- LOGIN
- MemberShip
- 2025-12-20 10:57:08
- Company
- Daiichi Sankyo exceeds ₩300B in sales…new drug drives
- by Son, Hyung Min Jun 18, 2025 06:01am
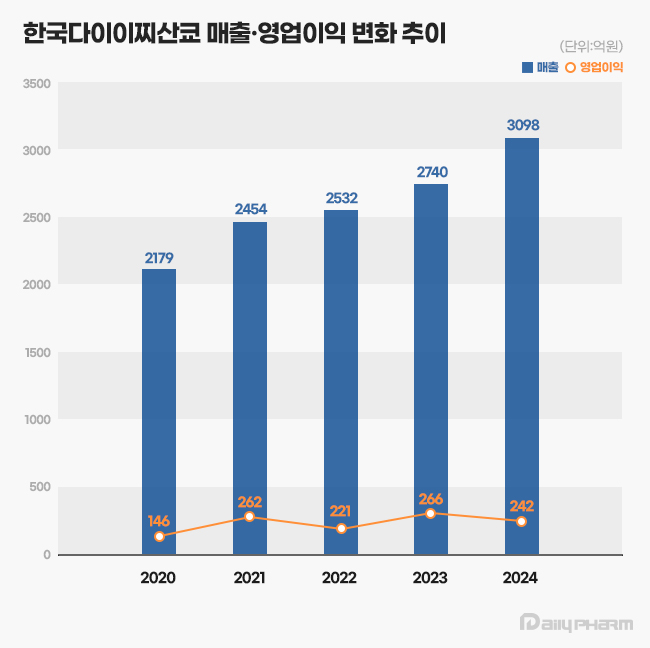
- Daiichi Sankyo Korea has exceeded KRW 300 billion in sales for the first time, led by its cardiovascular products, Antibody-Drug Conjugate (ADC), and new anticancer drugs. The company is successfully transitioning its portfolio towards new ADC drugs while maintaining robust growth from established cardiovascular products like Sevikar, Lixiana, and Olmetec. According to the Korea Financial Supervisory Service (FSS)'s electronic disclosure system on June 16, Daiichi Sankyo Korea's sales last year reached KRW 309.8 billion, a 13% increase from the previous year. During the same period, operating profit decreased by 9%, from KRW 26.6 billion to KRW 24.2 billion. Daiichi Sankyo Korea considers its sales for 2024 based on the Japanese fiscal year, covering April of last year to March of this year. Daiichi Sankyo Korea Daiichi Sankyo Korea's sales have been steadily increasing since 2020. The company first surpassed KRW 200 billion in revenue in 2020 with KRW 217.9 billion, followed by a continuous upward trend, reaching KRW 245.4 billion in 2021, KRW 253.2 billion in 2022, and KRW 274.0 billion in 2023. Notably, an analysis suggests that collaboration with the domestic pharmaceutical company Daewoong Pharmaceutical on some cardiovascular products, such as Lixiana and Sevikar, has created a synergistic effect. Daiichi Sankyo Korea signed co-promotion agreements for Sevikar in 2013 and Lixiana in 2015 with Daewoong Pharmaceutical, and this partnership continues to date. Among them, the highest revenue generator is the Direct Oral Anticoagulant (DOAC), Lixiana. According to market research firm UBIST, Lixiana's prescription sales last year was KRW 117.5 billion, a 12% increase compared to KRW 105.3 billion in 2023. DOACs are anticoagulants that prevent blood clots by directly acting on blood coagulation factors. They are increasingly being used in clinical settings as they replace warfarin, which inhibits Vitamin K metabolism. In Korea, Xarelto was approved in 2009, followed by Pradaxa and Eliquis in 2011, and Lixiana in 2015. Despite being the last to be launched among DOACs, Lixiana has rapidly increased its prescription performance, backed by demonstrated clinical data, and has maintained its market dominance since 2019. With annual growth of around 10%, its prescription performance nearly doubled in five years, from KRW 60.4 billion in 2019. Its market share in the overall DOAC market also expanded from 33% in 2019 to 45% last year. Sevikar, an olmesartan-based combination therapy for hypertension, continues to maintain its strong performance in prescription revenue. Sevikar's prescription revenue last year was KRW 68.8 billion, a 4% increase from the previous year. Despite numerous global and domestic pharmaceutical companies entering this market, Sevikar's prescription sales continue to grow. Sevikar's prescription revenue, which was KRW 53.4 billion in 2019, surpassed KRW 60 billion in 2022. In 2023, it recorded KRW 65.9 billion, demonstrating five consecutive years of increased prescription sales. The triple combination hypertension drug Sevikar HCT also maintained its growth trajectory. Sevikar HCT's prescription sales for the last year totaled KRW 42.1 billion, representing a 4% increase from the previous year. Daiichi Sankyo Korea generated approximately KRW 140 billion in prescription sales solely from olmesartan-based hypertension treatments, including Sevikar HCT (KRW 42.1 billion), Olmetec (KRW 30.6 billion), and Sevikar (KRW 68.8 billion). The 5 ADC Strategy...Will it achieve R&D success after Enhertu? Daiichi Sankyo Korea is working towards transitioning from a cardiovascular-focused company to a leader in oncology. The company is particularly concentrating its R&D capabilities on the ADC field, focusing on new growth engines. Following the already approved Enhertu, it is pursuing a '5 ADC strategy' and preparing for the launch of various other therapeutic agents, including Datroway, patritumab deruxtecan, DS-7300, DS-700, and DS-6000. An ADC is a new anticancer drug designed by linking an antibody that binds to a specific target antigen on the surface of cancer cells with a drug that has cell-killing capabilities using a linker. The advantage of ADCs is their ability to selectively target cancer cells by utilizing the antibody's target specificity and the drug's cytotoxic activity, thereby maximizing therapeutic efficacy while minimizing side effects. ADC anticancer agentWhile first-generation ADCs, such as Roche's Kadcyla, were initially limited to breast cancer indications, second-generation ADCs are successfully securing various other indications. Among these, Enhertu is a second-generation new ADC drug introduced by Daiichi Sankyo Korea. Enhertu is a next-generation ADC that links a monoclonal antibody with the same structure as trastuzumab (which binds to specific target receptors overexpressed on the surfaces of cancer cells) and a highly potent, novel topoisomerase I inhibitor payload via a tumor-selective, cleavable linker. Currently, Enhertu won domestic approval for HER2-positive breast cancer, gastric cancer, and non-small cell lung cancer, and is primarily used as a second-line treatment. Its potential as a first-line treatment for breast cancer is also currently being investigated. Daiichi Sankyo is also preparing to launch its second new ADC drug, Datroway. This ADC targets the Trop-2 protein and has been approved in the U.S. for the treatment of breast cancer. The Trop-2 protein is a cell membrane antigen overexpressed in breast cancer, particularly in over 90% of triple-negative breast cancer cases. Datroway binds to the Trop-2 protein and delivers cytotoxic substances into the cancer cells. It has the advantage of maximizing the benefits of targeted therapy and cytotoxic chemotherapy while minimizing damage to healthy cells. Currently, Daiichi Sankyo is co-developing and co-marketing Enhertu and Datroway with AstraZeneca. Daiichi Sankyo is also developing an ADC with Merck. patritumab deruxtecan, which targets HER3, showed efficacy in EGFR-mutated patients compared to platinum-based chemotherapy in the Phase 2 HERTHENA-Lung01 study. Daiichi Sankyo continues to conduct research for subsequent ADC candidates after Enhertu, which targets the HER2 biomarker. The company is also jointly conducting clinical studies with Merck on DS-7300, which targets B7-H3 (an emerging new biomarker in solid tumors), and DS-6000, a CDH6-targeting ADC.
- Company
- Zejula, a new standard ovarian cancer maintenance therapy
- by Whang, byung-woo Jun 18, 2025 06:00am
- Ovarian cancer is often diagnosed at an advanced stage due to the difficulty of early detection, and it is known for its high recurrence rate even after initial treatment. First-line maintenance therapy aimed at delaying recurrence as much as possible after surgery and chemotherapy became a key strategy that determines treatment outcomes for ovarian cancer. Recently introduced PARP inhibitors have emerged as a standard option for maintenance therapy, and the use of biomarkers to guide patient selection has been a major advancement, enabling better prediction of which patient subgroups are likely to benefit the most. In an interview with Dailypharm, Professor Jae Kwan Lee of the Department of Obstetrics and Gynecology at Korea University Guro Hospital, and Dr. Bradley Monk of the Florida Cancer Specialists & Research Institute stressed the need for institutional support for personalized treatment of ovarian cancer. Long-term efficacy of Zejula as first-line maintenance therapy for ovarian cancer proven Ovarian cancer is difficult to diagnose at an early stage and often recurs after initial treatment, raising the importance of maintenance therapy. This is why first-line maintenance therapy to delay recurrence as much as possible after surgery and chemotherapy has become a key strategy in ovarian cancer treatment. Professor Lee said, "First-line maintenance therapy is becoming a critical turning point in ovarian cancer treatment. HRd (homologous recombination deficiency)-positive patients showed an average progression-free survival period extension of approximately 2 years when receiving first-line maintenance therapy after surgery. Given the high recurrence rate of ovarian cancer, maintaining remission for as long as possible is key to successful outcomes, and first-line maintenance therapy serves as a highly effective strategy in this regard." Jae Kwan Lee, Professor of Obstetrics and Gynecology, Korea University Guro Hospital (President, Korean Society of Gynecologic Oncology)One of the changes in the domestic treatment environment for ovarian cancer came with the expansion of reimbursement criteria for the PARP inhibitor Zejula (niraparib) to HRd-positive ovarian cancer in October last year. The reimbursement extension of the PARP inhibitor Zejula was significant because of its biomarker. Approximately 50% of all ovarian cancer patients are HRd-positive, and about half of them, or 25%, have BRCA gene mutations. In addition, studies continue to demonstrate the efficacy of PARP inhibitors in HRd-positive patients. Professor Lee said, “In the past, reimbursement was limited to patients with BRCA mutations, so HRd-positive patients who were BRCA-negative could not choose to use Zejula due to the financial burden. However, since the reimbursement criteria were extended to include HRd-positive patients, many patients are actively starting Zejula treatment.” Zejula is currently one of the most promising PARP inhibitors for first-line maintenance therapy for ovarian cancer. In particular, the long-term follow-up data from the PRIMA study published last year has enhanced the reliability of Zejula. In the PRIMA trial, Zejula increased progression-free survival (PFS) by more than twofold in HRd-positive ovarian cancer patients compared to placebo. Additionally, at the time of clinical confirmation, the median PFS in the Zejula treatment group was 24.5 months, compared to 11.2 months in the placebo group, showing a significant difference. The 5-year PFS rate was also 35%, approximately twice as high as that of the placebo group. Dr. Monk stated, “Previously, there were concerns that long-term use of PARP inhibitors could lead to drug resistance, but this data confirms that such a possibility is low. These long-term follow-up results will serve as a strong source of reliability for doctors who have been hesitant about prescribing Zejula in the long term." He further explained, “Zejula can be used as a first-line maintenance therapy for all patients who respond to platinum-based chemotherapy (all-comer), but it is known to show the most effective results in HRd-positive patients. Since approximately half of all ovarian cancer cases are classified as HRd-positive, Zejula is increasingly being considered as a key option when setting treatment strategies.” “Diagnostic hurdles remain despite Zejula’s extended reimbursement for HRd-positive ovarian cancer” One of the main reasons for the popularity of Zejula is its ease of administration. While other PARP inhibitors require twice-daily dosing, Zejula can be taken once daily, improving medication adherence. Professor Lee said, “For patients to adhere to long-term maintenance therapy without becoming fatigued, treatment convenience is crucial. Zejula’s once-daily dosing regimen has had a positive impact on patients' ability to remain on therapy over the long term without discontinuation." Bradley Monk, MD, Medical Director of Late-Phase Clinical Research Program, Florida Cancer Specialists & Research Institute Dr. Monk added, "Zejula has the advantage of having relatively low drug-drug interactions, which makes it a safer option when used in combination with other drugs. This is a significant advantage for elderly patients with comorbidities or those receiving complex medication regimens.” Meanwhile, with the expansion of reimbursement criteria for HRd-positive ovarian cancer, it has become essential to determine whether a patient is HRd-positive before establishing a treatment strategy, but access to such HRd diagnostic tests remains a barrier. Currently, BRCA1/2 mutation testing for ovarian cancer patients is relatively affordable through national support programs and partial health insurance coverage. However, genomic panel testing required to confirm HRd status is not covered by insurance, leaving patients to bear the full cost of approximately KRW 2.5 million. Professor Lee pointed out, “HRd testing is essential for HRd-positive patients to receive Zejula treatment, but the fact that the test is not covered by insurance and must be paid out of pocket is a major institutional contradiction. Policy improvements should be made so that HRd diagnostic tests can settle as a diagnostic tool accessible under the same criteria as BRCA tests.” In contrast, access to HRd tests has been rapidly improving overseas. Dr. Monk stated, “Currently, more than 10 companies in the United States offer HRd tests, and some provide the service at very low costs. HRd diagnostic tests can serve as an important basis for predicting treatment response to PARP inhibitors such as Zejula.” For this reason, the Korean Society of Gynecologic Oncology is also known to be actively collecting supporting data to officially propose reimbursement for HRd tests to Korean health authorities. If HRd tests are promptly covered by health insurance, patients will be able to receive the necessary testing without financial burden and fully enjoy the benefits of targeted maintenance therapy such as Zejula. In addition, Professor Lee, who has been appointed as the President of the Korean Society of Gynecologic Oncology, emphasized his commitment to advancing precision medicine based on the genetic profiling of ovarian cancer. Professor Lee stated, “The society plans to focus on how to diagnose and manage the genetic characteristics of ovarian cancer. In other countries, there are already detailed clinical guidelines in place for individuals with BRCA mutations, and I believe similar protocols are needed in Korea as well." He concluded, "Since ovarian cancer often occurs alongside other cancers such as breast or endometrial cancer, collaboration with other specialties, including surgical departments, is essential. Establishing a multidisciplinary, patient-centered integrated care system through close coordination with various medical fields is another key priority for the society."
- Company
- Adstiladrin receives orphan drug designation in Korea
- by Eo, Yun-Ho Jun 18, 2025 05:59am
- The new bladder cancer drug Adstiladrin has been designated as an orphan drug in Korea. The Ministry of Food and Drug Safety recently announced the news in a orphan drug designation announcement. The specific indication for designation is “treatment of BCG-refractory high-risk non-muscle-invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papilloma.” Adstiladrin (nadofaragene firadenovec-vncg) received FDA approval in the United States in 2022. This drug uses a non-replicating adenovirus vector to deliver the human interferon alpha-2b gene, inducing an immune response by directly expressing the protein within the bladder epithelium. Adstiladrin demonstrated efficacy through the NCT02773849 clinical trial that involved 157 patients with bladder cancer. In the study, 51% of the 98 patients treated with Adstiladrin achieved complete response (CR). The median duration of response was 9.7 months. In addition, 46% of patients who achieved CR remained recurrence-free at 12 months after treatment. The most commonly reported side effects were instillation site discharge (33%), fatigue (24%), bladder spasms (20%), urinary urgency (19%), and hematuria (17%). The rate of discontinuation due to side effects was 1.9%. Non-muscle-invasive bladder cancer (NMIBC) is an early-stage bladder cancer confined to the bladder mucosa, accounting for approximately 70–80% of all bladder cancers. Among these, high-risk patients include those with carcinoma in situ (CIS) or multifocal high-grade tumors, which have a high risk of recurrence and invasion. Although BCG instillation therapy is used as first-line treatment, approximately 30–50% of patients eventually experience recurrence or become resistant within a few months. While radical cystectomy is considered the standard treatment thereafter, as it is a highly invasive surgery, there has been a continued demand for bladder-preserving therapeutic alternatives.
- Company
- Long-acting HIV treatment shifts HIV treatment paradigm
- by Whang, byung-woo Jun 18, 2025 05:59am
- With insurance reimbursement now available for the long-acting HIV (human immunodeficiency virus) treatment Vocabria+Rekambys injection therapy, expectations are high on how it will meet the unmet demand. Compared to existing treatments that require daily administration, the new treatment is administered only 6 times a year, offering overwhelming convenience. With its accessibility barriers removed with reimbursement approval, the treatment is expected to gain influence quickly in the market. On the 17th of this month, GSK Korea held a meeting to commemorate the domestic launch of the Vocabria+Rekambys injection therapy and highlighted the treatment's effects and significance. The Vocabria+Rekambys combination was approved by the Ministry of Food and Drug Safety in February 2022 as a combination therapy for the treatment of HIV-1 infection in adult patients who are virologically suppressed, have no history of virological failure, and have no known or suspected resistance to cabotegravir or rilpivirine. Jae-Phil Choi, Professor of Infectious Diseases at Seoul Medical Center Jae-Phil Choi, Professor of Infectious Diseases at Seoul Medical Center, who presented at the event, stated, “Thanks to advancements in treatment that allow effective suppression of the virus, HIV has become a chronic condition that can be managed for life, similar to diabetes or hypertension. However, despite advancements in HIV treatment, social discrimination and stigma against people living with HIV remain widespread in South Korea.” According to Professor Choi, negative perceptions toward HIV influence treatment adherence among infected individuals, leading many to hesitate about seeking active early treatment and serving as a barrier to continuing treatment. According to the 2024 HIV Treatment Awareness Survey conducted by Love4One, a group representing people living with HIV, 73% of respondents in Korea reported feeling anxious that taking HIV medication might expose their status to others or attract unwanted attention. While conventional HIV treatments require daily oral dosing (365 days a year), injectable therapy using Cabotegravir (Vocabria) can reduce the dosing frequency to as little as once a month or once every two months—up to just 6 times a year. These advantages are expected to be effective in alleviating the anxiety caused by social stigma, which is one of the major challenges faced by HIV-infected individuals. Professor Choi emphasized, “Domestic HIV-infected individuals feel a significant psychological burden when taking medications, and as a result, they prefer long-acting HIV injections over oral medications that require daily intake. The Vocabria+Rekambys injection therapy could serve as an option that reduces anxiety about disclosure of infection status and alleviates the inconvenience and concerns associated with daily dosing, thereby providing high treatment adherence and satisfaction.” “High demand for bi-monthly dosing, sufficient flexibility in administration” Next, Professor Yeon-Sook Kim of the Department of Infectious Diseases at Chungnam National University Hospital, who participated in the clinical trial of the Vocabria+Rekambys injection therapy, stated that considering the treatment efficacy of the regimen, it may be possible to adjust treatment options according to the lifestyles of people living with HIV. Yeon-Sook Kim, Professor of Infectious Diseases at Chungnam National University HospitalProfessor Kim stated, “Analysis of data from HIV-infected individuals in Asia (n=41), including Korean HIV-infected individuals in the Phase IIIb clinical trial of the Vocabria+Rekambys injection therapy, showed that 83% of participants maintained viral suppression at Week 96 of treatment, with no reported cases of defined virological failure. This suggests that the Vocabria+Rekambys injection therapy could be an effective treatment option for HIV-infected individuals in Korea.” Professor Kim added, “A survey of HIV-infected individuals in Korea showed a high demand for ‘long-acting treatment with less frequent dosing’ for HIV treatment. With the recent reimbursement approval, it is worth considering changing the treatment option to a long-acting HIV injection according to the lifestyle of infected individuals.” However, along with expectations for reimbursement for the Vocabria+Rekambys injection therapy, there is also the concern that patients may feel burdened by having to visit the hospital more frequently. In the case of the Vocabria+Rekambys injection therapy, there is a 7-day period before and after the standard administration cycle, allowing for a total of 14 days of flexibility in administration, but this means that some patients may find it difficult to switch depending on their circumstances. “From the patient's perspective, there are cases where it is difficult to take time off work, and these patients receive prescriptions for oral medication once every 6 months. In such cases, it may be difficult to recommend switching to an injection therapy. However, for patients who typically receive a prescription every 3 months, we can consider switching to a long-acting injection administered every 2 months.” Professor Kim added, “Additionally, in cases where it is difficult to align the administration schedule due to overseas business trips, it is possible to reintroduce oral therapy. Since it is possible to switch back to oral medication after using the injection therapy, we believe there is sufficient flexibility in its administration.”
- Company
- Lotte-Axcelead-Kanaph signs MOU for joint ADC development
- by Kim, Jin-Gu Jun 17, 2025 05:58am
- Lotte Biologics announced on the 16th that it has signed a three-party memorandum of understanding (MOU) with global new drug development company Axcelead and innovative new drug development company Kanaph Therapeutics to establish an ‘ADC Toolbox’ for the development of antibody-drug conjugates (ADC). Under the agreement, the three companies will collaborate on joint research and development of linkers and payload technologies, which are key for the development of antibody-drug conjugates, which are regarded as next-generation anticancer drugs. Axcelead is a global contract research organization (CRO) spun off from Takeda Pharmaceutical in Japan. It will utilize Takeda's library of over 1.2 million compounds and more than 1,000 new drug development data to identify novel payload candidates that have not been applied to existing ADCs. Kanaph Therapeutics will focus on building an innovative platform that overcomes the limitations of existing linkers and payloads in ADC development. The developed linkers, payloads, and other results will be transferred to Lotte Biologics, based on which the company will strengthen the competitiveness of its ADC platform, including SoluFlex Link. Through the collaboration, Lotte Biologics plans to provide an ADC toolbox service that allows customers to select and utilize various technologies according to their needs. This is expected to further strengthen its one-stop platform service, which covers everything from research and development to GMP production for ADC modalities. A Lotte Biologics representative said, “The agreement marks another step forward in establishing a differentiated ADC platform and toolbox. We will continue to strengthen our partnership with both companies to enhance our ADC competitiveness in the global market and provide patients with more innovative treatments.” An Axcelead representative said, “We are very pleased to be able to forge a strategic partnership for the development and advancement of ADC platform technology and services. Based on our proprietary new drug development platform, we will contribute to the development of innovative treatments.” A Kanaph Therapeutics representative added, “Through this collaboration, we will strive to build a diverse toolbox of linkers and payloads that can overcome the limitations of existing ADC drugs and accelerate the development of innovative new drugs.”
- Company
- New RNAi drug 'Rivfloza' gets ODD in KOR
- by Eo, Yun-Ho Jun 16, 2025 06:04am
- The rare genetic disorder treatment 'Rivfloza' has been designated as an orphan drug in South Korea. The Ministry of Food and Drug Safety recently announced this on its notification board. Rivfloza (nedosiran) is indicated to treat children over 9 years of age and above and adults with 'primary hyperoxaluria type 1 (PH1)' and relatively preserved kidney function. Rivfloza was approved by the U.S. Food and Drug Administration (FDA) in October 2023. It is the first RNAi-based rug developed using Novo Nordisk's GalXC RNAi technology platform. The efficacy of Rivfloza was demonstrated through the results obtained from the Phase 2 PHYOXTM 2 study and the interim analysis data from the Phase 3 PHYOXTM 3 extension study. In the PHYOXTM 2 study, the Rivfloza-treated group showed a significantly lower 24-hour urinary oxalate (Uox) excretion rate at 90-180 days compared with the start of the administration. The study measured the changes in the 24-hour Uox excretion rate from the starting date using the area under the curve (AUC) analysis method. The results have shown that the average linear squares (LS) difference in 24-hour Uox excretion rate between the patient group treated with Rivfloza for 90 days and the control group was 4976. Furthermore, the interim results of the PHYOX3 extension study confirmed that reduced 24-h Uox excretion was maintained in 13 patients who received additional treatment of Rivfloza for 6 months. Meanwhile, PH1 is a rare genetic disorder causing oxalate overproduction in the liverm with an estimated prevalence of 1 in 38,600 people in the world. PH1 is the most common clinically out of three types of primary hyperoxaluria (approximately 80%). It is a metabolic disorder primarily affecting the kidney and may cause progression of kidney damage.
- Company
- Anti-PD-1 immunotherapy 'Zynyz' gets ODD in KOR
- by Eo, Yun-Ho Jun 16, 2025 06:02am
- Product photo of Zynyz (retifanlimab) The checkpoint inhibitor immunotherapy 'Zynyz' has been designated as an orphan drug in South Korea. The Ministry of Food and Drug Safety (MFDS) has recently announced this through its notification board. It is indicated to treat relapsed or metastatic locally advanced Merkel cell carcinoma (MCC). Zynyz (retifanlimab) obtained U.S. approval in 2023 through the FDA's expedited approval program. MCC is associated with fast cancer cell growth and high metastatic rate, thus known for poor prognosis. Therefore, a first-line treatment option that can bring about a continuous response in metastatic MCC patients is necessary. The basis of accelerated approval of Zynyz is the Phase 2 PODIUM-201 study involving 65 patients with relapsed or metastatic locally advanced MCC who have not received prior systematic therapy. The study participants were treated with 500 mg Zynyz every 4 weeks for 24 weeks until they experienced progression of disease or non-tolerable toxicity level. The study's primary goal was set as the overall response rate (ORR) assessed by an independent review committee according to the RECIST guidelines (version 1.1). The secondary goals included the duration of response (DOR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS), and safety. The study results showed that the ORR of Zynyz was 52.2% (95% CI 40~65). In patients who showed responses, 18% of those had complete remission, and 34% had partial remission. The range of DOR was 1.1~24.9 months. 76% of the patients had DOR lasting over 6 months, and 62% had DOR lasting over 12 months. Meanwhile, Zynyz was recently approved by the FDA for additional indication to treat anal squamous cell carcinoma. It was the first immunotherapy to be approved. The basis of approval was the Phase 3 POD1UM-303 and the Phase 2 POD1UM-202 studies.
- Company
- Denosumab’s reimbursement denials increase due to stricter
- by Moon, sung-ho Jun 16, 2025 06:02am
- Although the government extended the reimbursed period for denosumab-based osteoporosis treatments last year, concerns about reimbursement denials continue in practice. Major medical societies, such as the Korean Endocrinology Society, have reached the point where they have issued guidelines to prevent insurance reimbursement cuts when prescribing treatments, raising awareness of the issue. According to the medical community on the 11th, the Korea Endocrine Society recently analyzed cases of insurance reimbursement cuts for denosumab prescriptions and issued guidelines to prevent such cuts to frontline healthcare providers. Previously, in May of last year, the Ministry of Health and Welfare expanded the reimbursement criteria for denosumab-based osteoporosis treatments, including Amgen's Prolia. The key change was that patients who had reached the treatment goal for osteoporosis—defined by their T-score—but remained at the borderline threshold, would continue to be eligible for reimbursement under the revised guidelines. Under the revision, a patient who was initially eligible for reimbursement based on a bone mineral density (BMD) T-score of ≤ -2.5 and shows improvement during treatment to a T-score between -2.5 and -2.0, can receive continued reimbursement for an additional year—up to a maximum of two years. The issue, however, lies in the increasingly strict reviews by the Health Insurance Review and Assessment Service (HIRA), which has led to a rise in reimbursement denials. In fact, HIRA has introduced new review guidelines following the Ministry of Health and Welfare’s extension of the reimbursement criteria in 2023. These include specific principles regarding the use of osteoporosis drugs, particularly focusing on the interpretation of lumbar spine BMD results. HIRA clarified the evaluation method for ‘central bone’ in DXA (dual-energy X-ray absorptiometry) scans and established new review criteria for early denosumab administration. The move appears to show the authorities’ intention to minimize confusion in reimbursement claims arising from the expanded coverage by providing more precise review standards to clinical practitioners. The guidelines issued by the Korean Endocrine Society also closely align with these principles set by HIRA. Specifically, the society emphasized in its preventive guidance that: ▲Selecting only two areas with the lowest T-scores is no longer acceptable, ▲ All measurable lumbar vertebrae from L1 to L4 must be included in the assessment. Also, ▲the Exclusion of a specific vertebra requires a clear medical rationale, supported by radiographic evidence, and, ▲ clinicians must fully understand both the diagnostic criteria based on BMD interpretation and the insurance reimbursement requirements for prescribed treatments. A representative from the Korean Endocrine Society stated, “Osteoporosis treatments are now being prescribed at local clinics and small hospitals, leading to a gradual increase in reimbursement cuts. Selecting only two vertebrae with the lowest T-scores for osteoporosis diagnosis is no longer acceptable. Unless there is a clear justification—such as a difference of more than 1.0 in T-scores compared to surrounding vertebrae, structural abnormalities, implants, or degenerative changes confirmed by radiographic imaging—all measurable lumbar vertebrae must be included in the bone density evaluation.” They further explained, “Denosumab should be administered on a 180-day (6-month) schedule, and early administration is only permitted within 2 weeks of the due date. Follow-up BMD testing must be conducted at exact 365-day intervals, and if testing is performed earlier, reimbursement will generally not be allowed. In cases where early testing within 4 weeks is unavoidable, the reason must be clearly documented in the medical record. Otherwise, it may be subject to reimbursement denial.” Meanwhile, following the expiration of Amgen's substance patent for its original denosumab-based therapy Prolia, biosimilar versions of the drug have entered the domestic clinical market. The first denosumab biosimilar, Stoboclo, has received reimbursement approval and is being co-marketed by Celltrion Pharm and Daewoong Pharmaceutical. Samsung Bioepis is also set to enter the Korean denosumab market, preparing to launch its biosimilar Obodence in partnership with Hanmi Pharmaceutical. As a result, intense competition in sales and marketing is expected between Amgen and its Korean partner Chong Kun Dang, Celltrion Pharm (with Daewoong), and Samsung Bioepis (with Hanmi), all vying for market share in the osteoporosis treatment market.
- Company
- Latecomer psoriasis drug Bimzelx enters competition in KOR
- by Whang, byung-woo Jun 13, 2025 06:03am
- The new psoriasis treatment Bimzelx (bimekizumab) has cleared the reimbursement hurdle and is now officially entering Korea’s market competition. With numerous psoriasis treatment options already on the market, the drug is expected to target new patients based on its relatively low drug price. On the 12th, UCB Korea held a press conference to celebrate the launch of Bimzelx in Korea and highlighted the product's competitiveness. ▲ (From left) Jeong-Eun Kim, Department of Dermatology, Hanyang University Hospital; Stevan Shaw, Head of Research at UCB UK Bimzelx is the first plaque psoriasis treatment that dually inhibits interleukin-17A and 17F (IL-17A and 17F). IL-17A and IL-17F are key cytokines that trigger the inflammatory process in psoriasis, and Bimzelx selectively and directly targets and inhibits both simultaneously. In the BE READY trial, the global Phase 3 clinical study that became the basis for the approval, 90.8% of patients in the Bimzelx group achieved PASI 90 at Week 16, and 68.2% of patients achieved PASI 100. In a clinical trial that compared Bimzelx with another biological agent, there was a clear difference in the percentage of patients who achieved complete clearance of skin lesions at Week 16, which is referred to as 'PASI 100'. Specifically, ▲BE VIVID: Bimzelx 59%, ustekinumab (Stelara) 21% ▲BE SURE: Bimzelx 60%. 8%, adalimumab (Humira) 23.9% ▲BE RADIANT: Bimzelx 61.7%, secukinumab (Cosentyx) 48.9%, etc. The introduction of Bimzelx as a new psoriasis treatment option is significant because of its dual inhibiting mechanism of action that blocks IL-17A and IL-17F. Dr. Stevan Shaw, Head of Research at UCB UK and developer of Bimzelx, said, "Bimzelx’s dual inhibition of interleukin-17A and interleukin-17F showed a higher skin lesion improvement rate in psoriasis patients compared to secukinumab, which only inhibits interleukin-17A.” He further explained, “The dosing regimen, which involves administration every 8 weeks for maintenance therapy, also enhances patient convenience, representing a significant advantage over existing interleukin-17A inhibitors.” In addition, Professor Jeong-Eun Kim of Hanyang University Hospital's Department of Dermatology said, “Even in a meta-analysis conducted over a long period of 52 weeks, Bimzelx showed better efficacy than other drugs in terms of the cumulative number of days achieving PASI 100. No new safety issues were reported during long-term treatment that continued for over three years, with no special events reported overall.” In other words, despite the emergence of various psoriasis treatments, there is still unmet demand due to resistance and other factors, for which Bimzelx is considered to be competitive. The reimbursement price for Bimzelx, which has been covered by health insurance since June, is KRW 801,332. In order to compare the specific drug prices with existing treatments, it is necessary to consider the dosage and administration, and Bimzelx does not have a significant advantage in terms of cost competitiveness, which is a strategy often chosen by later entrants. Bimzelx is administered subcutaneously at 320 mg (two 160 mg doses) at 0, 4, 8, 12, and 16 weeks, and then every 8 weeks thereafter. Considering that competing treatments have administration schedules ranging from 4 weeks to 12 weeks, Bimzelx has a moderate dosing schedule. Regarding this, Professor Kim stated that prescriptions will be tailored to individual patient characteristics and the doctors’ discretion. He added, “While consideration should be given to the patient's comorbidities and prior treatment history, there is no guideline on which drug must be used as the first or last option based on efficacy. However, as more treatment options become available, the therapeutic paradigm for psoriasis is expected to shift and become more segmented.” Finally, Professor Kim added, “Personally, I think Bimzelx should be considered first for patients who do not respond well to biological agents.”
- Company
- New ADC drug Padcev seeks reimb again in Korea
- by Eo, Yun-Ho Jun 13, 2025 06:03am
- The ADC bladder cancer drug Padcev is once again attempting reimbursement listing in Korea. According to Dailypharm coverage, Astellas Pharma Korea recently submitted a reimbursement application for its antibody-drug conjugate (ADC) Padcev (enfortumab vedotin). Accordingly, it will be interesting to see whether the company will be able to make progress in the discussion on insurance reimbursement for Padcev as a monotherapy and combination therapy. This is the company’s third attempt at reimbursement listing. Padcev was first approved in Korea in March 2023, and has remained non-reimbursed for over 2 years since. The monotherapy option passed the Health Insurance Review and Assessment Service's Cancer Disease Deliberation Committee review in February this year, but the application was rejected after the government and pharmaceutical companies failed to agree on the cost-effectiveness after the company completed the pharmacoeconomic evaluation. At the end of last year, Astellas Pharma applied for Padcev’s reimbursement as a monotherapy for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma who have previously received PD-1 or PD-L1 inhibitors and platinum-based chemotherapy, and as first-line therapy for advanced metastatic urothelial carcinoma in combination with the PD-1 inhibitor ‘Keytruda (pembrolizumab)’ However, the applications were also rejected by the Cancer Disease Deliberation Committee in February. Astellas Pharma plans to supplement the relevant data and reapply for reimbursement. The drug is recommended as Category 1 in the National Comprehensive Cancer Network (NCCN) guidelines. It is a new treatment option for urothelial cancer patients whose cancer has progressed or recurred even after receiving treatment with immunotherapy drugs and platinum-based chemotherapy. The drug was approved in March in Korea for the treatment of patients with locally advanced or metastatic urothelial cancer who have received prior treatment with PD-1 or PD-L1 inhibitors and platinum-based chemotherapy, then was approved in combination with Keytruda in July. Padcev’s efficacy as a monotherapy was demonstrated through the EV-301 study, an open-label, Phase III trial that was conducted on 608 patients with locally advanced or metastatic urothelial cancer who have previously been treated with platinum-based chemotherapy and PD-1 or PD-L1 inhibitors. Study results showed that Padcev reduced the risk of death by 30% compared to chemotherapy. The median overall survival (OS) of the Padcev group was 12.9 months, demonstrating a significant improvement in survival compared to chemotherapy's 9.0 months. In addition, Padcev significantly improved progression-free survival (PFS) with a 38% reduction in disease progression or death risk, with the median progression-free survival (PFS) for Padcev being 5.6 months and 3.7 months for the control group. In the case of the Keytruda-Padcev combination, its efficacy was demonstrated through the randomized Phase III EV-302 trial that was presented at the European Society for Medical Oncology Annual Meeting (ESMO 2023). The trial evaluated Padcev+Keytruda versus conventional chemotherapy in 886 patients in 25 countries. Trial results showed, that at a median follow-up of 17.2 months, the median overall survival in the Padcev combination therapy group was 31.5 months, approximately twice as long as the 16.1 months in the platinum-based chemotherapy group, reducing the risk of death by 53%.