- LOGIN
- MemberShip
- 2025-12-22 02:43:12
- Company
- ‘Leqvio reduces LDL-C with twice-yearly dose’
- by Son, Hyung Min Feb 07, 2025 05:51am
- While the “the lower, the better” mantra in LDL-cholesterol (LDL-C) treatment is gaining traction around the world, achieving and maintaining target levels is challenging due to the patients’ poor adherence to existing therapies. In the United States, 50% of patients who are prescribed statins stop taking them within one year, and only 5% remain on treatment after 5 years. Long-term management of LDL-C is crucial, but the burden of traditional oral medications has been leading to poor patient adherence, which is why demand has been rising for new treatment options that can address this unmet need. Dr. Michael D. Shapiro. Profesosr of Cardiology at Wake Forest University School of Medicine Michael D. Shapiro. Professor of Cardiology at Wake Forest University School of Medicine explained that the siRNA therapy Leqvio could serve as an alternative in this aspect, at a recent meeting with Dailypharm. Leqvio uses siRNAs that are naturally present in the body to reduce LDL-C in the blood by inhibiting the production of the PCSK9 protein, which raises LDL-C. SiRNAs work by binding in a sequence-specific manner to messenger ribonucleic acids (mRNAs) that make disease-causing proteins and then degrade them to block the underlying cause of the disease. Through RNA interference, siRNA causes the degradation of PCSK9 mRNA, which inhibits protein production. When PCSK9 is inhibited, increased low-density lipoprotein receptors (LDLRs) on the cell surface bind LDL-C and reduce LDL-C levels in the blood. Leqvio was approved in the U.S. in December 2021 and has been available since 2022. In Korea, it was approved last year for the treatment of heterozygous familial hypercholesterolemia (HeFH) in patients with atherosclerotic cardiovascular disease (ASCVD) or at risk of ASCVD and has been gradually being used in the field. Leqvio’s greatest strength is its ease of administration. Leqvio can be administered twice a year for effective LDL-C control, compared to daily oral medications or biweekly injections. Professor Shapiro believes that Leqvio will allow more patients to benefit from effective LDL-C reduction. High LDL-C levels are associated with increased cardiovascular risk...“Expectations rise on the use of Leqvio” Cardiovascular disease is the number one cause of death worldwide, and LDL-C management has become an important goal in ASCVD as lowering LDL-C is associated with a lower risk of cardiovascular disease. However, only 24.4% of Korean patients with ASCVD achieved their LDL-C goal, and about 78% of Korean patients with acute myocardial infarction did not achieve their LDL-C goal within 1 year after myocardial infarction. Adherence is also one of the challenges of LDL-C treatment. Patients need to take medication for the rest of their lives to treat LDL-C, but adherence is difficult to maintain. U.S. data shows that 50% of patients stop taking statins within 1 year of being prescribed them, and only 5% remain on treatment at 5 years. “There has been rising hope that siRNA therapies may address the unmet need for adherence in patients with dyslipidemia,” said Professor Shapiro. In particular, Leqvio has the potential to change the landscape of LDL-C treatment with its ability to effectively lower LDL-C and maintain lower levels for longer with twice-yearly dosing.” In three Phase III studies in patients diagnosed with primary hypercholesterolemia, mixed dyslipidemia, or at-risk or heterozygous familial hypercholesterolemia, Leqvio demonstrated LDL-C reductions of up to 52% compared to placebo. In particular, the drug demonstrated 60.5% lower LDL-C compared to placebo in the ORION-18 study in Asian patients, 24% of which were Koreans. “Leqvio may be considered for patients who are not achieving adequate LDL-C control with the maximum tolerated dose of statin,” said Professor Shapiro. “The efficacy and safety of Leqvio in clinical studies have been consistent in real-world practice. The LDL-C reduction effect has been high, around 50%.“ Twice-yearly dosing a major strength...”We need to improve patient access” Leqvio’s twice-yearly dosing regimen dramatically increases the interval between doses compared to oral statins, ezetimibe, and other injectables that require daily dosing. The next hurdle is reimbursement. Novartis, Leqvio’s developer, has reportedly begun reimbursement discussions with the government. “It's not just about lowering LDL-C, it's about maintaining lower LDL-C levels for a longer period of time,” said Professor Shapiro. As LDL-C increases, the risk of cardiovascular disease increases, so we need to improve the care setting to allow consideration of aggressive treatment early on.” However, the burden of cardiovascular disease is underestimated in general due to a lack of understanding of its risk. For example, once a patient suffers a heart attack, there is a high likelihood of heart failure, and the prognosis is much worse than cancer, yet there is a lack of awareness of the seriousness of the disease. “It is important to actively manage LDL-C with drugs as soon as possible,” said Professor Shapiro. In Korea, cardiovascular disease is the second leading cause of death, but it can change to take the lead anytime, so we want to create an environment where people can manage their disease more actively,” said Dr. Shapiro. “Patients with familial hypercholesterolemia have very high cholesterol levels from birth, and if not diagnosed and treated early, they can experience cardiovascular disease at a young age. Statin and ezetimibe therapy alone is not enough, so Leqvio’s effect in achieving and maintaining stable LDL-C reductions renders the drug a quite significant treatment option.”
- Company
- China rises as global R&D leader, K-bio not in top 50
- by Cha, Jihyun Feb 06, 2025 05:57am
- The R&D investment gap between Korean biopharmaceutical companies and Big Pharma is increasing. Analysis suggests that Korean companies must seek measures to respond to competition for next-generation technology as the R&D investment centers around the United States and China. On February 5, the Korea Chamber of Commerce and Industry (KCCI) announced the results of analyzing analyzed the top 2,000 companies as part of the 2024 EU Industrial R&D Investment Scoreboard report, which was reported on December 2024 by the EU Joint Research Centre. According to the results, the United States ranked No.1 in both the number of companies and investment amount in the 2023 list of top 2,000 companies for R&D investment worldwide. The number of US companies included in the top 2,000 companies for R&D investment totaled 681. In 2023, the US accounted for 42.3% of the investment amount, totaling EUR 531.9 billion. Table: Top 10 countries by the number of companies and investment amount in the 2023 list of top 2,000 companies for R&D investment worldwide. Notably, China's growth rate last year was outstanding. In 2023, China ranked No.2 with 524 companies and an investment amount of EUR 215.8 billion. Compared to the 2013 record, 119 companies and EUR 18.8 billion, the number of Chinese companies added to the top 2,000 companies increased to 405 and the investment amount increased 11.5-fold over 10 years. Out of the top 10 countries, China is the only country that has continuously increased both the number of companies and investment over 10 years. Analysis suggests that the investment trend tipped toward the US, an unchallenged No.1, and China, with robust growth. The number of US and Chinese companies included in the top 2,000 R&D investments totaled 1,205, which accounts for 60.3%. The net R&D investment amount is EUR 747.7 billion, close to 59.5%. The number of Korean companies on the list decreased from 54 in 2013 to 40 in 2023. However, South Korea maintained No.8 in rank, defending the position relatively well. As per the investment amount, South Korea ranked No.7 in 2013 with EUR 19.3 billion, then increased to No.5 with EUR 42.5 billion in 2023. For pharmaceutical companies, Big Pharma's investment showed marketed increases. Compared to 10 years before, the R&D investment increases were 3.4-fold for the US-based Gilead Sciences, 3.1-fold for AbbVie, 3.1-fold for Bristol Myers Squibb, and 3-fold for AstraZeneca. Following the COVID-19 pandemic, R&D investment increased significantly. Big Pharma Among Korea's biopharmaceutical companies, Hanmi Pharmaceutical invested the most in R&D. R&D investment over 10 years increased twofold. Hanmi Pharmaceutical's R&D investment increased from EUR 70 billion in 2023 to EUR 130 billion in 2023. The top 50 companies included Roche, Johnson & Johnson, Merck, Pfizer, AstraZeneca, Eli Lilly, Bristol Myers Squibb, Oracle, Novartis, Sanofi, AbbVie, GSK, Siemens, Boehringer Ingelheim, Bayer, Gilead Sciences, and Takeda Pharmaceutical. No Korean biopharmaceutical companies were listed among the top 50 out of the 2,000 ranked companies. Among the top 40 of 2,000 ranked companies, Korean companies included Hanmi Pharmaceutical, GC Corp, Daewoong, Daewoong Pharmaceutical, Yuhan Corp, Celltrion, Chong Kun Dang, and Ildong Pharmaceutical. Hanmi Pharmaceutical, which ranked first in R&D investment, was followed by GC Holdings with EUR 126 million, Daewoong with EUR 124 million, and Yuhan Corporation with EUR 116 million in R&D. Kim Hyun-soo, head of the KCCI's Economic Policy Team, said, "As the introduction of China-based Artificial Intelligence (AI) start-up's DeepSeek shocking the world shows, the competition between companies over the industry's leading technologies intensifies." Kim added, "South Korea must enhance R&D through tax supports, such as increasing tax exemption for investing in the next-generation R&D facilities, a measure under review in the National Assembly, and increasing the deduction rate for basic R&D."
- Company
- Baxter’s Renal Care Unit spinoff Vantive is launched
- by Whang, byung-woo Feb 06, 2025 05:56am
- With the acquisition process complete for Baxter's Renal Care business, it has newly launched as Vantive, a new company specializing in kidney and life-sustaining organ therapies. The spin-off follows the acquisition of Baxter's Kidney Care business by funds managed by global investment firm The Carlyle Group. As an independent company, Vantive will focus on raising the standard of kidney and vital organ care to help patients around the world live richer, longer lives. Its strategy for advancing life-sustaining organ care will empower patients and healthcare providers to take more control of their care by helping to break down barriers to starting and maintaining treatment. In particular, Vantive will build on its 70-year legacy of innovation in kidney care to fulfill its mission of “Extending Lives, Expanding Possibilities.” “For patients starting dialysis and for caregivers struggling to save lives in the intensive care unit, it's critical to provide the right treatment at the right time,” said Chris Toth, CEO of Vantive. ”Our core focus is to support patients and caregivers in these moments by providing better choices, greater autonomy, and greater possibilities. “Vantive is ushering in a new era of innovation in life-sustaining long-term care, and we look forward to working with our 23,000 employees around the world to make a difference for millions of patients and families.” Vantive provides innovative products, digitally enhanced solutions and advanced services to support dialysis at home and in the hospital, and treatment options to support kidney and vital organ function in critically ill patients. Patients in more than 100 countries around the world use Vantiv's solutions and services more than one million times every day, representing an opportunity to deliver more than one million improved treatment experiences every day. Through innovative integrated devices, Vantive also aims to help healthcare providers deliver care more efficiently and patients receive treatment while maintaining their daily routines. “The launch of Vantive marks a significant milestone in the continued evolution of kidney care and life-sustaining organ support,” said Kieran Gallahue, Chairman of the Board of Directors at Vantive. ”Vantive is focused on providing better connectivity, visibility, and insight throughout the patient's care journey. Robert Schmidt, Global Co-Head of Carlyle Healthcare, added, “We are excited to partner with Vantive to expand its global impact and realize sustainable growth. In this new journey, we look forward to actively supporting Vantive's ongoing innovation to improve patient access to care, quality and outcomes, and clinician efficiency.
- Company
- Imminent launch of the 3rd new CAR-T-cell therapy 'Yescarta'
- by Eo, Yun-Ho Feb 06, 2025 05:56am
- Product photo of Gilead Sciences The third CAR-T-cell therapy 'Yescarta' is expected to be commercialized in South Korea. According to industry sources, Gilead Sciences submitted documentation for the approval of the CAR-T-cell therapy, Yescarta (axicabtagene ciloleucel), and the Ministry of Food and Drug Safety (MFDS) is currently conducting the review. Yescarta was designated an orphan drug by the MFDS in September last year. The designated indication includes ▲Adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal B-cell lymphoma (PMBCL) who have undergone two or more systemic treatments ▲Adult patients with DLBCL who have relapsed or refractory within 12 months after the first-line chemoimmunotherapy. Yescartav is a CAR-T cell therapy that received the first approval from the U.S. Food and Drug Administration (FDA) as the third-line treatment in October 2017. After receiving approval from the European Commission (EC), the drug has expanded to the second-line treatment. In 2021, it became available for use in treating follicular lymphoma. The efficacy of Yescarta as the third-line treatment has been confirmed through the ZUM-1 clinical study. The study reported a 5-year survival rate, showing that 42.6% of all patients treated with Yescarta survived for five years, and 92% of those no longer needed additional cancer treatment. The ZUMA-7 Phase 3 clinical trial confirmed the drug's efficacy for the second-line treatment. It was the first-in-class for a CART-T cell therapy, the largest in scale, and the longest follow-up trial. 359 patients world-wide were randomly assigned to receive one-time treatment of Yescarta or the existing standard-care second-line therapy. The analysis results presented at the American Society of Clinical Oncology conference (ASCO 2023) last year included a median follow-up at 47.2 months, at which the median value of overall survival (OS) with Yescarta was not reached. However, Yescarta's death rate was 27%, which was statistically lower than the control group's 31.1 months. The OS at 48 months was 54.6% for Yescarta and 46.0% for the control group. Yescarta treatment showed consistent survival benefits in pre-assigned subgroups, including age groups, refractory at the first-line therapy, early relapse, or high-grade B-cell lymphoma (HGBL). Additionally, excluding the impact of treatment switching using the pre-assigned method, Yescarta's risk of death was 39% lower than the control group. In South Korea, Novartis Korea's 'Kymriah (tisagenlecleucel)' was the first CAR-T-cell therapy to receive approval. In March 2024, Janssen Korea's 'Carvykti (ciltacabtagene autoleucel)' also received approved. Kymriah is now added to the insurance reimbursement list, and Carvykti is still a non-reimbursed drug.
- Company
- 1st to exceed KRW 1T sales…Pfizer's ESG initiatives
- by Whang, byung-woo Feb 05, 2025 05:52am
- Health Insight by Reporter Whang, byung-woo Pfizer Korea, the first to exceed KRW 1 trillion in sales from COVID-19 vaccine·treatments, continues strengthening its sustainable business management based on the ESG initiative. After recording KRW 3 trillion in 2022 during the COVID-19 pandemic, Pifzer Korea's sales robustly decreased. However, despite unusual circumstances, the industry assesses that the company ensured internal stability afterward. Also, Pfizer Korea launched the 'An Accord for a Healthier World (Health Equity)' study and appears to be expanding its influence in all ESG aspects, including pursuing long-term projects. Major achievements during the COVID-19 pandemic…anticipates securing a stable source of sales Pfizer Korea's past few years can be summarized with the keyword 'COVID-19.' The company's sales robustly increased since 2021 after supplying the COVID-19 vaccine Comirnaty and the COVID-19 treatment Paxlovid. Pfizer Korea's sales reached ▲KRW 391.9 billion in 2020 ▲KRW 1.694 trillion in 2021 ▲KRW 3.2254 in 2022. However, the 2023 sales amounted to KRW 1.6018 trillion, down 50.3% from the previous year. Analysis suggests that the sales were reduced in half due to decreased COVID-19 patients transitioning to the endemic. Because distribution and marketing of the COVID-19 vaccine·treatment required minimal SG&A costs since the government primarily handled these, Pfizer Korea could generate high revenue. Pfizer Korea The sales trend for 2024 is yet unavailable. However, based on global sales, a significant drop, such as those observed in 2023 compared to 2022, seems unlikely. Pfizer's Q3 sales last year recorded US$ 17.7 billion (approximately KRW 24.4 trillion), up 31.2% Year-over-Year (YoY). Pfizer's net sales from Q1 to Q3 last year amounted to US$45.864 billion (approximately KRW 63.47 trillion), up 2.0% from the same period of the previous year. Such sales growth was driven by the COVID-19 pharmaceuitcals. The COVID-19 treatment Paxlovid generated US$ 2.7 billion in Q3, which robustly increased by 1238.1% YoY. Based on these statistics, Pfizer believes that the sales index for the COVID-19 vaccine·treatment has entered a stable period. Despite experiencing robust increases and decreases in sales during the COVID-19 pandemic, Pfizer believes its potential sales are now predictable as the COVID-19 vaccination became mandated for the high-risk group. The South Korean government also actively secured treatments by adding Paxlovid to the reimbursement list. Pfizer recently estimated 2025 sales to reach around US$ 61 billion (KRW 88 trillion) to US$ 64 billion (KRW 93 trillion). It may not be close to the sales of US$ 100 billion (KRW 145 trillion) in 2022, but the company is likely to continue growth compared to the sales of US$ 58.5 billion (KRW 85 trillion) in 2023. Sales trend of Paxlovid and Comirnaty (unit: US$ 1 million). BLUE: Paxlovid, Gray: Comirnaty Cash cow sources following the pandemic…hopes for pneumococcal‧RSV vaccines대 Because COVID-19-related sales are expected to be limited, Pfizer needs a new cash cow to drive sales growth. As part of its mid-to-long-term growth strategy, Pfizer aims to strengthen its position in the oncology sector by launching eight new drugs expected to generate over US$1 billion (approximately KRW 1.3 trillion) in sales by 2030. For this goal, Seagen, an antibody-drug conjugate (ADC) specialist company that Pfizer acquired for US$ 43 billion (approximately KRW 55 trillion), is involved. Seagen has developed various ADC-based cancer therapies, including Adcetris, Padcev, and Tukysa. Pfizer plans to establish a blockbuster drug portfolio centered on Seagen’s ADC therapies alongside its existing targeted cancer treatments, such as Ibrance for breast cancer and Lorbrena for lung cancer. Given the momentum from the Seagen acquisition, Pfizer projects revenue from non-COVID-19 products to reach US$ 70 billion to US$ 84 billion (approximately KRW 95 trillion to KRW 114 trillion) by 2030. Additionally, Pfizer has launched a global cost reduction initiative. Through its 'Cost Realignment Program,' the company aimed to cut US$ 4 billion (approximately KRW 5.2 trillion) in spending by 2024. Pfizer has also announced plans to further reduce costs by an additional US$ 1.5 billion (approximately KRW 2.1 trillion) by 2027. CEO of Pfizer Albert Bourla stated, "Through the Cost Realignment Program, we successfully achieved our goal of reducing US$ 4 billion (KRW 5.8332 trillion) in net operating costs by 2024. We also expect to secure an additional US$ 500 million (KRW 729.1 billion) in 2025." Bourla added, "To further improve profitability, we will continue advancing our manufacturing optimization program this year." Pfizer In the Korean market, many new products are expected to challenge the market. One notable product is Prevenar 20, which received approval from the MFDS in November last year. It is a new pneumococcal vaccine that Pfizer is introducing in 14 years. The vaccine added seven new serotypes (serotypes 8, 10A, 11A, 12F, 15B, 22F, 33F) to the existing Prevenar 13. The vaccine is projected to launch in the early half of the year. Since it has established solid ground in the global market, Prevenar 20 is anticipated to land quickly. Mylotarg (gemtuzumab ozogamicin) for acute myeloid leukemia (AML), which has not been reimbursed, and 'VYNDAMAX (tafamidis)' for cardiomyopathy, which is near drug price negotiations with the HIRA, is expected to affect the sales for 2025. Also, Pfizer's RSV vaccine is expected to be submitted for approval process this year. RSV is regarded as a new market opportunity. Products have been approved or launched, GSK for adults and Sanofi for children, experts analyze that Pfizer, with adults and children indications, still has a chance in the competition. Driven ESG initiatives, strengthened sustainable business management Additionally, Pfizer conducts long-term projects by expanding all aspects of ESG initiatives to meet the goal to pursue social value and continue efforts. Pfizer Korea launched the ESG initiative, 'Moves for a Healthier World,' in 2022, and has included it as one of the company goals. For instance, the company has chosen six priority areas, including ▲Climate change action ▲Pharmaceutical innovation ▲Equitable Access to medicines ▲Diversity, Equity, and Inclusion ▲Pharmaceutical quality and safety ▲Company ethics, and has taken action on sub-projects. As part of the Green Moves Campaign, Pfizer Korea recently donated KRW 10 million to the 'School Forest Project' hosted by the Forest for Life. In collaboration with Pusan National University's Research and Business Development Foundation, Pfizer also commenced the 'Study to Develop Index and Analyze Social Determinants for Health Equity Across the Life Course' involving Korean citizens. This project aims to assess healthcare inequality and enhance societal interest in alleviating the healthcare gap by focusing on increased healthcare inequality and losses in socially disadvantaged classes after the COVID-19 pandemic. Additionally, Pfizer also holds various activities to enhance social value, including the 'Pfizer Medical Research Award,' which is in its 22nd year, and the Work Program for Young Disabled People. Pfizer Korea CEO Dong-Wook Oh stated, "Pfizer Korea aims to realize 'Moves for a Healthier World' and continues efforts to bring positive changes to Korean society." Oh added, "The program covering all ESG aspects reflects Pfizer's goal and efforts to bring changes so that all people can experience a healthier world." "The 'Health Equity' project launched last year will be our first crucial step in assessing Korea's healthcare equity state and establishing measures to improve the system," Oh added. "By closely collaborating with Korean researchers, Pfizer will strive to help people to stay healthy."
- Company
- Multiple sclerosis drug Ocrevus may soon be reimbursed
- by Eo, Yun-Ho Feb 05, 2025 05:52am
- The new multiple sclerosis drug Ocrevus is expected to be listed for reimbursement in Kore. According to industry sources, Roche Korea has completed negotiations with the National Health Insurance Service on the drug price of Ocrevus (ocrelizumab) for relapsing multiple sclerosis (MS). As a result, the drug is expected to be reimbursed once it passes the Health Insurance Policy Review Committee review. The company is moving quickly to finalize the reimbursement process, given that it submitted the application for reimbursement after its domestic approval in May last year. Ocrevus targets CD20-expressing B cells that affect the demyelinating process that causes neurological disorders in MS patients. MS is a chronic disease in which myelin is damaged by an autoimmune inflammatory response. Damage to the myelin sheath causes symptoms such as muscle weakness, fatigue, and vision impairment, and can lead to non-traumatic disability. As of 2022, an estimated 2,674 patients in Korea are known to be suffering from MS, with those in the 20-40 age group accounting for more than 62% of the total. Antibody therapies such as Tysabri (natalizumab), Gilenya (fingolimod), and Mabthera (rituximab) have been utilized in the disease area, but there has been a steady demand for additional high-efficacy drugs. Various new drugs have been developed overseas, including Novartis' Kesimpta (ofatumumab) and TG Therapeutics' Briumvi (ublituximab), but Roche's Ocrevus is the only one introduced in Korea. Ocrevus also has the advantage of offering a shorter dosing period. Ocrevus can be administered once every 6 months, which is more convenient than Kesimpta (administered once every month). The approval was based on the Phase III OPERA-I and II studies. The studies evaluated the efficacy and safety of Ocrevus versus Biogen's interferon therapy Plegridy (interferon beta-1a) in patients with relapsing MS. In the trial, Ocrevus reduced the annualized relapse rate (ARR) by nearly half compared to Plegridy. Specifically, in the OPERA I study, the ARR was 0.156 for 96 weeks of Ocrevus versus 0.292 for the control arm, and in OPERA II, the ARR was 0.155 for 96 weeks of Ocrevus versus 0.290 for the control arm. Ocrevus also showed efficacy in the Phase III ORATIORIO study in patients with primary progressive MS. In this study, Ocrevus reduced the risk of confirmed disability progression (CDP) by 24% over 12 weeks compared to the control group. Ho Jin Kim, professor of Neurology at the National Cancer Center, said, “In MS, even small differences in the early stages can have huge cumulative consequences. This is why the benefits of early access to highly effective therapies are significant. These treatments will not only improve patients’ quality of life but also help reduce the cost of the disease to society and the economy. Ocrevus is well positioned to be utilized because it has sufficient data established not only in terms of efficacy but also on long-term treatment administration.”
- Company
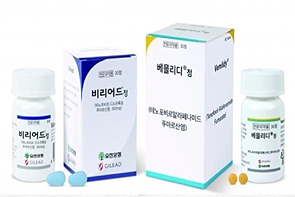
- Hepatitis B drug market remakes ₩300B after 6 years
- by Kim, Jin-Gu Feb 04, 2025 05:55am
- The outpatient prescription market for hepatitis B drugs expanded again last year to exceed KRW 300 billion. This is the first time the market has exceeded the mark in 6 years since 2018. Gilead Sciences’ Vemlidy drove the market growth with a 15% increase in sales from KRW 61.9 billion to KRW 71.3 billion in 1 year, while the combined prescription volume of tenofovir-based generic products increased by 21%. Hepatitis B drug market re-enters KRW 300 billion range for the first time in 6 years According to the market research institution UBIST on Wednesday, the outpatient prescription market for hepatitis B drugs was KRW 303 billion last year. This is a 4% increase from the KRW 292.3 billion in 2023. The market has re-entered the KRW 300 billion range for the first time in six years since 2018. The market was valued at over KRW 300 billion in 2018 but declined to KRW 273.1 billion in 2019. This was due to the patent expiry of Gilead Science’ Viread, the market leader at the time, and the corresponding reduction in Viread’s drug price. The market further shrank to KRW 266 billion in 2020. In 2021, the market rebound. In 2021, it grew by 4% to KRW 275.6 billion. In 2022, it grew to KRW 283.8 billion and in 2023 to KRW 292.3 billion, a 3% increase for 2 consecutive years. It expanded further to exceed KRW 300 billion last year. Vemlidy grows 15% in one year...No. 2 in the market Gilead's Vemlidy led the market growth. Last year, Vemlidy’s prescriptions totaled KRW 71.3 billion, up 15% from the previous year. Vemlidy is Gilead’s new hepatitis B drug that was developed as a successor to Viread. While the original Viread was highly effective in suppressing the hepatitis B virus, it was criticized for its side effects, including kidney dysfunction and decreased bone density. Vemlidy overcame these shortcomings. In clinical trials, no adverse events, including renal dysfunction and decreased bone density, were found in patients using Vemlidy. Long-term safety was also highlighted as an advantage given the difficult-to-cure nature of hepatitis B. In fact, sales of Vemlidy have steadily increased since its launch in Korea in 2017, making up for the decline in Viread’s prescription performance. In 2019, it surpassed KRW 10 billion in prescriptions, and in 2021, it expanded to exceed KRW 30 billion. This was followed by a further increase to KRW 49.2 billion in 2022 and KRW 61.9 billion in 2023. In particular, last year, it reached KRW 71.3 billion, overtaking BMS Baraclude (KRW 71.9 billion), the No. 2 product in the market. Given Baraclude’s recent sales decline, the industry expects Viread will overtake Baraclude and become the No. 2 product within this year. While Gilead's prescription sales were down 1% YoY, the company’s still stays strong at more than KRW 90 billion. By 2019, the company was generating more than KRW 100 billion in annual prescription sales. Since then, it has switched to Vemlidy, and its prescription performance has been declining moderately. In addition, original items from domestic and foreign pharmaceutical companies have recently seen a slowdown in prescription sales. Ildong Pharmaceutical's Besivo (besifovir) generated KRW 2.3 billion in sales last year, the same as in 2023. The sales of Bukwang Pharm’s Sebivo (telbivudine) decreased from KRW 1.2 billion to KRW 1 billion, and the sales of Levovir (clevudine) remained at KRW 800 million each in 2023 and 2024. Levovir’s patent term expires in April 2022. Sales of GSK’s Zeffix (lamivudine) fell slightly from KRW 3.3 billion to KRW 3.2 billion in 1 year. Sales of GSK’s other product, ‘Hepsera (adefovir)’ have not been counted since the company withdrew the drug’s domestic license in 2022. Vemlidy generics enter market in full scale…sales of tenofovir-based generics jump 21% in the US Generic tenofovir drugs have also seen a significant increase in sales. Last year, the combined prescription value of tenofovir generics was KRW 20.7 billion, up 21% from KRW 17.1 billion in 2023. In Korea, generic versions of tenofovir-based hepatitis B treatments have been launched in succession since 2018 for Viread and 2023 for Vemlidy. In the case of tenofovir-based generics, the growth was somewhat slower, with the existing Viread generics generating KRW 15.9 billion in 2020, KRW 16.5 billion in 2021, KRW 17.7 billion in 2022, and KRW 17.1 billion in 2023. However, the entry of Vemlidy generics has grown the market significantly to reach KRW 20.7 billion. The prescription performance of existing Viread generics has mostly declined. Chong Kun Dang’s Tenofobell fell from KRW 3.7 billion in 2023 to KRW 3.3 billion last year. Dong-A ST’s Virreal fell from KRW 2.8 billion to KRW 2.6 billion. On the other hand, Vemlidy generics saw a significant increase in prescriptions. Samil Pharmaceutical's Vemlino generated only KRW 300 million in 2023 but increased eightfold to KRW 2.4 billion last year. Sales of Dong-A ST’s Vemlia also surged from KRW 300 million to KRW 1.7 billion in one year. Generic versions of another hepatitis B drug, Baraclude, generated KRW 33.6 billion last year. The combined prescription value of Baraclude generics has been growing moderately since exceeding KRW 30 billion in 2020. Among Baraclude generics, Dong-A ST’s Baracle was the highest prescribed at KRW 10.4 billion. It was followed by Samil Pharmaceutical's Enped at KRW 3.8 billion, Daewoong Pharmaceutical's Baracross at KRW 3.4 billion, Bukwang Pham’s Bukwang Entecavir at KRW 3.2 billion, and Hanmi Pharmaceutical's Cavir at KRW 3 billion.
- Company
- 'Tibsovo' reapplies for bile duct cancer indication reimb
- by Eo, Yun-Ho Feb 04, 2025 05:55am
- Product photo of Tipsovo 'Tibsovo,' which is indicated for the treatment of bile duct cancer (cholangiocarcinoma) and acute myeloid leukemia (AML), reapplies for obtaining insurance reimbursement. According to industry sources, Servier Korea has submitted a reimbursement application for cholangiocarcinoma indication of Tibsovo (ivosidenib), a drug targeting the isocitrate dehydrogenase 1 (IDH-1) gene mutation. If a patient is tested positive for IDH1 mutation, Tibsovo can be used as a ▲Monotherapy in patients with locally advanced or metastatic AML and had prior therapy ▲Combination therapy with 'azacytidine' in adult patients over 75 years with accompanying disease that cannot be treated with chemotherapy. Tibsovo's AML indication passed the Health Insurance Review and Assessment Service (HIRA)'s Cancer Disease Review Committee (CDRC) in October of last year. Yet, the cholangiocarcinoma indication has not passed the CDRC review. It remains to be seen if Tibsovo, effective in treating cholangiocarcinoma, where treatment options are limited, will be considered for reimbursement. Cholangiocarcinoma is a cancer with a poor prognosis. The five-year relative survival rate is only 28.9%. 65% of the patients with cholangiocarcinoma of the liver are found be non-operable when diagnosed. Tibsovo is the only targeted drug recommended as a Category 1, the highest grade, by the National Comprehensive Cancer Network (NCCN) for a second-line treatment for cholangiocarcinoma. According to ClarlDHy Phase 3 clinical trial, Tibsovo reduced the disease progression by 63% compared to a placebo and had a median progression-free survival (PFS) of 2.7 months (placebo 1.4 months). Also, patients treated with Tibsovo had a median overall survival (OS) of 10.3 months, which was longer over twice than 5.1 months of those treated with a placebo. Do-Youn Oh, Professor of Department of Hematology-Oncology at Seoul National University Hospital, said, "Over the last five years, the development of treatments for cholangiocarcinoma got fast. Along with new drug development, many companies are focusing on developing drugs for cholangiocarcinoma. Patients with cholangiocarcinoma need to follow physician's advice, receive treatments, and seize new opportunities such as participating in clinical trials." Meanwhile, in the AGILE Phase 3 trial involving patients with AML, Tibsovo was demonstrated to improve event-free survival (EFS) when combined with azacytidine, and the overall survival (OS) was significantly improved. The patients treated with Tibsovo had a median OS of 24.0 months (placebo 7.9 months). In a long-term follow-up study, the median OS of Tibsovo combination therapy was 29.3 months, over 3.7-fold longer than that of placebo combination therapy.
- Company
- Lixiana leads the DOAC market..generics make advances
- by Kim, Jin-Gu Feb 04, 2025 05:55am
- (Clockwise from top left) Lixiana, Eliquis, Pradaxa, and Xarelto Daiichi Sankyo's Lixiana (edoxaban) is further solidifying its dominance in the direct-acting oral anticoagulant (DOAC) market. Prescriptions grew 12% year-on-year, and its share of the total market expanded to 45%. Sales of Eliquis (apixaban) and Xarelto (rivaroxaban), on the other hand, have faltered. Eliquis saw its first year-over-year prescription decline due to generic re-entry. Xarelto generics have expanded prescriptions by 47% in a year, fiercely chasing the original. Lixiana’s sales near KRW 120 billion a year...doubled in 5 years, accelerating its dominance in the market According to the market research institution UBIST, the outpatient prescription market for DOAC was KRW 260.1 billion last year. This is a 7% increase from the KRW 242.8 billion in 2023. DOACs are anticoagulants that prevent blood clots by directly acting on blood clotting factors. It has replaced warfarin, which inhibits the metabolism of vitamin K, and expanded its use in the prescription field. Xarelto was approved in Korea in 2009, followed by Pradaxa-Eliquis in 2011 and Lixiana in 2015. When the products were first introduced, they were commonly referred to as NOACs (New Oral Anti-Coagulants), but as more than a decade has passed after their first approval, they are now referred to as DOACs (Direct Oral Anti-Coagulants), which means they act directly on clotting factors. Major DOAC prescriptions by year (Unit: KRW 100 million, Source: UBIST) Lixiana's dominance has been growing in the market. Last year, prescriptions for Rixiana totaled KRW 117.5 billion, up 12% from KRW 105.3 billion in 2023. Although Lixiana was one of the last DOACs to be launched in the market, it quickly ramped up prescriptions and has risen to become the market leader since 2019. With annual growth of around 10%, prescription sales have nearly doubled in 5 years from KRW 60.4 billion in 2019. Its share of the total DOAC market has also expanded from 33% in 2019 to 45% last year Eliquis’s sales decline for the first time...generics re-entry impact The remaining original drugs, on the other hand, have seen a recent decline. Eliquis, the No. 2 product in the market, posted prescription sales of KRW 74.3 billion last year. This is down 4% from KRW 77.3 billion in 2023. Last year was the first time Eliquis' prescription sales declined year-on-year. This decline in prescription sales is attributed to the reentry of generics and drug price cuts that followed. Last September, Eliquis' substance patent expired. This was followed by the re-entry of Eliquis generics into the market after a three-and-a-half-year break. Eliquis generics have been launched into the market one after another since June 2019. At that time, generic companies launched the product based on the verdict of the first and second material patent trials. Since then, Eliquis generics have steadily increased prescriptions in the market. However, in April 2021, the tide turned when the Supreme Court overturned the first and second trial rulings and ruled in favor of BMS. The generic companies immediately withdrew their products from the market, and Eliquis regained its monopoly position in the apixaban-based DOAC market. Quarterly prescription performance of Eliquis originals and generics (Unit: KRW 100 million, Source: UBIST) The generic drugs returned last year upon the expiry of Lixiana’s substance patent. At the same time, the price of the original product was reduced. When the generic was launched in 2019, the price cut was suspended due to BMS's administrative litigation, but last year, the 30% price cut was applied. Inluence of Xarelto generics expand...market share of rivaroxaban reach 46% Xarelto generated KRW 31.5 billion in sales last year. That's up 2% year-over-year, but the long-term decline is steady. Xarelto's prescription sales steadily declined from KRW 60.9 billion in 2021 to KRW 49.4 billion in 2022, then to KRW 31 billion won in 2023. The decline in Xarelto prescriptions was driven by patent expiry and subsequent generic launches. Xarelto generics have been available since the second quarter of 2021. Then Xarelto's price cut, which had been blocked by administrative litigations, was enforced in the third quarter of 2022. Xarelto prescriptions plummeted at the time of the price cut. Meanwhile, Xarelto generics are rapidly gaining influence in the market. Sales of Xarelto generics surpassed KRW 10 billion in prescriptions in 2022, their second year on the market. By 2023, the market had grown to KRW 18.3 billion, and last year, it rose 47% to KRW 26.9 billion. Generics' share of the rivaroxaban-based DOAC market expanded from 37% in 2023 to 46% last year. The pharmaceutical industry expects sales of Xarelto generics to surpass the original in combined prescriptions this year. Quarterly prescription performance of Xarelto original and generics (Unit: KRW 100 million, Source: UBIST) Prescription sales of major generic products increased simultaneously. Hanmi Pharmaceutical's Riroxban more than doubled from KRW 3.9 billion in 2023 to KRW 8.1 billion last year. Sales of Samjin Pharm’s Rivoxaban increased 64% from KRW 3.2 billion to KRW 5.2 billion. Sales of Chong Kun Dang’s Riroxia grew 10% year-on-year to KRW 5 billion. Sales of Pradaxa (dabigatran) have been in a prolonged slump. Last year, prescription sales fell to less than KRW 10 billion. Will Eliquis generics return to the market and replicate past gains The future of this market will be determined by how quickly generic Eliquis products, which returned to the market in September last year, will expand their influence. Some industry observers believe that the major generics companies will quickly expand their presence in the market, as they already have experience launching the products. Eliquis generics generated prescription sales of KRW 1.2 billion in 2019 and KRW 9.3 billion in 2020. In the first quarter of 2021, just before the Supreme Court ruling, the company's quarterly prescriptions expanded to KRW 3.6 billion. Since most companies have Xarelto generics in stock, the expectation is that the two products will bring a synergistic effect. On the contrary, some believe that it is unlikely that these generics will be able to achieve similar levels of prescription growth as Xarelto generics. As Xarelto generics are already well established in the DOAC market, it is unlikely that they will be able to generate the same level of prescriptions as expected. In addition, some believe that even if generic prescriptions of Eliquis do increase, this will then lead to a decline in Xarelto generic sales.
- Company
- ROS1 targeted cancer drug 'Augtyro' nearing mkt entry
- by Eo, Yun-Ho Feb 03, 2025 05:52am
- Product photo of Augtyro (repotrectinib)The ROS1-targeting anticancer drug, 'Augtyro,' is expected to be commercialized in South Korea. According to industry sources, Bristol Myers Squibb (BMS) Korea is close to receiving approval from the Ministry of Food and Drug Safety (MFDS) for Augtyro (repotrectinib), an anticancer drug that can be used regardless of cancer types. This drug was designated as an orphan drug from the MFDS in early June 2024. This drug was indicated for ▲the treatment of patients with ROS-1 positive topical advanced or metastatic non-small cell lung cancer (NSCLC) ▲the treatment of patients with NTRK(Neurotrophic tyrosine receptor kinase) fusions in topical advanced, metastatic solid cancer or who have a high likelihood of severe morbidity upon surgical removals. Augtyro initially received U.S. FDA approval for the treatment of NSCLC in November 2024. Its indication for the treatment of solid cancer accompanying NTRK fusions was recently approved. In November of the same year, it also successfully received a recommendation for marketing authorization from the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP). The drug’s efficacy was confirmed through multinational Phase 1/2 TRIDENT-1 studies. The results showed that 71 patients who had not previously received TK1 treatments had an objective response rate (ORR), which was the primary endpoint, of 79% after Augtyro treatment. Progression-free survival (PFS) doubled compared to conventional targeted therapy. The ORR was defined by the percentage of patients showing decreased tumor sizes (partial response) or no more cancer symptoms (complete response) during the specified treatment period. The median duration of response (DOR) was 34.1 months. 56 patients who had previously undergone ROS1 TK1 therapy and no chemotherapy had an ORR of 38% and a median DOR of 14.8 months. The study also demonstrated the drug’s effectiveness in patients who had developed drug tolerance to previously administered targeted therapies. 56 patients with drug tolerance had an ORR of 38% and a PFS of 9 months. Notably, 17 patients who acquired G2032R mutation had an ORR of 59% and a PFS of 9.2 months. The TRIDENT-1 study was published in the New England Journal of Medicine (NEJM, IF 176.082) with Byoung Chul Cho (Director of the Lung Cancer Center at Yonsei Cancer Hospital) as the corresponding author. Meanwhile, lung cancers with ROS1 mutation account for 2% of all lung cancers. Conventional therapy includes targeted anticancer therapies that target the mutated gene. The common drugs are 'crizotinib' and 'entrectinib.' 'Repotrectinib' is gaining attention as the next-generation drug.