- LOGIN
- MemberShip
- 2025-12-21 21:44:27
- Company
- Expanded reimb for 'Zejula'
- by Son, Hyung Min Jan 17, 2025 05:53am
- Dr. Jae-Weon Kim of Seoul National University Hospital Following the reimbursement expansion of Zejula for ovarian cancer, patient access to treatment has been improved. Experts suggest that Zejula will be more widely used in clinical practices based on demonstrated benefits in efficacy and safety in long-term treatment studies. On January 16, Takeda Pharmaceutical Korea held a press conference commemorating the reimbursement expansion of Zejula monotherapy as a first-line maintenance therapy of HRd-positive ovarian cancer. As of October 2024, the national health insurance reimbursement criteria for Zejula have been expanded to include treatment of homologous recombination deficiency (HRD)-positive ovarian cancer. Previously, Zejula has been covered by reimbursement only as a maintenance therapy for the first-line treatment of patients with ovarian cancer associated with BRCA who respond to platinum-based therapy. Due to the reimbursement expansion, Zejula became the only PARP (Poly ADP-ribose Polymerase)-inhibitor covered by reimbursement for the first-line maintenance therapy used in patients with HRd-positive ovarian cancer. HRd refers to homologous recombination deficiency, a DNA damage repair mechanism. When HRd is positive, cancer cells cannot efficiently repair DNA damage. It is particularly associated with BRCA1/2 gene mutations frequently observed in breast and ovarian cancers. Experts suggest that the clinical prevalence of HRd expression in ovarian cancer is over 50%. Based on a long-term follow-up study of 6.2 years, Zejula demonstrated significant benefits for patients with HRd-positive ovarian cancer. In the clinical study, the HRd patient group treated with Zejula recorded a progression-free survival of 24.5 months, which was markedly different from the 11.2 months of the placebo group. At the five-year mark of treatment, the number of patients who survived without disease progression in the Zejula group was twice as high as that in the placebo group. In the HRd patient group, a statistically significant difference in progression-free survival was observed between those treated with Zejula and those given a placebo up to five years of treatment. "Zejula has demonstrated long-term PFS benefits through the PRIMA trial, its approval study, and follow-up studies. In terms of safety, adverse events were consistent with previous clinical results, confirming its safety even with long-term use," Dr. Jae-Weon Kim of Seoul National University Hospital's Department of Obstetrics and Gynecology stated. In clinical practices, prescriptions for Zejula have been increasing since its reimbursement expansion. Ovarian cancer patients take two 100 mg tablets once daily, while Zejula is the only ovarian cancer treatment available with a once-daily dosing regimen. "Since the reimbursement expansion notice, no severe adverse events have been reported among HRd-positive patients continuing first-line maintenance therapy with Zejula monotherapy. Zejula, as an oral medication administered once daily, significantly enhances patient convenience," Dr. Jung-Yun Lee of Severance Hospital's Department of Obstetrics and Gynecology emphasized. Dr. Lee added, "HRd is a biomarker commonly observed during first-line maintenance therapy. With the reimbursement expansion of Zejula, there has been an increase in cases involving HRd testing, leading to higher diagnosis rates. Through diagnostic testing, many patients are expected to benefit from Zejula." Dr. Jung-Yun Lee of Severance Hospital
- Company
- Ultomiris is reimbursed for aHUS in Korea
- by Son, Hyung Min Jan 16, 2025 06:14am
- Dr. Jinseok Kim, professor of hematology at Severance Hospital. Ultomiris has been reimbursed in Korea for atypical hemolytic uremic syndrome (aHUS) since January. While experts have welcomed the news of its reimbursement, they have also raised the need for systematic improvements to the stringent conditions for reimbursement, including the preliminary review requirement. On the 10th, AstraZeneca Korea held a press conference at JW Marriott Dongdaemun Square Seoul to celebrate the reimbursement coverage of Ultomiris for aHUS in Korea. Starting this month, Ultomiris will be reimbursed by health insurance for patients with aHUS with thrombotic microangiopathy (TMA) and kidney damage. This is expected to improve access to treatment for patients with aHUS whose symptoms can worsen rapidly and lead to end-stage renal disease (ESRD). Ultomiris is a next-generation C5 complement inhibitor with a half-life approximately four times longer than Soliris. While Soliris requires dosing every 2 weeks, Ultomiris has an extended dosing interval of 8 weeks, improving treatment convenience. When complement C5 is activated on the surface of bacteria, it produces a membrane-attacking complex that causes holes in the cell membrane. If the normal immune system process of complement activation continues, vascular endothelial cells are disrupted, causing related diseases. Ultomiris has a mechanism of action that inhibits this process. aHUS is an acute rare disease in which the immune system's complement is overactivated due to a genetic defect and causes thrombotic microangiopathy, which can lead to severe damage to multiple organs, especially the kidneys. aHUS refers to hemolytic uremic syndrome not associated with E. coli. The efficacy and safety of Ultomiris were confirmed in Phase III Study 311 in adult patients who were not previously treated with complement inhibitors. At 26 weeks of treatment, Ultomiris demonstrated improvement in TMA-related markers, including platelet and LDH levels, in 53.6% of patients. The treatment also demonstrated sustained terminal complement inhibition by maintaining serum-free C5 concentrations of < 0.5 μg/ml. In the Phase III Study 312 in pediatric patients, Ultomiris demonstrated complete resolution of TMA in 94.4% of patients at 50 weeks of treatment. “In a study of pediatric aHUS patients who switched from Soliris to Ultomiris, the renal and hematologic parameters remained stable over one year, confirming the efficacy of the switch,” said Dr. Jinseok Kim, professor of hematology at Severance Hospital. “As clinicians, we are excited to be able to offer hope to our patients with the introduction of this new treatment option.” Ultomiris and Soliris are currently available for aHUS in Korea, but both agents are only available to patients who have been approved through the prior review process. Clinicians have called for improved reimbursement, including moving to a post-review process, as patients' conditions can worsen if they are not properly dosed. The coverage of aHUS was implemented in 2018 with the introduction of Soliris. However, according to an analysis of the results of the preliminary review system from July 2018 to October last year, 56 out of 321 cases were approved, showing an average approval rate of 18%. Kim added, “Although Ultomiris has been reimbursed, like Soliris, it is subject to the same restrictions as Soliris, which requires preliminary review. We hope that systemic improvements are also made so that aHUS patients can receive treatment in a timely manner.”
- Company
- Last year's pharma export sales recorded KRW 11T
- by Kim, Jin-Gu Jan 16, 2025 06:14am
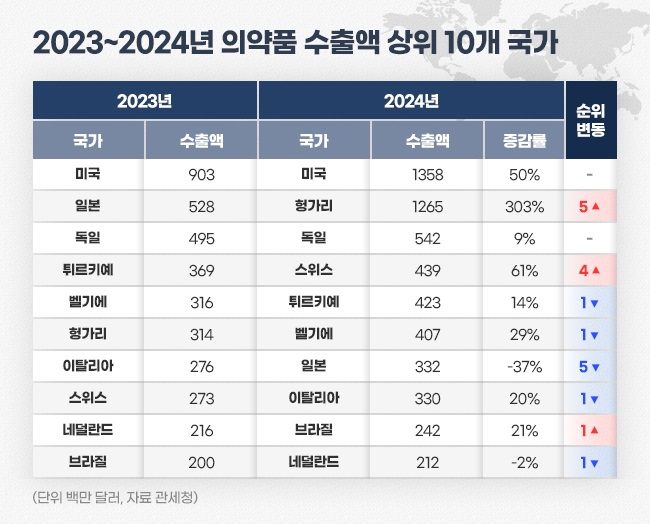
- Last year's export sales of Korea-made pharmaceuticals amounted to US$ 7.5 billion (approximately KRW 11 trillion), up 29% from the previous year. Analysis indicates that a significant increase in biopharmaceutical exports of Samsung Biologics and Celltrion has contributed to the growth. The largest exported country was the United States, with exports totaling US$ 1.3 billion (approximately KRW 1.98 trillion), up 50% from the previous year. Export sales to Hungary increased more than fourfold over the year, reaching US$ 1.2 billion (approximately KRW 1.85 trillion), making it the second largest after the United States. Pharmaceutical export sales reached US$ 7.54 billion last year…second largest in the history According to the Korea Customs Service on January 16, last year's Korean pharmaceutical export sales amounted to US$ 7.53 billion. After the announcement of the endemic, export sales expanded to the largest size. The export sales of domestically produced pharmaceuticals significantly increased during the COVID-19 pandemic. In 2019, the export value was US$ 3.69 billion, which soared by 81% over the year to reach US$ 6.68 billion in 2020 as the pandemic intensified. By 2021, this figure had further increased by 22%, reaching US$ 8.12 billion, setting an all-time high, primarily driven by the export sales of domestically developed COVID-19 vaccines. After that, the sales decreased for two consecutive years until 2023. In 2022, it decreased to US$ 6.27 billion, down 23% compared to the previous year. In 2023, it further shrank to US$ 5.85 billion. Last year, export sales rebounded successfully. Compared to 2023, export sales rose by 29%. It is the second largest after 2022. Yearly pharmaceutical export sales (unit: US$ 1 million, source: Korea Customs Service) The pharmaceutical industry considers that the export growth resulted from a significant increase in biopharmaceutical exports to the United States and Europe, primarily driven by Samsung Biologics and Celltrion. As of Q3 last year, Samsung Biologics' cumulative export sales reached KRW 3.29 trillion, up 26% compared to KRW 2.62 trillion in the Year-over-Year (YoY). This represents an annual export growth of over KRW 600 billion. Considering its order backlog, Samsung Biologics is projected to achieve record-breaking yearly export performance. Since 2015, Samsung Biologics has secured US$ 14.23 billion in orders as of Q3 last year. Among this, US$ 7.49 billion of products have been delivered, leaving an order backlog of US$ 6.73 billion. If the clients successfully develop their products, the estimated value of the order backlog could rise to US$ 12.31 billion. Celltrion's cumulative export sales for Q3 also increased by 30%, from KRW 623.5 billion in 2023 to KRW 810 billion in 2024. Additionally, SK Biopharm, GC Biopharma, Yuhan, Hugel, Dongwha, Daewon, and Boryung also had significant increases in export sales, contributing to the overall growth of pharmaceutical exports. Pharmaceutical import sales reached US$ 8.98 billion, marking a 5% decrease compared to the previous year. The pharmaceutical trade deficit narrowed with a significant increase in exports and a decline in imports. The trade deficit improved from US$ 3.58 billion in 2023 to US$ 1.45 billion last year. Exports to the US represent largest amount over three consecutive years…Hungary, a four-fold increase over the year Korea has exported the most to the United States the most. Last year's pharmaceutical exports to the United States reached US$ 1.35 billion. The United States accounts for 18% of the total pharmaceutical export sales. Export sales to the United States quickly rose over the past two years. It increased 7% from KRW 843.94 million in 2022 to KRW 933.0 million in 2023. Last year, it increased over 50%. In 2022, the United States became the largest export destination, replacing Germany. Since then, it has maintained the top rank for three consecutive years. Hungary ranked second in export sales after the United States. Last year's export sales to Hungary reached US$ 1.26 billion, surging more than four-fold compared to US$ 316.27 million in 2023. The top countries by pharmaceutical export sales 2023-2024 (unit: US$ 1 million, source: Korea Customs Service) One of the reasons for the significant increase in exports to Hungary is Celltrion's expanded biosimilar export to Europe. Hungary is Celltrion's export hub for the European market. The company announced its plans to establish a direct sales system using its Hungarian subsidiary as the gateway for European exports. As part of this strategy, shipments of Celltrion's biosimilars for the European market have been concentrated through its Hungarian subsidiary. Celltrion's biosimilars, primarily Remsima SC, performed strongly in Europe last year. The company's cumulative European export sales for Q3 of last year reached KRW 444.9 billion, up 57% compared to KRW 283.5 billion during the same period the previous year. This growth is attributed to the expanded export coverage of Remsima SC to additional countries. Followed in ranking were Germany, Switzerland, Türkiye, Belgium, Japan, Italy, Brazil, and the Netherlands. Export sales increased YoY for all countries except Japan and the Netherlands. Exports to Germany amounted to US$ 542.14 million, up 9% from US$ 495.40 million in 2023. Exports to Switzerland recorded US$ 438.78 million, up by 61%. Exports to Türkiye rose 14% to US$ 422.80 million, while Belgium saw a 29% increase, recording US$ 406.59 million. Exports to Japan declined by 37% from US$ 528.47 million in 2023 to US$ 331.77 million. In 2023, exports to Japan ranked second after the United States. However, it went down to No.7 for last year.
- Company
- Elahere receives orphan drug designation in Korea
- by Eo, Yun-Ho Jan 16, 2025 06:13am
- The new ovarian cancer drug Elahere has received an orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced the designation through the first orphan drug designation announcement of the new year. Specifically, the drug is indicated as monotherapy in adult patients with folate receptor-alpha (FRα) positive ovarian cancer, fallopian tube cancer, or primary peritoneal cancer who have received one to three prior systemic therapies and have not responded to or are no longer responding to treatment with platinum-based chemotherapy. AbbVie’s Elahere(mirvetuximab soravtansine) first received accelerated approval in the U.S. in November 2022 and then full approval in March of this year based on the MIRASOL clinical data, and was also recently approved in Europe. Ovarian cancer patients initially receive platinum-based anticancer drugs as treatment. However, many patients develop resistance to platinum-based anticancer drugs, which makes it difficult for patients to achieve anti-cancer effects with just platinum-based anticancer drugs. Antibody-drug conjugates (ADCs), which combine an antibody with a platinum-based drug (payload), have been developed in recent years to overcome this issue. Elahere, is a first-in-class ADC drug that combines a folate receptor alpha antibody and the tubulin inhibitor ‘maytansinoid payload DM4’ with a linker. The efficacy of the drug was demonstrated through the Phase III MIRASOL study. In the trial, Elahere improved the primary endpoint of progression-free survival (PFS), with a 35% reduction in the risk of disease progression or death compared to chemotherapy. Elahere also improved the secondary endpoint of overall survival (OS) compared to chemotherapy, with a 33% reduction in the risk of death in the Elahere arm. The objective response rate (ORR) was 42% in the Elahere arm and 16% in the chemotherapy arm. Among those who responded, 5% showed complete responses and 37% showed partial responses. AbbVie added Elahere to its oncology portfolio when it acquired the U.S. biopharmaceutical company ImmunoGen in 2023 for approximately USD 10.1 billion (KRW 14.7561 trillion).
- Company
- Companies develop new drugs for Fabry disease
- by Son, Hyung Min Jan 16, 2025 06:13am
- Domestic and foreign pharmaceutical companies are speeding up the development of new drugs for the rare Fabry disease. They aim to develop treatments that improve not only efficacy and safety but also administration methods compared to existing treatments. According to industry sources on the 15th, the Ministry of Food and Drug Safety recently approved the Phase I/II clinical trial plan (IND) for LA-GLA, a new drug candidate for Fabry disease being jointly developed by GC Biopharma and Hanmi Pharmaceutical. The two companies entered multinational clinical trials in August last year after receiving approval in the U.S. and Korea. Fabry disease is a rare disease that is inherited as a sex chromosome and is a type of Lysosomal Storage Disease (LSD). It is caused by a deficiency of the enzyme ‘alpha-galactosidase A,’ which breaks down glycolipids in lysosomes, an organelle in cells that remove unnecessary substances. The continuous accumulation of unprocessed glycolipids in the body leads to cytotoxic and inflammatory reactions, and progressive damage to various organs, eventually leading to death. LSD is caused by a genetic deficiency of certain enzymes, resulting in metabolic abnormalities. Lysosomes, which are organelles within cells, contain enzymes that break down substances that the body no longer needs. When these enzymes are impaired or not produced, substances that should be broken down gradually accumulate in the cell. Fabry disease, mucopolysaccharidosis, Pompe disease, and Gaucher disease, are typical examples of LSD. In 2020, Hanmi Pharmaceutical and GC Pharma signed an agreement to jointly develop next-generation innovative drugs for the treatment of LSD and are conducting joint research with the goal of developing global innovative drugs in the field of rare diseases. LA-GLA, a new drug candidate for Fabry disease being developed by the two companies, is a next-generation long-acting enzyme replacement therapy that improves the limitations of existing treatments. GC Biopharma and Hanmi Pharmaceutical plan to develop LA-GLA into a subcutaneous injection formulation that can be administered once a month. Most Fabry disease patients are treated with enzyme replacement therapy (ERT), an intravenous injection of enzymes developed by gene recombination technology. Sanofi Currently, Sanofi's Fabrazyme, Takeda's Replagal, Handok's newly introduced drug Galafold, and ISU Abxis’ Fabrazyme biosimilar Fabagal are being currently used in Korea. All but Galafold are intravenous ERT formulations. Galafold is an oral formulation that is more convenient to administer, but it is not available until after a year of ERT-based therapy. ERT-based therapies are known to have limitations, including the inconvenience of long intravenous infusions every 2 weeks in the hospital and the lack of efficacy in controlling advanced kidney disease. In preclinical studies, LA-GLA has been shown to improve kidney function and fibrosis compared to existing therapies, and in an animal model with Fabry disease, LA-GLA significantly improved peripheral sensory function and histologic lesions of the nerve cells that control it and was effective in improving the increase in blood vessel wall thickness caused by glycolipid accumulation. LA-GLA was designated as an orphan drug (ODD) by the U.S. Food and Drug Administration (FDA) in May based on its efficacy in improving kidney function, vascular disease, and peripheral nerve disorders compared to existing therapies in the clinical stage. Gene therapy also in development The global pharma and biotech industry also has the potential for new drugs for Fabry disease through single-dose gene therapy. Last month, the FDA granted orphan drug designation to Exegenesis Bio’s Fabry disease drug candidate 'EXG110'. EXG110 is a single-dose gene therapy candidate designed to clone the GLA gene and deliver it to liver and heart cells. The drug candidate utilizes an adeno-associated virus (AAV) to deliver the therapeutic gene. AAV is a virus that does not cause disease in humans and is characterized by its ensured safety. Exogenesis is currently conducting clinical trials in Fabry disease patients in China. The first patient has now been dosed with EXG110. The company plans to seek approval in the U.S. for a multinational trial. In September of last year, Unicure's Fabry disease drug candidate AMT-191 was granted orphan drug designation in the United States. AMT-191 is a gene therapy designed to target the liver to produce the GLA protein. Unicure is conducting a Phase I/II clinical trial of AMT-191 in the United States. Unicure plans to administer AMT-191 to 6 patients with Fabry disease to measure the expression of the lysosomal enzyme aGLA-A and evaluate the safety, tolerability, and efficacy of the candidate.
- Company
- JW Pharma's hemophilia assay for patients using 'Hemlibra'
- by Kim, Jin-Gu Jan 16, 2025 06:13am
- Product photo of Hemlibra. JW Pharmaceutical announced on January 15 that its chromogenic assay designed to assess the severity of 'hemophilia' in patients using 'Hemlibra' has been commercialized in South Korea. The Ministry of Health and Welfare (MOHW) has newly established a non-reimbursable CSA testing criterion, effective January 1. CSA test is used to measure the activity of clotting factors in patients with hemophilia to diagnose the severity of the disease and to monitor the effectiveness of the treatment. It is particularly effective in accurately measuring factor VIII, which is deficient in patients with Hemophilia A. It is suitable for patients who use non-coagulant agents like Hemlibra. The primary test used in South Korea is the One-Stage Clotting Assay (hereafter, OSA test). However, the OSA test is limited in accurately diagnosing patients with mild symptoms and confirming the severity of hemophilia in patients treated with Hemlibra. For this reason, the World Federation of Hemophilia (WFH) recommends a combination of OSA and CSA. CSA testing is recommended for testing the severity of hemophilia in patients using Hemlibra and hemostasis testing after factor VIII combination therapy. "The commercialization of the CSA test allows for assessing hemophilia severity in patients treated with Hemlibra," said Dr. Park Young-sil, professor of pediatrics at Kyung Hee University Hospital at Gangdong. "It will also improve the diagnostic environment for patients with mild symptoms and female hemophilia patients (carriers) who could not be accurately diagnosed using the existing OSA test." "The commercialization of the CSA test will significantly advance hemophilia diagnosis and treatment," said a representative from JW Pharmaceutical. "We will strive to improve patient access to treatments and the quality of life for patients with hemophilia A."
- Company
- China-made new drugs actively enter the global market
- by Son, Hyung Min Jan 16, 2025 06:13am
- Many Chinese pharmaceutical and biotech companies are demonstrating results from their new anti-cancer drug development in the global market. Companies like Jiangsu Hengrui Pharmaceuticals and BeiGene have obtained approvals for their immunotherapy and targeted anti-cancer agents from the regulatory authorities globally, including South Korea, the United States, and Europe. The gap between Chinese and Korean pharmaceutical and biotech companies has increased as Chinese companies succeeded in commercialization and Phase 3 clinical trial entries. It has been reported that most new anti-cancer drugs from Korean pharmaceutical and biotech companies remain in the preclinical stages or phase 1 clinical trials. Two immunotherapy drugs have been commercialized in the U.S….bispecific antibodies have entered phase 3 trials According to industry sources on January 14, the US Food and Drug Administration (FDA) recently approved 'Tevimbra,' an immunotherapy developed by a Chinese pharmaceutical company, BeiGene, as a first-line treatment for HER2-positive gastric cancer. Following the approval, Tevimbra became the third immunotherapy following MSD's Keytruda and BMS·Ono Pharmaceutical's Opdivo to be used as a first-line treatment for HER2-positive gastric cancer. In November 2023, Tevimbra received domestic approval as a monotherapy for the treatment of adult patients with unresectable, recurrent, locally advanced, or metastatic esophageal squamous cell carcinoma who are unable to continue platinum-based chemotherapy or have experienced recurrence or progression following such treatment. BeiGene has started over 17 clinical trials for potential indications for Tevimbra. The company has already confirmed positive results from 11 phase 3 trials and 4 phase 2 trials. To date, over 900,000 patients have been treated with Tevimbra. Tevimbra obtained approvals from over 40 countries worldwide. Chinese pharmaceutical companies had previously failed to obtain approvals from global regulatory authorities when only enrolling Chinese patients. Now, these companies are overcoming the approval hurdles by enrolling patient groups of various ethnicities and genders. In November 2023, 'Loqtorzi,' anti-PD-1 immunotherapy from China's Junshi Biosciences, was approved in the United States. Loqtorzi has been approved as the first-line treatment in combination with platinum-based chemotherapy for the treatment of metastatic or locally advanced nasopharyngeal carcinoma (NPC). Junshi Biosciences entered into an out-licensing deal with the US-based Coherus BioSciences for Loqtorzi with an upfront payment of US$ 150 million, totaling US$ 1.1 billion (approximately KRW 1.6 trillion). Jiangsu Hengrui Pharmaceuticals aims to receive approval for its camrelizumab in combination with the HLB group's targeted anti-cancer, Rivoceranib. In May 2024, Jiangsu Hengrui Pharmaceuticals and HLB received a Complete Response Letter (CPL) from the FDA but received a decision of 'not required to take additional actions' during the site monitoring. The combination therapy containing Rivoceranib and Jiangsu Hengrui Pharmaceuticals' immunotherapy, camrelizumab, demonstrated the longest survival extension benefit as the first-line treatment for liver cancer. China's Innovant is knocking on the door of the FDA in collaboration with the global pharmaceutical company Eli Lily. Following obtaining approval for the immunotherapy sintilimab in China, Innovant signed an out-licensing agreement with Eli Lily in 2015 worth US$ 1 billion (approximately KRW 1.3375 trillion). Both companies applied for approval of sintilimab for the treatment of non-small cell lung cancer (NSCLC) but failed to obtain the FDA approval. Lily and Innovant plan to conduct multi-regional clinical trials comparing sintilimab+chemotherapy to the existing standard therapy as requested by the FDA. Chinese pharmaceutical companies are showing results in bispecific antibodies. Last year, the PD-1/VEGF bispecific antibody ivonescimab demonstrated superior effects than Keytruda. Based on a Phase 3 clinical trial involving 398 patients with PD-L1-positive NSCLC, ivonescimab reduced the tumor progression risk by 49% than Keytruda. Ivonescimab is a bispecific antibody developed by China's Akeso, and Akeso signed a partnership agreement with the US-based Summit Therapeutics. Summit Therapeutics has the right to develop ivonescimab in the U.S., Canada, Europe, Japan, and Latin America. Akeso is developing AK112, a new drug candidate, in China and Australia. Chinese biotech company SystImmune is developing 'BL-B01D1,' targeting EGFR and HER3, which are commonly found in solid cancers. The company has confirmed the efficacy and safety in clinical trials and out-licensed the candidate to BMS for US$ 8.4 billion (KRW 11.7 billion). Korean companies' candidates are in the early phases of clinical trials…increasing gap between China Chinese companies have successfully commercialized their immunotherapies and bispecific antibodies. However, Korean pharmaceutical and biotech industry candidate products are still in the early phases of clinical trials. TiumBio is conducting a Phase 2ab trial of its immunotherapy candidate TU2218. TU2218 blocks pathways of transforming growth factor beta (TGF-ß) and vascular endothelial growth factor (VEGF), which are known to hinder cancer immunotherapy activation. In a Phase 1a trial, TU2218 treatment reduced the blood concentration of major biomarkers of connective tissue growth factor (CTGF), whose expression is known to be regulated by TGF-ß. After seven days, CTGF blood concentration decreased by 27% on average compared to baseline levels. Currently, in addition to the TU2218 monotherapy, TiumBio is also conducting clinical trials for a combination therapy that includes Keytruda. GI Innovation recently modified its Phase ½ clinical trials in the United States studying immunotherapy candidate 'GI-102' to confirm its combination with Enhertu. GI-102, which acts on CD80 and interleukin (IL)-2. IL-2 is involved in immune cell proliferation and activation, and CD80 blocks CTLA-4, a receptor preventing immune cells from attacking cancer cells. GI-102 is being developed as intravenous (IV) and subcutaneous (SC) formulations. GI-102 demonstrated potential in a monotherapy clinical trial. According to Phase 1/2a clinical trial data presented by the company, Skin melanoma patients treated with GI-102 had an objective response rate (ORR) of 43%. Under GI-102 treatment, lymphocyte proliferation was found, and there has been no significant drug toxicity regarding the safety profile. ImmuneOnsia confirmed the drug safety profile of its immunotherapy candidate, IMC-002, is a dose-escalation Phase 1a trial. IMC-002 works by blocking the signal between CD47 of cancer cells and macrophages. The company monitored the effect of IMC-002 in 12 patients, and there was no drug toxicity in each dosage. Six out of 12 patients had steady disease (SD). The company plans to determine the recommended dosage for the phase 2 trial based on the results from the phase 1 trials. ABL Bio has started a phase 1 trial to evaluate the efficacy and safety of its immunotherapy candidate ABL103 in patients with advanced·metastatic solid cancer. The company aims to evaluate the safety and tolerability of ABL103 monotherapy and determine the recommended dosage and maximum tolerated dose for a subsequent phase 2 clinical trial. The phase 1 trial will be conducted in both South Korea and the United States. In preclinical studies, ABL103 demonstrated to induce 4-1BB activation in tumor microenvironments expressing B7-H4. Additionally, it showed complete eradication of cancer cells and the suppression of recurrence in homologous cancer cells. Pharmaceutical personnel said, "New drug candidate discovery capacity of Korean companies is at the global level. However, they have difficulty entering a later phase of clinical trials. To secure global approval, these companies need to conduct multi-national clinical trials and clinical trials across numerous sites. Therefore, raising the funds can be difficult." "Companies must demonstrate the benefits of new drug candidates and how to address their weaknesses in clinical trials. They should identify unmet needs in clinical practices before conducting trials and set goals for the development of new drugs based on intended purpose," he added.
- Company
- ‘Global healthcare M&A to recover this year... AI-China’
- by Cha, Jihyun Jan 15, 2025 05:53am
- Artificial intelligence (AI) and China are expected to be the keywords for mergers and acquisitions (M&A) in the global bio and healthcare sector this year. On the 14th, KoreaBIO disclosed a report by global accounting consulting firm Ernst & Young (EY) that contained the prospects above. According to the report, 130 M&A deals were made in the global bio-healthcare sector last year, including 95 for biopharmaceuticals and 36 for medical devices. This was similar to the previous year's 130 (81 biopharma and 49 medical devices). Deal values were down significantly from the previous year. The value of M&A deals last year was USD 130 billion (approximately KRW 190 trillion), down 41% from the previous year. Unlike 2023, which was dominated by large deals in safe-haven (risk-free) assets such as government bonds, last year was full of smaller deals. Bio-Healthcare M&A Deals (Source: Bio-Healthcare M&A) The report characterizes 2024 as a “reset year” for big pharma that was digesting and integrating acquisitions made the previous year. Regulation by the U.S. Federal Trade Commission (FTC) and the implementation of the Inflation Reduction Act (IRA) contributed to a slowdown in M&A activities last year. The average M&A deal size in 2024 was USD 1 billion (about KRW 1.5 trillion), which was down 42% from the previous year. Rather than invest billions of dollars to acquire less risky, market-ready assets, companies are looking to innovate by acquiring early-stage assets that are in pre-Phase III clinical development. “This means that global bio-healthcare M&A last year was smaller but smarter than in previous years,” the report concludes. The report predicted that the market will likely recover this year, but uncertainty remains. The industry has USD 1.3 trillion (KRW 190 billion) of M&A firepower, but some regulations and policies could be in the way, it said. The report cited emerging areas of ▲AI and ▲Chinese partnerships as trends in M&A this year. Over the past 5 years, the value of healthcare AI M&A deals has exceeded USD 60 billion. Most leading companies have formed at least one partnership for AI collaboration. In 2024, the number of deals reached a record high. The number and value of deals over the past 5 years reached 41 deals in 2020 (USD 5 billion), 54 deals in 2021 (USD 16.4 billion), 77 deals in 2022 (USD 15.5 billion), 55 deals in 2023 (USD 13.9 billion), and 87 deals in 2024 (USD 13.6 billion). Trends in AI-related Bio-Healthcare M&A Deals (Source: Bio-Healthcare M&A) The largest AI-related deal to date was the August 2024 acquisition of Exscientia by Recursion Pharmaceuticals. Recursion acquired Exscientia for USD 712 million. “The surge in AI partnerships and acquisitions over the past 5 years is indicative of the opportunities that AI presents to life sciences companies,” the report said, noting that the biggest focus is on using AI to discover new drugs and optimize development. The report added, “AI is delivering benefits across the value chain, from operations to commercial strategy, and the EY CEO Confidence Index shows that life sciences CEOs see emerging technologies, including AI, as the biggest disruptors over the next 12 months, along with talent acquisition.” The report also found that China is becoming a more important partner for companies looking to transfer technology for antibody-drug conjugates (ADCs) and other novel oncology therapies. In 2023, deals involving new modalities, such as ADCs, next-generation radiopharmaceuticals, and multi-specific antibodies, had been made in the Chinese market. The report also found that 43% of M&A with Chinese companies were aimed at acquiring ADCs. AstraZeneca paid USD 1.2 billion to acquire China's Gracell Biotech. It was the first outright acquisition of an innovative Chinese company by a global Big Pharma. Novartis' purchase of Shanghai Argo Biopharmaceuticals last year was also considered potentially the largest deal. However, the U.S. Biosecure Act is seen as the biggest challenge to the growth of China's life sciences sector. “This could limit the ability of companies to collaborate across borders,” the report said, adding that “the U.S.-China relationship also faces uncertainty under the Trump administration in 2025.”
- Company
- Cholangiocarcinoma·AML targeted anticancer drug 'Tibsovo'
- by Eo, Yun-Ho Jan 15, 2025 05:53am
- Product photo of Tibsovo 'Tibsovo,' a targeted anticancer drug for cholangiocarcinoma and acute myeloid leukemia (AML), is now available for prescription in general hospitals. According to industry sources, Servier's Tibsovo (ivosidenib), a drug targeting the isocitrate Dehydrogenase 1 (IDH-1) gene mutation, has passed the drug committees (DC) of the 'Big 5' tertiary general hospitals, including Samsung Medical Center, Seoul National University Hospital, and Seoul Asan Medical Center, and medical centers, including, Gangnam Severance Hospital, National Cancer Center, Gangnam Severance Hospital, and Chungbuk National University Hospital. Following receiving approval from the Ministry of Food and Drug Safety (MFDS) in May 2024, it was officially launched in September of the same year. Since then, it has expanded prescription areas. If a patient is tested positive for IDH1 mutation, Tibsovo can be used as a ▲Monotherapy in patients with locally advanced or metastatic AML and had prior therapy ▲Combination therapy with 'azacytidine' in adult patients over 75 years with accompanying disease that cannot be treated with chemotherapy. Cholangiocarcinoma is a cancer with a poor prognosis. The five-year relative survival rate is only 28.9%. 65% of the patients with cholangiocarcinoma of the liver are found be non-operable when diagnosed. Tibsovo is the only targeted drug recommended as a Category 1, the highest grade, by the National Comprehensive Cancer Network (NCCN) for a second-line treatment for cholangiocarcinoma. According to ClarlDHy Phase 3 clinical trial, Tibsovo reduced the disease progression by 63% compared to a placebo and had a median progression-free survival (PFS) of 2.7 months (placebo 1.4 months). Also, patients treated with Tibsovo had a median overall survival (OS) of 10.3 months, which was longer over twice than 5.1 months of those treated with a placebo. Do-Youn Oh, Professor of Department of Hematology-Oncology at Seoul National University Hospital, said, "Over the last five years, the development of treatments for cholangiocarcinoma got fast. Along with new drug development, many companies are focusing on developing drugs for cholangiocarcinoma. Patients with cholangiocarcinoma need to follow physician's advice, receive treatments, and seize new opportunities such as participating in clinical trials." Meanwhile, in the AGILE Phase 3 trial involving patients with AML, Tibsovo was demonstrated to improve event-free survival (EFS) when combined with azacytidine, and the overall survival (OS) was significantly improved. The patients treated with TIbsovo had a median OS of 24.0 months (placebo 7.9 months). In a long-term follow-up study, the median OS of Tibsovo combination therapy was 29.3 months, over 3.7 fold longer than that of placebo combination therapy.
- Company
- Samsung Biologics signs largest CMO deal…KRW 2 Trillion
- by Cha, Jihyun Jan 15, 2025 05:52am
- Pic of Samsung Biologics (Source: Samsung Biologics) Samsung Biologics announced on the 14th that it has signed a contract manufacturing organization (CMO) agreement worth USD 1.41 billion (approximately KRW 2.74 trillion) with a Europe-based pharmaceutical company. This is the largest contract in Samsung Biologics' history. It represents 40% of the total order value of KRW 5.4035 trillion last year. The contract will run through December 31, 2030. The customer and product names were not disclosed due to confidentiality. With the contract, Samsung Biologics has broken its record for the largest order. The record renewal comes just 3 months after the company signed a contract worth KRW 1.7028 trillion with an Asian pharmaceutical company in October last year. Last year, Samsung Biologics further solidified its position in the global market by signing 3 “big deals” worth KRW 1 trillion in major markets including the U.S., Europe, and Asia. Last year's annual orders totaled at KRW 5.4035 trillion, the largest ever amount, a 1.5 times increase compared to the previous year. Samsung Biologics currently has 17 of the top 20 global pharmaceutical companies as customers. Based on its core order-winning competitiveness, including overwhelming production capacity, quality competitiveness, and multiple track records, the company's cumulative order total has exceeded USD 17.6 billion since its establishment. Samsung Biologics is expanding its production capacity to proactively prepare for the growing demand for biopharmaceuticals. Plant 5, a 180,000-liter production plant that will incorporate the best practices of Plants 1 through 4, is under construction and is scheduled to be operational in April. Upon completion, Samsung Biologics will have a total production capacity of 784,000L. In terms of quality, Samsung Biologics has proven its competitiveness in all stages of drug manufacturing and management, including a 99% batch success rate. As of December 2024, the company has obtained a total of 340 global regulatory approvals, including 41 from the U.S. Food and Drug Administration (FDA) and 36 from the European Medicines Agency (EMA). Its regulatory on-site inspection pass rate is also among the highest in the industry. Samsung Biologics has been participating in a series of large-scale pharmaceutical and biotech industry conferences in the U.S., Europe, and Asia to strengthen business networking and order acquisition activities. Samsung Biologics is participating in the '2025 JP Morgan Healthcare Conference', the largest investment event in the pharmaceutical and biotechnology industry that is being held in San Francisco, U.S.A., from April 13-16 to strengthen networking for business expansion.