- LOGIN
- MemberShip
- 2025-12-22 01:08:19
- Companies show mixed responses to patent term limitation law
- by Kim, Jin-Gu | translator Alice Kang | 2024-12-30 05:57:23
A bill to amend the Patent Act to limit the patent period for new drugs has passed the plenary session of the National Assembly.
The amendment will limit the upper limit of the remaining patent term to a maximum of 14 years from the time a new drug is approved and allow only one of several patents registered for a single drug to be extended.
The pharmaceutical industry has expressed both excitement and concern.
Multinational pharmaceutical companies that own a large number of original drugs are worried that their patent life will be shortened.
Domestic pharmaceutical companies, on the other hand, are generally excited about the prospect of being able to launch generics sooner.
The amendment to the Patent Act raises concerns among multinational pharmaceutical companies...“will rather reduce access to drugs” According to industry sources on the 30th, the National Assembly held a plenary session on the 27th and passed the bill to amend the Patent Law.
The amendments to the Patent Law are implemented through the government's promulgation process.
Its effective date will be 6 months from the date of promulgation.
The amendments will apply to patent applications registered after the amendment is enacted.
Assuming that the revised law is promulgated early next year, this means that the new patent term system will be implemented as early as the second half of next year.
Conversely, this means that generics will be available in Korea sooner.
The advantages and disadvantages that the amendment will bring to original and generic companies will be sharply divided.
Multinational pharmaceutical companies with a large number of original drugs are expected to be disadvantaged by the revised law.
The shorter patent period will allow generics to be launched sooner, which will result in lower drug prices.
It is also expected to partially neutralize the original companies’ strategy of delaying generic entry by extending multiple drug patents.
When the actual amendment was being discussed by the National Assembly, the multinational pharmaceutical companies submitted a statement opposing the amendment.
Korean Research-based Pharmaceutical Industry Association (KRPIA) criticized the amendments, saying, “Adopting foreign practices for only some elements, rather than revising the entire regulation on patent term extension, undermines the public's access to medicines and undermines international harmonization.” A representative of a multinational pharmaceutical company said, “Patent challenges by generic companies have been frequent in Korea due to the first generic prior authorization (first generic) system, and the probability of winning patent lawsuits has been higher for the generic companies than in the US and Europe.” “Under these circumstances, shortening the effective patent term will just further damage the companies that own the original drugs.” Expenctations rise among domestic companies on “launching generics faster”…some play ‘caution’ On the other hand, domestic pharmaceutical companies generally welcome the passage of the Patent Act amendment bill during the National Assembly’s plenary session.
As the patent term of original drugs will be shortened, the domestic pharmaceutical companies expect this will accelerate the early release of generics, which already own a fair share of the market.
“Compared to advanced countries such as the U.S.
and Europe, Korea has been overprotective of the patent term of original drugs,” said an official from a Korean pharmaceutical company.
”I think it's good that the law has been revised so that we can keep pace with the global pharma and biotech industry.”
“The extended patent life was virtually impossible to overcome,” the official said, adding, ”The revised law is expected to play a positive role in the national health insurance finances by accelerating the time to market generics.”

“Compared to the past, more domestic pharmaceutical companies have succeeded in developing original drugs.
In the future, there will be more companies that own originals,” he said, adding, ”In such a situation, competition with other domestic pharmaceutical companies will inevitably intensify if the patent term is shortened.” 14-year upper limit wet on patent term after marketing authorization...will shorten the total patent term The Patent Act passed by the National Assembly has two main objectives.
One is to place a cap of 14 years on the remaining term from the date of drug approval.
Previously, the limit for extending the patent term was stipulated to 5 years, but there was no provision for a cap on the total term, including post-authorization patent extensions.
On the other hand, the United States and China limit the effective patent term to 14 years and Europe to 15 years.
The Korea Intellectual Property Office promoted the introduction of a cap on the effective patent term for equity and international harmonization with other countries.
Even if a particular pharmaceutical company is granted a patent term of 20+5 years (normal patent term + extension period), the cap is set at 'up to 14 years from the time of marketing authorization, thus shortening the overall patent term.
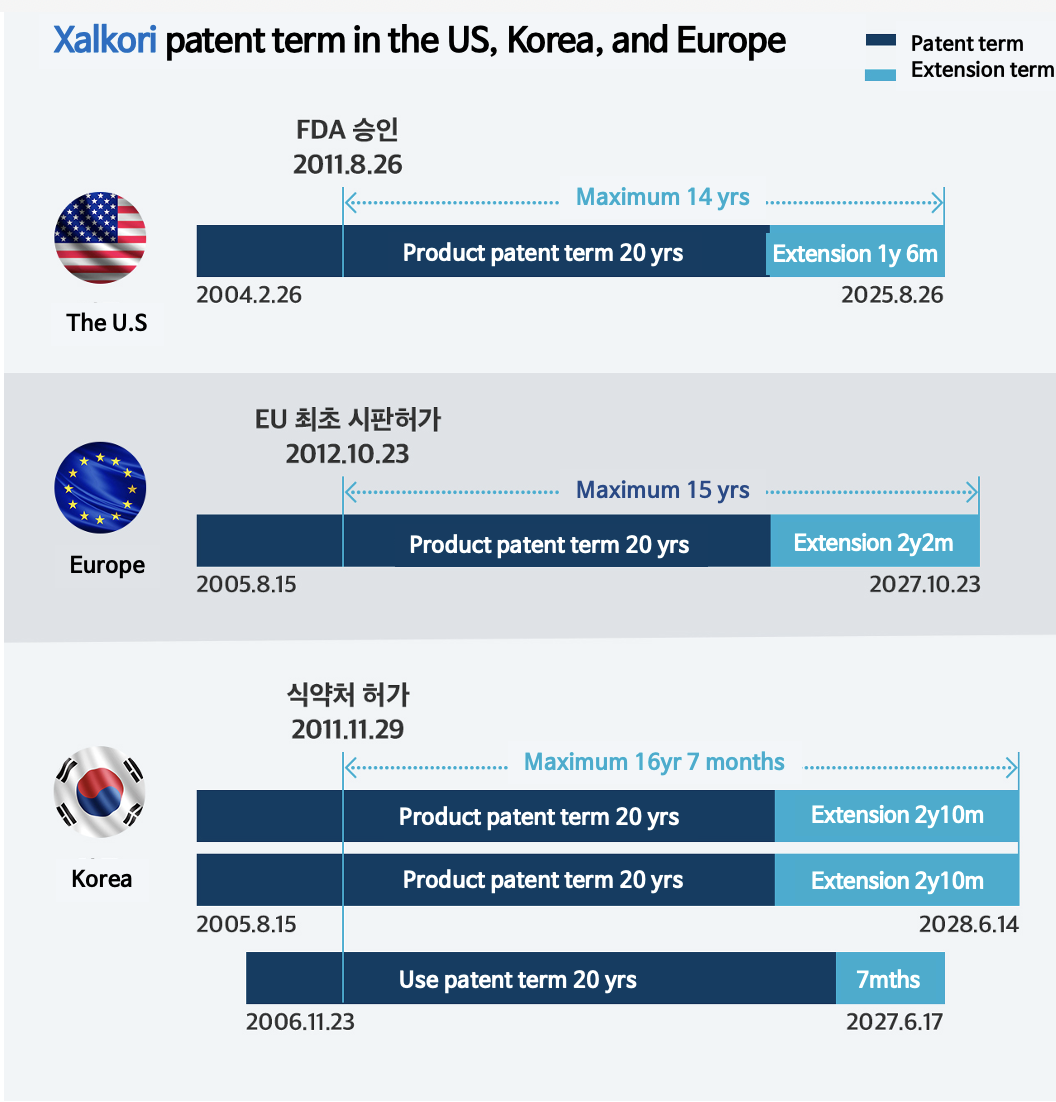
after FDA approval.
Due to this cap, Xalkori was only granted a patent extension of 1 year and 6 months (547 days) in the US.
In Korea, however, there is no such cap, so Pfizer was granted a full patent extension.
The patent extension period for Xalkori in Korea is 2 years and 10 months (1,034 days), which is about 1 year and 4 months (16 months) longer than in the US.
1One patent extension granted per item...favorable for generic companies The law was also amended to allow generic companies to choose only one patent to extend out of the many patents registered for a single drug.
Generally, the original company registers as many patents as possible when developing a drug.
A single drug may have more than 10 patents, including product patents, use patents, dosage forms patents, method and dosage patents, formulation patents, and crystalline form patents.
The more patents a company has, the better it is to defend its product against generic challenges.
This is why only one of the patents registered for a drug can be extended in the U.S.
and Europe.
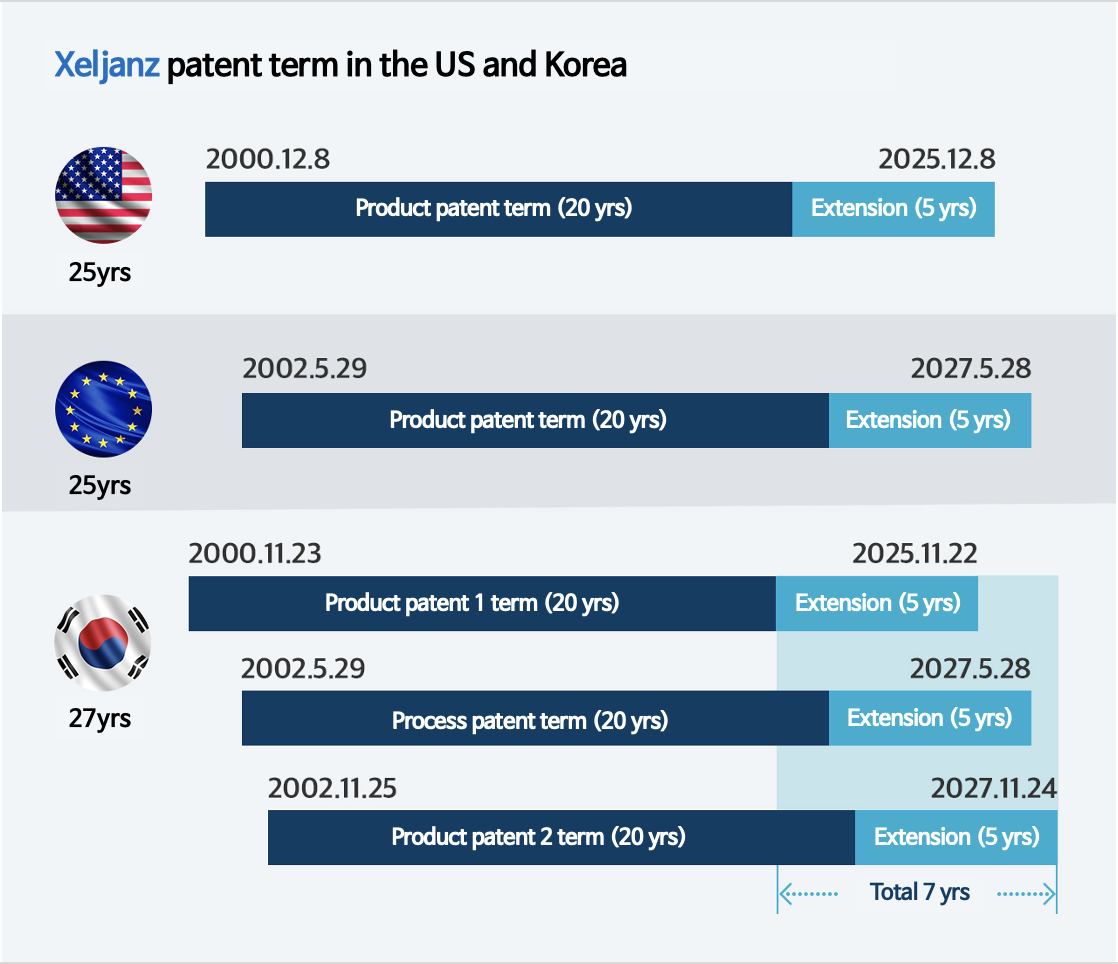
for its product patent from among several patents.
As a result, the patent term for Xeljanz became 20+5 years.
In Korea, on the other hand, if multiple patents are registered for a drug, each patent can be extended.
In fact, Pfizer applied for an extension (up to five years) for 2 product patents and 1 formulation patent in Korea.
An overlap occurred when all 3 patent extensions were granted simultaneously.
As a result, the patent term for Xeljanz in Korea is approximately 2 years (732 days) longer than in the US and Europe.
The benefits to Pfizer of having 2 more years of Xeljanz patent term in South Korea are significant.
For one thing, the company can avoid the price reductions that would come with the introduction of a generic version of Xeljanz.
The price of the original drug was reduced by 70% in the first year and 53.55% in the second year upon the release of the generic, but Pfizer delayed this price cut by 2 years.
By delaying the launch of the generic, the company can maintain its market monopoly for 2 more years.
Considering how the annual sales of Xeljanz are around KRW 15 billion, Pfizer avoided a loss of KRW 12.8 billion + α for 2 years.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










