of the combination comes just 1 month after the CHMP's recommendation. The approval is based on results from the Phase III MARIPOSA study. In the study, the combination of Rybrevant-Leclaza reduced the risk of disease progression or death by 30% compared to Tagrisso (osimertinib) monotherapy. In addition, progression-free survival (PFS) was 23.7 months, compared to 16.6 months with osimertinib, and duration of response (DOR) was 25.8 months, 9 months longer than Tagrisso's 16.8 months. Even in high-risk patients with TP53 mutations, brain metastases, or liver metastases, the combination of Rybrevant and Leclaza demonstrated a consistent PFS benefit over Tagrisso, with a trend toward superior overall survival (OS). This renders Leclaza the first homegrown anticancer drug to be marketed in Europe following approval in the U.S. The Rybrevant-Leclaza combination was previously approved by the U.S. Food and Drug Administration (FDA) in August. In Korea, it was approved by the Ministry of Food and Drug Safety in January 2021 for the treatment of non-small cell lung cancer. As the Rybrevant-Leclaza combination passed the European threshold, the company will receive the European launch milestone of USD 30 million (approximately KRW 44.2 billion). This brings the total amount received from Janssen for the export of Leclaza technology to USD 240 million (approximately KRW 353.7 billion). In November 2018, the company received USD 50 million in upfront payment for the export of Leclaza’s technology. In April 2020, Yuhan Corp received a milestone payment of USD 35 million from Janssen. At that time, Johnson & Johnson paid Yuhan Corp an additional milestone payment after initiating clinical trials for the Rybrevant and Leclaza combination. In November 2020, Johnson & Johnson paid an additional milestone of USD 65 million to Yuhan Corp as it began recruiting patients for the trial. Subsequent successful completion of the trial and FDA approval resulted in an additional USD 60 million in additional milestone payments. Sales royalties are also expected to be recognized once the Rybrevant-Leclaza combination is launched in Europe. The royalty rate on sales of Leclaza is expected to be in the range of 10% to 12%. Lung cancer is the leading cause of cancer-related death, which causes an estimated 1.8 million deaths worldwide each year. Patients with non-small cell lung cancer, which is targeted by the Rybrevant-Leclaza combination, account for 80-85% of lung cancer patients. In addition, Yuhan Corp may receive additional milestones upon regulatory approval in Japan, China, and other Asian countries. The total amount of additional technology fees that Yuhan may receive for future development and sales of Leclaza amounts to USD 740 million.
- Company
- K-Bio to showcase CDMO·new drugs at the JP Morgan
- by Son, Hyung Min Jan 02, 2025 06:10am
- The Korean pharmaceutical and biotech industry is set to participate in the new year's first global event, the JP Morgan Healthcare Conference. Attention has been drawn to what the Korean pharmaceutical and biotech industry will accomplish during the event, which will include discussions of blockbuster technology transfers and partnering agreements. According to industry sources on January 2, Samsung Biologics, Lotte Biologics, Celltrion, Bridge Biotherapeutics, Onconic Therapeutics, and others will attend the JP Morgan Healthcare Conference. The Healthcare Conference, hosted by the US investment bank JP Morgan, is the largest pharmaceutical and biotech industry investment event, where venture capitals (VC) and hedge funds attend. The event will be held in San Francisco, US, for four days from January 8. Korean CDMO companies gear up to land contracts During the event, Samsung Biologics, Lotte Biologics, Celltrion, and other participants will showcase their contract development and manufacturing organization (CDMO) competitiveness. Samsung Biologics will showcase its biopharmaceutical CDMO competitiveness based on its new manufacturing plant, Plant 5, which will be completed in April. The company is constructing a plant at the Bio Campus in Songdo, Incheon, for antibody-drug conjugates (ADC) production, which is aimed to be completed within this year. Samsung Biologics currently has a total production capacity of 600,000 liters. The company has invested KRW 1.98 trillion in constructing Plant 5 in Songdo. Samsung Biologics will hold a total capacity of 784,000 liters across Plant 1-5. Celltrion will showcase its new drug development achievements, including a new ADC anti-cancer drug, and CDMO vision. The company unveiled the new ADC drug result of the Phase 1 clinical trial at the 'World ADC 2024' held in November last year. Celltrion Celltrion is conducting a clinical trial for 'CT-P70,' an ADC candidate product for non-small cell lung cancer (NSCLC), and 'CT-P71,' which targets many solid cancer indications, including bladder cancer. The safety of these new drug candidates has been confirmed in a phase 1 clinical trial and a pre-clinical trial, respectively. Additionally, Celltrion will initiate the business operations of its subsidiary, 'Celltrion BioSolutions,' which was launched in December 2024. Celltrion aims to build manufacturing facilities and a research center and generate sales from 2028. Lotte Biologics will attend the conference and plans to showcase its CDMO competitiveness. The company plans to build three bio plants in Songdo by 2023 with a total production capacity of 360,000 liters for antibody pharmaceutical production. The new Plant 1 will have a cell culture system and an API system for producing clinical agents. The company will design a 3,000-liter bioreactor capable of fulfilling the demand for both stainless steel bioreactors (cell culture system) and high-potent active pharmaceutical ingredients (HPAPI). Lotte Biologics aims to achieve global biopharmaceutical production competitiveness by investing up to KRW 4.6 trillion by 2030. To showcase achievements in new drug development…will this result in out-licensing opportunities? Onconic Therapeutics and Bridge Biotherapeutics have been officially invited to the JP Morgan Healthcare Conference, where they will showcase their competitiveness in new drug development. Onconic Therapeutics launched 'Ja Q Bo Tab,' a P-CAB for the treatment of gastroesophageal reflux disease (GERD), last year. Ja Q Bo Tab has been designated the 37th new drug in Korea. Additionally, Onconic Therapeutics successfully out-licensed Ja Q Bo to twenty-one foreign countries. Onconic Therapeutics plans to focus on holding strategic meetings with global pharmaceutical companies and investors during the upcoming JP Morgan Healthcare Conference. In addition to Ja Q Bo, the company plans to introduce the targeted anticancer agent, 'nesuparib,' to the global market. Onconic Therapeutics has been developing 'nesuparib,' a dual-targeting inhibitor of PARP·tankyrase, as the follow-up pipeline after Ja Q Bo. Bridge Biotherapeutics plans to present its key R&D projects, including the idiopathic pulmonary fibrosis (IPF) treatment candidate 'BBT-877,' and potential company growth strategy. BBT-877 is an innovative new drug candidate that selectively inhibits the new targeted protein autotaxin. Autotaxin is a protein involved in pathophysiological mechanisms, such as tumorigenesis, by binding with receptors in the cell. Bridge Biotherapeutics has completed enrolling 120 patients in the Phase 2 clinical trial for BBT-877. The comparative Phase 2 clinical trial will evaluate the effectiveness, safety, and drug tolerance by administering BBT-877 and placebo in patients with IPF. STCube will hold business meetings with multinational pharmaceutical companies to out-license 'nelmastobart.' The company is developing the candidate immune checkpoint inhibitor nelmastobart, which targets a new biomarker, BTN1A1. BTN1A1 is a protein that regulates the immune responses to cancer cells by suppressing the activity of T cells, which are immune cells. This biomarker is not expressed in healthy cells but is strongly expressed in cancer cells. BTN1A1 expression is mutually exclusive to that of PD-L1. By targeting BTN1A1, STCube is developing an immune checkpoint inhibitor that could be a new treatment option for intractable cancer. D&D Pharmatech will showcase the glucagon-like peptide 1 (GLP-1) receptor agonist for obesity. In November, D&D Pharmatech began the clinical trial for DD02, an oral GLP-1 drug for obesity, through its US partner, Metsera. D&D Pharmatech also plans to showcase the new drug candidate for treating MASH, DD01, which is undergoing a phase 2 clinical trial.
- Company
- Yuhan Corp will distribute Canesten·Bepanthen
- by Nho, Byung Chul Dec 30, 2024 05:57am
- Yuhan Corp has been selected as the new distribution partner for Bayer’s Canesten and Bepanthen. According to industry sources, Bayer Korea recently terminated its 10-year co-promotion agreement with Ildong Pharmaceutical and signed a new distribution agreement with Yuhan Corp. Under the new agreement, Yuhan Corp will take over the domestic distribution of Bayer's flagship products ▲ Canesten Cream (antifungal), Vaginal Tablets (vaginitis treatment), Powder (infant antifungal), and Bepanthen Ointment (diaper rash treatment). Based on drug distribution performance, Bepanthen and Canesten recorded sales of KRW 8.9 billion and KRW 3.7 billion, respectively, last year. This is a simple wholesale distribution standard data, and the actual company-based performance is expected to be higher than this, so Yuhan Corp’s OTC drug performance is expected to further increase with the addition of this new lineup. The Canesten lineup consists of Canesten 1 vaginal tablet, Canesten cream, and Canesten powder, which are used to treat vaginal candida infections, athlete's foot, and ringworm. The dexpanthenol-based Bepanthen cream is effective for burns, bedsores, dermatitis, eczema, diaper rash, and sunburn, and its competitors include Hanpoong’s Jaungo. Yuhan Corp is a traditional OTC pharmaceutical powerhouse alongside DongKook Pharmaceutical, Donghwa Pharmaceutical, Ildong Pharmaceutical, Daewoong Pharmaceutical, and GC Biopharma, and has recently pursued a sustained and aggressive consumer product marketing strategy. In particular, Yuhan Corp secured the exclusive rights to fatigue recovery drug Lalaola from Vivozon Pharmaceutical in May 2023, and is making considerable efforts to secure a pipeline of OTC drugs, and has been conducting aggressive marketing such as TV-CFs and the appointment of broadcaster Dong-Yup Shin as its exclusive model. In addition, to secure a foothold in the OTC painkiller market that is worth KRW 150 billion, the company launched liquid soft capsule painkillers Yuhan Vfend and Yuhan Acetaminophen soft capsules last year to expand sales. It is also noteworthy that the company has secured more than 15,000 direct-dealer pharmacies for its multinutrient Beecom-Megatrue, which has improved performance through continuous lineup-strengthening activities.
- Company
- Companies show mixed responses to patent term limitation law
- by Kim, Jin-Gu Dec 30, 2024 05:57am
- A bill to amend the Patent Act to limit the patent period for new drugs has passed the plenary session of the National Assembly. The amendment will limit the upper limit of the remaining patent term to a maximum of 14 years from the time a new drug is approved and allow only one of several patents registered for a single drug to be extended. The pharmaceutical industry has expressed both excitement and concern. Multinational pharmaceutical companies that own a large number of original drugs are worried that their patent life will be shortened. Domestic pharmaceutical companies, on the other hand, are generally excited about the prospect of being able to launch generics sooner. The amendment to the Patent Act raises concerns among multinational pharmaceutical companies...“will rather reduce access to drugs” According to industry sources on the 30th, the National Assembly held a plenary session on the 27th and passed the bill to amend the Patent Law. The amendments to the Patent Law are implemented through the government's promulgation process. Its effective date will be 6 months from the date of promulgation. The amendments will apply to patent applications registered after the amendment is enacted. Assuming that the revised law is promulgated early next year, this means that the new patent term system will be implemented as early as the second half of next year. The revision is expected to result in a shorter patent term for original drugs in Korea. Conversely, this means that generics will be available in Korea sooner. The advantages and disadvantages that the amendment will bring to original and generic companies will be sharply divided. Multinational pharmaceutical companies with a large number of original drugs are expected to be disadvantaged by the revised law. The shorter patent period will allow generics to be launched sooner, which will result in lower drug prices. It is also expected to partially neutralize the original companies’ strategy of delaying generic entry by extending multiple drug patents. When the actual amendment was being discussed by the National Assembly, the multinational pharmaceutical companies submitted a statement opposing the amendment. Korean Research-based Pharmaceutical Industry Association (KRPIA) criticized the amendments, saying, “Adopting foreign practices for only some elements, rather than revising the entire regulation on patent term extension, undermines the public's access to medicines and undermines international harmonization.” A representative of a multinational pharmaceutical company said, “Patent challenges by generic companies have been frequent in Korea due to the first generic prior authorization (first generic) system, and the probability of winning patent lawsuits has been higher for the generic companies than in the US and Europe.” “Under these circumstances, shortening the effective patent term will just further damage the companies that own the original drugs.” Expenctations rise among domestic companies on “launching generics faster”…some play ‘caution’ On the other hand, domestic pharmaceutical companies generally welcome the passage of the Patent Act amendment bill during the National Assembly’s plenary session. As the patent term of original drugs will be shortened, the domestic pharmaceutical companies expect this will accelerate the early release of generics, which already own a fair share of the market. “Compared to advanced countries such as the U.S. and Europe, Korea has been overprotective of the patent term of original drugs,” said an official from a Korean pharmaceutical company. ”I think it's good that the law has been revised so that we can keep pace with the global pharma and biotech industry.” “The extended patent life was virtually impossible to overcome,” the official said, adding, ”The revised law is expected to play a positive role in the national health insurance finances by accelerating the time to market generics.” However, some domestic pharmaceutical companies that own original products were not so quick to welcome the change. “Compared to the past, more domestic pharmaceutical companies have succeeded in developing original drugs. In the future, there will be more companies that own originals,” he said, adding, ”In such a situation, competition with other domestic pharmaceutical companies will inevitably intensify if the patent term is shortened.” 14-year upper limit wet on patent term after marketing authorization...will shorten the total patent term The Patent Act passed by the National Assembly has two main objectives. One is to place a cap of 14 years on the remaining term from the date of drug approval. Previously, the limit for extending the patent term was stipulated to 5 years, but there was no provision for a cap on the total term, including post-authorization patent extensions. On the other hand, the United States and China limit the effective patent term to 14 years and Europe to 15 years. The Korea Intellectual Property Office promoted the introduction of a cap on the effective patent term for equity and international harmonization with other countries. Even if a particular pharmaceutical company is granted a patent term of 20+5 years (normal patent term + extension period), the cap is set at 'up to 14 years from the time of marketing authorization, thus shortening the overall patent term. For example, Pfizer's ALK-targeted anti-cancer drug Xalkori (crizotinib) has a 14-year patent term in the U.S. after FDA approval. Due to this cap, Xalkori was only granted a patent extension of 1 year and 6 months (547 days) in the US. In Korea, however, there is no such cap, so Pfizer was granted a full patent extension. The patent extension period for Xalkori in Korea is 2 years and 10 months (1,034 days), which is about 1 year and 4 months (16 months) longer than in the US. 1One patent extension granted per item...favorable for generic companies The law was also amended to allow generic companies to choose only one patent to extend out of the many patents registered for a single drug. Generally, the original company registers as many patents as possible when developing a drug. A single drug may have more than 10 patents, including product patents, use patents, dosage forms patents, method and dosage patents, formulation patents, and crystalline form patents. The more patents a company has, the better it is to defend its product against generic challenges. This is why only one of the patents registered for a drug can be extended in the U.S. and Europe. For example, for the rheumatoid arthritis drug Xeljanz (tofacitinib), Pfizer applied for a patent extension in the U.S. for its product patent from among several patents. As a result, the patent term for Xeljanz became 20+5 years. In Korea, on the other hand, if multiple patents are registered for a drug, each patent can be extended. In fact, Pfizer applied for an extension (up to five years) for 2 product patents and 1 formulation patent in Korea. An overlap occurred when all 3 patent extensions were granted simultaneously. As a result, the patent term for Xeljanz in Korea is approximately 2 years (732 days) longer than in the US and Europe. The benefits to Pfizer of having 2 more years of Xeljanz patent term in South Korea are significant. For one thing, the company can avoid the price reductions that would come with the introduction of a generic version of Xeljanz. The price of the original drug was reduced by 70% in the first year and 53.55% in the second year upon the release of the generic, but Pfizer delayed this price cut by 2 years. By delaying the launch of the generic, the company can maintain its market monopoly for 2 more years. Considering how the annual sales of Xeljanz are around KRW 15 billion, Pfizer avoided a loss of KRW 12.8 billion + α for 2 years.
- Company
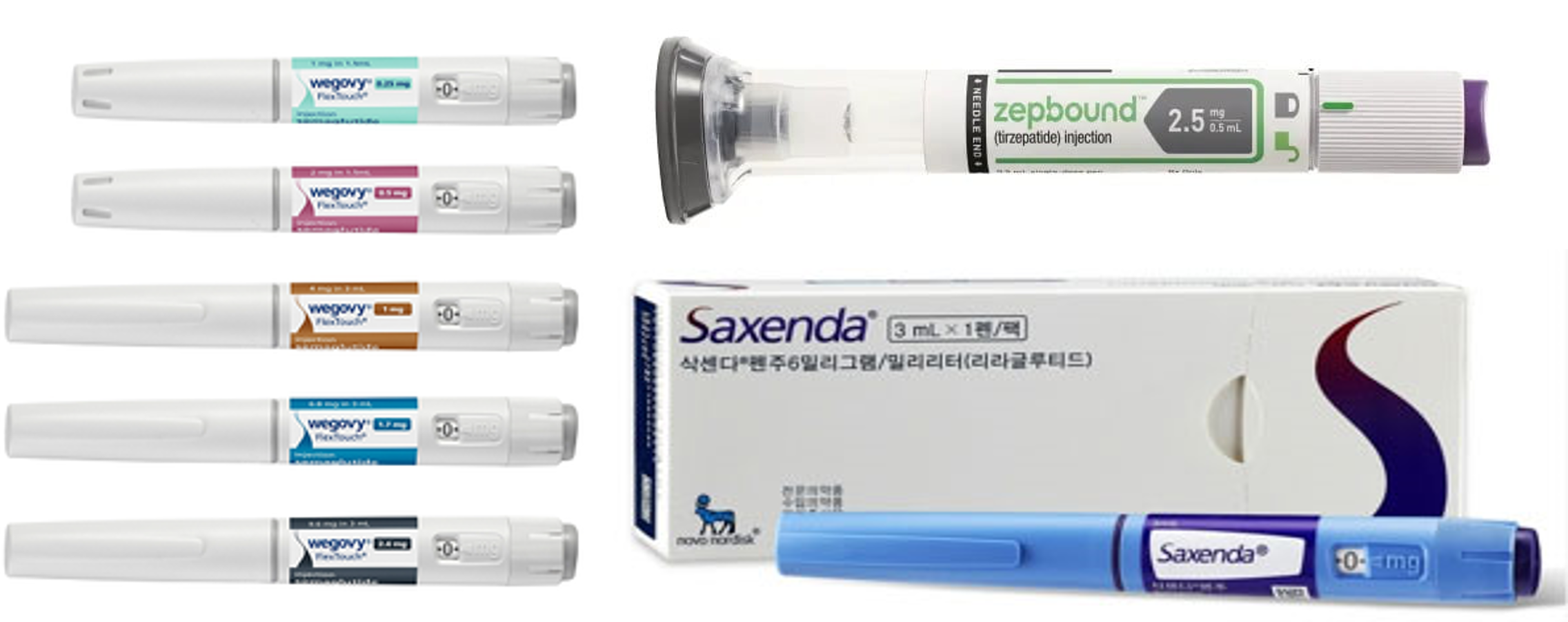
- Generic drugs mkt heat up as GLP-1 obesity meds demand soar
- by Son, Hyung Min Dec 30, 2024 05:57am
- It has been reported that not only new drugs but also generics have joined the competition in the market for glucagon-like peptide-1 (GLP-1)- containing treatment for diabetes and obesity. In the United States, generic versions of liraglutide, which is an active ingredient of the diabetes obesity·treatments Victoza·Saxenda that are GLP-1 receptor agonists. In addition to the development of generic versions of liraglutide, generics of dulaglutide, an active ingredient of Trulicity, are under development. Due to the benefits of effective weight loss with GLP-1 agonists, obesity treatment usage is significantly increasing. Several GLP-1 agonists for treating obesity, including semaglutide and liraglutide, are in shortage worldwide. The shortage in supply is due to a surge in demand for the drug. Consequently, regulatory authorities expect that generic versions of diabetes·obesity drugs that are GLP-1 agonists will likely to resolve drug shortages. The second diabetes drug containing liraglutide has been approved in the United States Novo NordiskAccording to industry sources on December 30, the U.S. Food and Drug Administration (FDA) has granted approval to a Victoza generic developed by a British pharmaceutical company, Hikma Pharmaceuticals. This approval is the second for a generic GLP-1 agonist following Teva's generic for Victoza. Teva Pharmaceuticals launched an authorized generic of Victoza after signing an agreement with Novo Nordisk. Victoza is a GLP-1 agonist for diabetes treatment developed by Novo Nordisk. It was first introduced to the market after obtaining approval in the United States in 2010. After discovering the drug's weight loss effect during a clinical trial, Novo Nordisk introduced obesity drug containing the same active ingredient to the market in 2014. Currently, GLP-1 agonist drugs, including liraglutide and semaglutide, are in global shortage. Regulatory authorities, such as the FDA, have announced that they will prioritize the review of approval applications for generic versions of originals, which will resolve the issue of limited patient access. The latecomers to the market anticipate gaining a market presence due to a surge in GLP-agonist demands. These developers plan to challenge the market by introducing the drugs at lower prices than the originals. Pre-launched Teva's Victoza generic is sold at a 15% lower price than the original drug. Analysis suggests that not only latecomer new drugs but generics will continue to compete fiercely in the market, as GLP-1 agonists are demonstrating effectiveness in treating metabolism dysfunction-related MASH and brain diseases, in addition to diabetes and obesity. Currently, GLP-1 agonist drugs are leading the market for diabetes and obesity treatments. Lily's Mounjaro, a GLP-1 agonist for diabetes, recorded sales of KRW 6.8482 last year, up 971.2% from 2022. The sales of Mounjaro, which was launched in May 2022 and generated the sales of KRW 640 billion in the same year, soared last year. Novo Nordisk's GLP-1 agonist drugs for diabetes also showed growth last year. Ozempic recorded sales of KRW 5.048 trillion last year, up 52% from the previous year. The sales of Rybelsus, containing the same active ingredient, amounted to KRW 1.44 trillion, up 140% from 2022. Saxenda generated sales of KRW 1.2252 trillion last year, up 9.8% from the previous year. Despite the shortage, Wegovy exceeded KRW 300 billion in sales last year. Pharmaceutical companies are closely watching the market for generic versions of GLP-1 agonists Korean companies are also closely watching the market for GLP-1 agonist generics. Handok has successfully launched a generic version of Saxenda, developed by the Indian biotech company Biocon, in South Korea. Both company signed an exclusive domestic sales·distribution agreements for Saxen generic in May. Biocon obtained approval for its first generic of Saxenda in the UK in March and launched the drug this month. Along with Biocon, the Indian companies Dr. Reddy's and Cipla and the Swiss company Sandoz are developing a generic version of liraglutide for diabetes and obesity treatments. Korean and overseas pharmaceutical companies are also focusing on semaglutide and dulaglutide, in addition to liraglutide. Semaglutide is an active ingredient of Novo Nordisk's diabetes treatment, Ozempic, and obesity treatment Saxena. Dulaglutide is an active ingredient of Lily's diabetes treatment Trulicity. Wegovy·Zepbound·Saxenda, GLP-1 agonists for obesity In June, the Chinese company Boan Biotech applied to the Chinese regulatory authority for approval of its generic version of Trulicity. This represents the first approval application for a generic version of Trulicity. Currently, generic versions of GLP-1 agonists for diabetes and obesity are continuing to be developed. Last year, a Chinese company successfully received approval for its Saxenda generic. Sam Chun Dang Pharm is targeting semaglutide generic. In June, the company signed an exclusive sales agreement with a Japanese pharmaceutical company for an oral generic version of semaglutide. The deal entails sales condition after the substance patent of semaglutide expires. In Japan, semaglutide's substance patent expires in 2031. Since 2018, Sam Chun Dang Pharm has been developing a generic version of liraglutide using its formulation-changing technology, called 'S-PASS.' Sam Chun Dang Pharm states that the company can avoid the formulation patent because it has been developing biosimilars and generics that avoid the patents of original formulations. The company recently confirmed the drug equivalence through a pharmaceutical equivalence test.
- Company
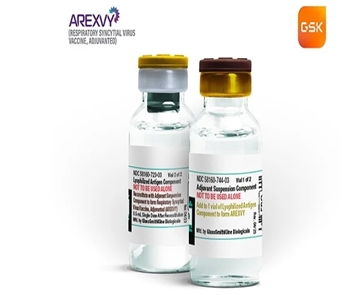
- First RSV vaccine Arexvy Inj lands in Korea
- by Whang, byung-woo Dec 27, 2024 05:56am
- Arexvy, GSK's global sales driver as a No. 1 adult RSV vaccine, is set to launch in Korea. Pic of Arexvy GSK Korea's Respiratory Syncytial Virus (RSV) vaccine Arexvy was recently approved by the Ministry of Food and Drug Safety (MFDS) for the prevention of lower respiratory tract disease (LRTD) caused by RSV in adults aged 60 years and older. After setting the milestone as the first RSV-LRTD vaccine, the vaccine was approved by the U.S. Food and Drug Administration (FDA) in May last year and then extended to those 50-59 years of age in June. The approval was based on results from two Phase III studies, RSV OA=ADJ-006 and RSV OA=ADJ-004, in adults aged 60 years and older. Study results showed, Arexvy significantly reduced the risk of RSV-LRTD by 82.6% and the risk of severe RSV-LRTD by 94.1% compared to placebo in subjects 60 years of age and older. In addition, vaccine efficacy for RSV-A-associated LRTD events and RSV-B-associated LRTD events was 84.6% and 80.9%, respectively. In addition, in 2 RSV seasons (from 15 days after the first dose in the Northern Hemisphere to the end of the second season), the mean follow-up period was 17.8 months, with 67.2% efficacy for RSV-LRTD and 78.8% efficacy for severe RSV-LRTD in patients aged 60 years and older. “RSV infections can be severe and even fatal in high-risk groups, such as the elderly, and bring a major social burden,” said Dr. Won Seok Choi, professor of infectious diseases at Korea University Ansan Hospital. ”Global health authorities in the U.S., U.K., and elsewhere are continuing efforts to prevent simultaneous outbreaks of RSV, influenza, and COVID-19.” “Older adults are at greater risk of infection due to age-related immune decline and a greater likelihood of complications,” said Professor Choi. ”Cross-infection can occur between young children and elderly grandparents, so practicing good personal hygiene and getting vaccinated early can help keep you, your family, and society safe.” Arexvy generated KRW 2 trillion in global sales last year...Records No. 1 in global share Currently, Arexvy’s global sales are on an upward trajectory, driven by its title as the first RSV vaccine. According to GSK's earnings release, Arexvy’s global sales in the second quarter were GBP 62 million (KRW 108 billion). Given that the RSV season is in the third and fourth quarters, sales are expected to become greater. Last year, the company's total revenue was GBP1.15205 billion (approximately KRW 2 trillion) GSK has raised its revenue forecast for 2024 based on the growth of its RSV vaccine and expects revenue to increase by 7-9% over last year. In particular, the company’s share is expected to grow despite the arrival of competing vaccines from Pfizer and Moderna. In the short term, the company plans to increase its global presence outside of the U.S. and European markets, including Japan. This includes South Korea, where it recently received approval from the Ministry of Food and Drug Safety. Arexvy is available in a vial containing the antigen (powder) in pre-fusion form of the RSV F-protein and a vial containing the immunostimulant (AS01E, suspension), where 0.5 mL of the reconstituted vaccine is administered intramuscularly using a needle. It can be administered in combination with the non-immune-boosting inactivated seasonal influenza vaccine, and the CDC recommends RSV vaccination for adults aged 75 and older or high-risk groups between 60 to 74 years of age. “RSV infections are a significant physical and economic burden for high-risk groups, including the elderly, and GSK is committed to the successful launch of Arexvy to not only prevent infection in adults but also to reduce the burden of disease for patients in Korea,” said Hyunji Kwon, Head of the Vaccines Business Unit at GSK Korea.