- LOGIN
- MemberShip
- 2025-12-22 04:30:56
- Hepatitis B drug market remakes ₩300B after 6 years
- by Kim, Jin-Gu | translator Alice Kang | 2025-02-04 05:55:37
The outpatient prescription market for hepatitis B drugs expanded again last year to exceed KRW 300 billion.
This is the first time the market has exceeded the mark in 6 years since 2018.
Gilead Sciences’ Vemlidy drove the market growth with a 15% increase in sales from KRW 61.9 billion to KRW 71.3 billion in 1 year, while the combined prescription volume of tenofovir-based generic products increased by 21%.
Hepatitis B drug market re-enters KRW 300 billion range for the first time in 6 years According to the market research institution UBIST on Wednesday, the outpatient prescription market for hepatitis B drugs was KRW 303 billion last year.
This is a 4% increase from the KRW 292.3 billion in 2023.
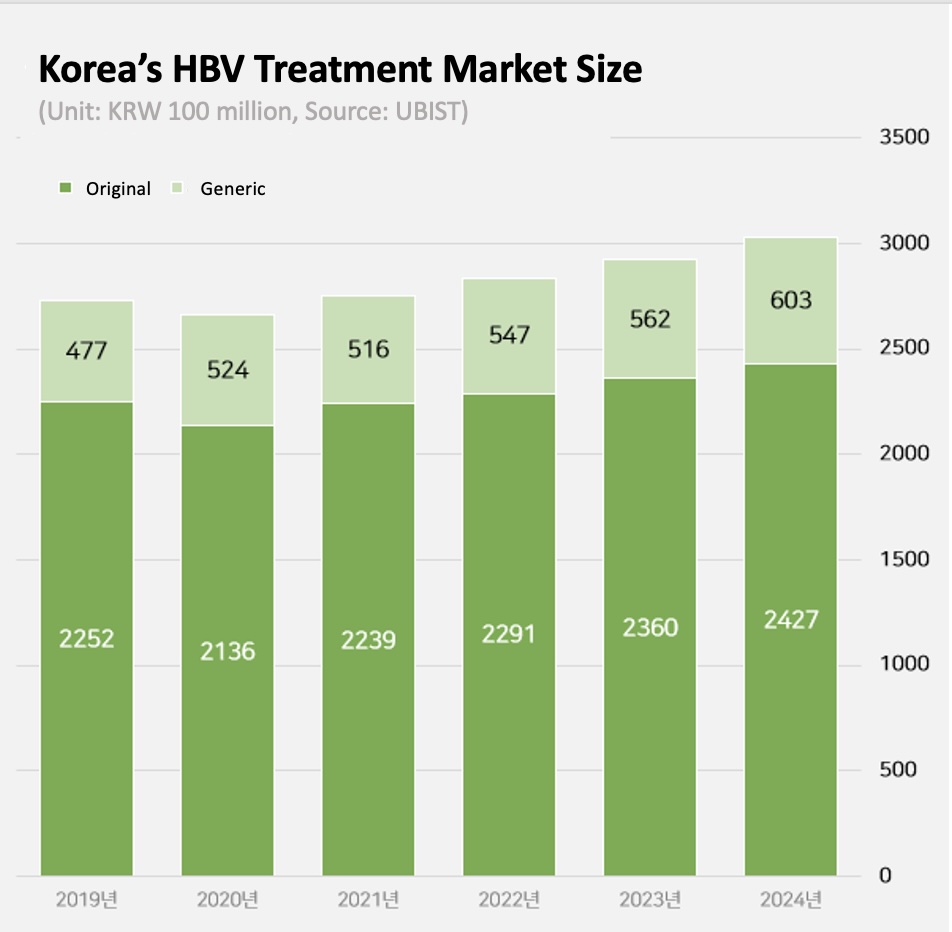
The market was valued at over KRW 300 billion in 2018 but declined to KRW 273.1 billion in 2019.
This was due to the patent expiry of Gilead Science’ Viread, the market leader at the time, and the corresponding reduction in Viread’s drug price.
The market further shrank to KRW 266 billion in 2020.
In 2021, the market rebound.
In 2021, it grew by 4% to KRW 275.6 billion.
In 2022, it grew to KRW 283.8 billion and in 2023 to KRW 292.3 billion, a 3% increase for 2 consecutive years.
It expanded further to exceed KRW 300 billion last year.
Vemlidy grows 15% in one year...No.
2 in the market Gilead's Vemlidy led the market growth.
Last year, Vemlidy’s prescriptions totaled KRW 71.3 billion, up 15% from the previous year.
Vemlidy is Gilead’s new hepatitis B drug that was developed as a successor to Viread.
While the original Viread was highly effective in suppressing the hepatitis B virus, it was criticized for its side effects, including kidney dysfunction and decreased bone density.
Vemlidy overcame these shortcomings.
In clinical trials, no adverse events, including renal dysfunction and decreased bone density, were found in patients using Vemlidy.
Long-term safety was also highlighted as an advantage given the difficult-to-cure nature of hepatitis B.
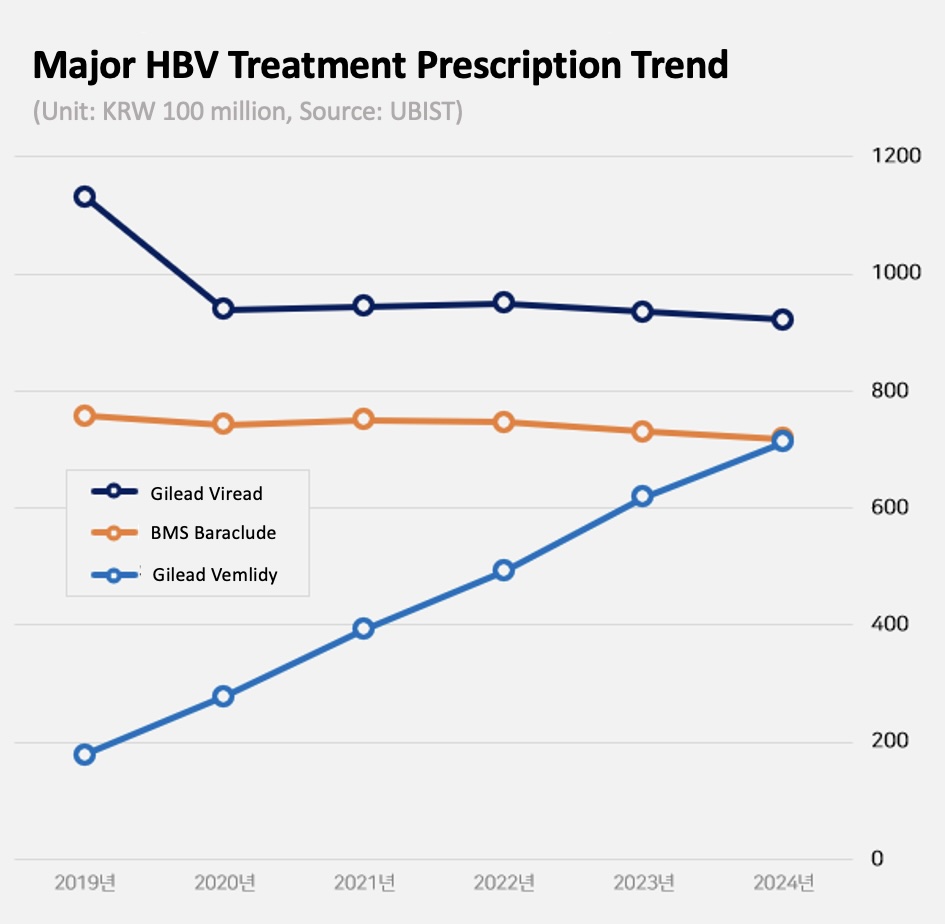
In 2019, it surpassed KRW 10 billion in prescriptions, and in 2021, it expanded to exceed KRW 30 billion.
This was followed by a further increase to KRW 49.2 billion in 2022 and KRW 61.9 billion in 2023.
In particular, last year, it reached KRW 71.3 billion, overtaking BMS Baraclude (KRW 71.9 billion), the No.
2 product in the market.
Given Baraclude’s recent sales decline, the industry expects Viread will overtake Baraclude and become the No.
2 product within this year.
While Gilead's prescription sales were down 1% YoY, the company’s still stays strong at more than KRW 90 billion.
By 2019, the company was generating more than KRW 100 billion in annual prescription sales.
Since then, it has switched to Vemlidy, and its prescription performance has been declining moderately.
In addition, original items from domestic and foreign pharmaceutical companies have recently seen a slowdown in prescription sales.
Ildong Pharmaceutical's Besivo (besifovir) generated KRW 2.3 billion in sales last year, the same as in 2023.
The sales of Bukwang Pharm’s Sebivo (telbivudine) decreased from KRW 1.2 billion to KRW 1 billion, and the sales of Levovir (clevudine) remained at KRW 800 million each in 2023 and 2024.
Levovir’s patent term expires in April 2022.
Sales of GSK’s Zeffix (lamivudine) fell slightly from KRW 3.3 billion to KRW 3.2 billion in 1 year.
Sales of GSK’s other product, ‘Hepsera (adefovir)’ have not been counted since the company withdrew the drug’s domestic license in 2022.
Vemlidy generics enter market in full scale…sales of tenofovir-based generics jump 21% in the US Generic tenofovir drugs have also seen a significant increase in sales.
Last year, the combined prescription value of tenofovir generics was KRW 20.7 billion, up 21% from KRW 17.1 billion in 2023.
In Korea, generic versions of tenofovir-based hepatitis B treatments have been launched in succession since 2018 for Viread and 2023 for Vemlidy.
In the case of tenofovir-based generics, the growth was somewhat slower, with the existing Viread generics generating KRW 15.9 billion in 2020, KRW 16.5 billion in 2021, KRW 17.7 billion in 2022, and KRW 17.1 billion in 2023.
However, the entry of Vemlidy generics has grown the market significantly to reach KRW 20.7 billion.
The prescription performance of existing Viread generics has mostly declined.
Chong Kun Dang’s Tenofobell fell from KRW 3.7 billion in 2023 to KRW 3.3 billion last year.
Dong-A ST’s Virreal fell from KRW 2.8 billion to KRW 2.6 billion.
On the other hand, Vemlidy generics saw a significant increase in prescriptions.
Samil Pharmaceutical's Vemlino generated only KRW 300 million in 2023 but increased eightfold to KRW 2.4 billion last year.
Sales of Dong-A ST’s Vemlia also surged from KRW 300 million to KRW 1.7 billion in one year.
Generic versions of another hepatitis B drug, Baraclude, generated KRW 33.6 billion last year.
The combined prescription value of Baraclude generics has been growing moderately since exceeding KRW 30 billion in 2020.
Among Baraclude generics, Dong-A ST’s Baracle was the highest prescribed at KRW 10.4 billion.
It was followed by Samil Pharmaceutical's Enped at KRW 3.8 billion, Daewoong Pharmaceutical's Baracross at KRW 3.4 billion, Bukwang Pham’s Bukwang Entecavir at KRW 3.2 billion, and Hanmi Pharmaceutical's Cavir at KRW 3 billion.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










