- LOGIN
- MemberShip
- 2025-12-20 10:54:04
- Company
- Tevimbra adds esophageal, gastric, lung cancer indications
- by Whang, byung-woo Jun 27, 2025 06:02am
- Pic of TevimbraBeiGene Korea (Name to be changed to BeOne Medicine Korea) announced that its immuno-oncology drug Tevimbra (tislelizumab) has been approved by the Ministry of Food and Drug Safety for additional indications for esophageal cancer, gastric cancer, and non-small cell lung cancer. With the additional approval, Tembriva can now be used as a first- or second-line treatment for a total of 5 indications across 3 solid tumor types. The approved cancer types are: esophageal squamous cell carcinoma (ESCC), gastric or gastroesophageal junction adenocarcinoma (G/GEJ), and non-small cell lung cancer (NSCLC). Tevimbra demonstrated efficacy and safety for the indications in the RATIONALE clinical trial series (RATIONALE-303, 304, 305, 306, 307), which served as the basis for this approval. In particular, the drugs’ clinical benefits were observed in the overall patient population for esophageal squamous cell carcinoma and gastric or gastroesophageal junction adenocarcinoma and showed consistent results in pre-specified subgroups based on PD-L1 expression levels. Such benefits of Tevimbra were also reflected in global treatment guidelines and is recommended at a high level by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO). Tevimbra employs a dual mechanism of action that effectively blocks PD-L1 while minimizing binding to Fc-gamma receptors (FcγR), thereby inducing potent antitumor responses through a mechanism distinct from that of existing immuno-oncology agents. Also, the drug demonstrated superior PD-1/PD-L1 blocking efficacy (>99%) compared to other immunotherapy agents of the same class, and according to the company, it has a higher binding affinity and a half-life 30-80 times longer than existing drugs, suggesting a more sustained therapeutic effect. In addition, by minimizing binding to the Fc gamma (Fcγ) receptor of the antibody, the drug enhanced the sustainability and stability of the immune response. Ji-Hye Yang, General Manager of BeOne Medicines Korea, explained, “Based on its differentiated mechanism and long-term clinical data, Tevimbra is gaining attention as a new standard of care that surpasses the limitations of existing immunotherapy drugs by offering treatment performance comparable to global benchmarks, as well as treatment sustainability and financial predictability.” Yang added, “We are particularly pleased to offer new treatment opportunities for underserved patients. in the first-line setting for esophageal squamous cell carcinoma, as Tevimbra is the only approved immunotherapy in Korea that can be used regardless of PD-L1 expression levels.” Meanwhile, BeiGene changed its corporate name to BeOne Medicines under the vision of “Overcoming Cancer Together” and is accelerating the expansion of its next-generation anti-cancer portfolio, including ADC (antibody-drug conjugates) and protein degraders, with Switzerland as its global hub. The domestic subsidiary will also complete its rebranding as ‘BeOne Medicines Korea’ by June 30 and plans to accelerate its growth in the solid tumor and blood cancer treatment markets based on its 2 products and 11 indications.
- Company
- KPTA ‘KOR-CHN-JPN supply cooperation to bring $12B effect'
- by Kim, Jin-Gu Jun 27, 2025 06:02am
- The Korea Pharmaceutical Traders Association (KPTA), China Chamber of Commerce for Import & Export of Medicines & Health Products (CCMPHIE), and Japan Pharmaceutical Traders Association (JPTA) announced on June 25 that they signed a memorandum of understanding (MOU) for the stabilization of the pharmaceutical supply chain at the Korea Pavilion in the CPHI & PMEC CHINA exhibition hall in Shanghai, China. The signing ceremony was attended by Hyung-seon Ryu, Chairman of the KPTA; Zhou Hui, President of CCCMHPIE; Ichiro Fujikawa, President of JPTA; Young-soo Jeong, Director of the KOTRA Shanghai Trade Office; and over 20 representatives and officials from each country. The MOU was signed to improve the global pharmaceutical supply chain, which has become unstable following the COVID-19 pandemic, and to enhance the three countries' capability to respond to health crises through cooperation. The three associations play leading roles in the pharmaceutical trade and distribution sectors. Through the MOU, the parties agreed to collaborate on ▲ the export, import, development, and supply of essential and active pharmaceutical ingredients; ▲ exchange of pharmaceutical research personnel, technology, and information; and ▲ joint hosting of seminars, academic conferences, and workshops to promote pharmaceutical trade. They plan to concretize the outcomes of the MOU through the implementation of practical joint projects. The KPTA stated that if supply chain stabilization is realized through this agreement, it will bring an annual economic effect of approximately USD 12 billion through ▲the reduction of raw material inventory costs ▲reduction of procurement costs through joint purchasing ▲reduction of health crisis response costs ▲trade creation effects from the activation of pharmaceutical trade among the three countries ▲trade diversion effects from replacing overseas pharmaceutical imports with intra-regional trade. Also, based on this agreement, it is anticipated that a three-country contract manufacturing (CDMO) model may be forged, enabling Korean companies to produce pharmaceuticals at competitive prices in China, register them in Japan, and enter the European market. Cases where domestic pharmaceutical companies experienced disruptions in imported raw material supplies also also expected to decrease, as the partnership will allow Korean companies to secure stable supply through emergency supply contracts with Chinese and Japanese companies. Hyung-seon Ryu, Chairman of the KPTA, stated, “Korea has strengths in producing high-quality drugs, Japan in precision manufacturing technology and rare drug raw material technology, and China in large-scale production and supply capabilities. This MOU will greatly contribute to the stability of the entire Northeast Asian supply chain and the enhancement of global competitiveness. We ask Korean companies to actively participate in various areas, including follow-up technical collaboration, research and development, and contract manufacturing.” Zhou Hui, Chairman of CCCMHPIE, said, “This agreement is the result of close collaboration between the 3 countries and will contribute not only to the stable supply of essential and raw pharmaceuticals but also to the creation of new business opportunities. It will serve as a leading model for cooperation in the East Asian pharmaceutical industry.” Ichiro Fujikawa, President of the JPTA, commented, “Korea and China are Japan’s core partners for raw materials and finished pharmaceutical products. This agreement will help alleviate the shortage of pharmaceutical supplies in Northeast Asia and positively impact the growth and development of each country’s pharmaceutical industry.”
- Company
- ‘Wegovy, a game-changer for high-risk obesity patients’
- by Whang, byung-woo Jun 26, 2025 06:08am
- Obesity is a cause of various metabolic syndromes and a major risk factor for cardiovascular disease. In fact, approximately 80% of patients hospitalized for cardiovascular disease are obese, and studies have shown that the risk of cardiovascular events in obese patients is up to twice as high as in those of normal weight. Over the past 20 years, the mortality rate from obesity-related cardiovascular diseases has increased significantly, with approximately two-thirds of obesity-related deaths attributed to cardiovascular diseases. Recently, semaglutide (brand name: Wegovy), a GLP-1 receptor agonist, has opened a new treatment paradigm in obesity treatment, demonstrating efficacy in reducing the risk of major cardiovascular events in high-risk obese patients. Kim Kyung-hee, professor of cardiology at Incheon Sejong Hospital (Director of the Heart Transplant Center), met with Dailypharm and emphasized the importance of obesity treatment for the prevention of cardiovascular disease. Obesity increases the risk of early onset of cardiovascular disease... “The number of young patients is also on the rise” Obesity is a precursor to various metabolic diseases, and inflammatory substances secreted from visceral fat reduce blood vessel elasticity. According to Professor Kim, these changes lead to hypertension, diabetes, and hyperlipidemia, which in turn greatly increase the risk of early onset of coronary artery disease and heart failure even in younger age groups. Kyung-hee Kim, Professor of Cardiology, Incheon Sejong Hospital (Director of the Heart Transplant Center) Professor Kim said, “Obesity can be a cause of all cardiovascular diseases, and there is a recent trend of an increase in patients with high blood pressure or symptoms of heart failure from a young age. Recently, the prognosis of severely obese patients is generally worse than that of the general population, but even in lean individuals, and those with sufficient muscle mass tend to have a better prognosis.” Kim further explained, “In cases of cardiovascular diseases such as angina or heart failure, there is a tendency for weight loss and reduced muscle mass due to decreased appetite and nutrient intake. Therefore, obesity typically occurs first, followed by cardiovascular diseases in most cases.” In other words, obesity often acts as a precursor to cardiovascular disease. In this regard, semaglutide is regarded a game changer in the fields of obesity treatment and cardiovascular disease prevention. Semaglutide was approved by the Ministry of Food and Drug Safety in April 2024 as an anti-obesity treatment for patients with a BMI of 27 kg/m² or higher (with comorbidities) or 30 kg/m² or higher, and in July of the same year, it was additionally approved for reducing the risk of cardiovascular events in overweight and obese adult patients with confirmed cardiovascular disease. Professor Kim emphasized the clinical value of semaglutide not merely as a weight-loss aid but as a preventive therapy for cardiovascular disease. In particular, KIM highlighted findings from the SELECT trial, a pivotal clinical study on semaglutide, where the drug demonstrated an additional 20% reduction in the risk of major adverse cardiovascular events (MACE) when added to standard care in patients already receiving conventional treatments. Professor Kim explained, “In the SELECT study, approximately 90% of participants were already receiving standard treatment, but when they added semaglutide, an additional 20% reduction in MACE risk was observed. This result demonstrates that semaglutide can make a substantial contribution to improving outcomes in high-risk patient populations where existing treatments have limitations.” According to the detailed results of the SELECT trial, over an average follow-up period of approximately 3.3 years, the semaglutide 2.4 mg group showed a statistically significant 20% reduction in the risk of cardiovascular death, nonfatal myocardial infarction, or stroke compared to the placebo group. Professor Kim added, “Semaglutide regulates the appetite center in the brain, delays gastric emptying to induce weight loss, and further improves cardiovascular risk factors through its anti-inflammatory effects. While weight loss may also play a role, we believe that the drug’s anti-inflammatory effect plays a very significant role in cardiovascular health.” In particular, Professor Kim emphasized that semaglutide is not simply a weight loss drug, but a scientifically proven cardiovascular treatment option. He said, “Semaglutide is a must-use drug for patients who are severely obese or have a BMI of 27 kg/m² or higher with cardiovascular disease.” “Limitations remain on its reimbursement... Selective reimbursement support needed for high-risk obese patients” Although semaglutide has emerged as an important drug that should be considered as part of a standard treatment for obese patients at high risk of cardiovascular disease, there are currently practical limitations to its access in Korea. This is because it is not yet covered by insurance in Korea. Professor Kim said, “Drugs such as semaglutide carry a certain risk of misuse, so caution should be exercised when expanding reimbursement to all patient groups. However, I believe it is desirable to allow reimbursement through strict criteria and screening procedures for high-risk groups, such as patients with severe obesity and cardiovascular complications, for whom clear therapeutic effects can be expected.” In fact, limiting reimbursement to groups with a clear clinical need for reimbursement, such as patients with a body mass index (BMI) of 27 kg/m² or higher and obesity-related complications or cardiovascular disease, may be a realistic alternative. Professor Kim also predicted that discussions on whether to continue reimbursement will be necessary when semaglutide significantly improves a patient’s BMI. He said, "If patients with a BMI of 30 kg/m² or higher are administered semaglutide and their BMI falls below 26 kg/m² due to weight loss, it may be possible to consider limiting the reimbursement period to the initial 4-6 months. However, since there is currently insufficient long-term data in Korea and some patients experience weight regain after 6 months of treatment based on clinical experience, further review of long-term management strategies is necessary.” To address current issues surrounding reimbursement coverage and costs, Professor Kim is currently conducting an economic evaluation study. “We are analyzing how much the number of medications taken by patients can be reduced when they lose weight after 6 months or a year through bariatric surgery or semaglutide treatment, and we expect this to be significant in terms of establishing future treatment strategies and fiscal efficiency.” Ultimately, Professor Kim believes that obesity treatment should not end with medication alone but must include comprehensive management to help patients fundamentally improve their lifestyle habits. Professor Kim emphasized, “Patients with a BMI of 30 kg/m² or higher often find exercise difficult, so they should be actively educated to combine medication with walking exercises, maintain a high-quality diet, reduce carbohydrate intake, and abstain from alcohol and smoking.” “Early intervention in obesity treatment is necessary to maximize preventive effects” Professional counseling and lifestyle education support are essential to increase the effectiveness of obesity treatment. However, the reality of how difficult it is to provide sufficient counseling in the current outpatient setting is also pointed out as an issue. Professor Kim said, “It is difficult to check blood pressure, assess the patient's condition, perform a physical examination, and explain lifestyle correction measures within the 5 minutes of consultation time allocated per patient. At least 7-10 minutes are necessary for proper treatment.” In this regard, Professor Kim proposed the establishment of a lifestyle education program and a new fee schedule to hire dedicated personnel to overcome such limitations. He explained, “Under the current system, separate reimbursement rates for education provided by specialized nurses need to be introduced, and institutional and financial support would also be needed to manage these personnel. Overall, I believe that establishing an environment where lifestyle education can be systematically implemented is essential to improving the quality of care for obese patients. In particular, Professor Kim emphasized, “Patients with hypertension are at high risk of developing heart failure over time. Starting medication early and educating young obese patients with hypertension on proper lifestyle habits can prevent serious complications and repeated hospitalizations.” In other words, Kim believes preventive treatment and lifestyle improvement efforts targeting young obese patients are expected to lead to macro-level medical cost savings in the future. Finally, Professor Kim emphasized that “Patients should always be treated with scientifically validated medications first. Drug treatment alone is not sufficient and must be accompanied by lifestyle modifications and proper dietary management.”
- Company
- The 2nd KRAS-targeted cancer drug 'Krazati' expected
- by Eo, Yun-Ho Jun 26, 2025 06:08am
- Product photo of Krazati The second KRAS inhibitor is expected to be commercialized in South Korea. Bristol Myers Squibb (BMS) Korea recently submitted a marketing authorization application to the Ministry of Food and Drug Safety (MFDS) for its anti-cancer drug, Krazati (adagrasib). Krazati was also designated as an orphan drug in January. It is indicated for the treatment of 'locally advanced or metastatic non-small cell lung cancer (NSCLC) with a KRAS G12C mutation, previously treated with at least one prior therapy.' Krazati received accelerated approval from the U.S. FDA in December 2022. It is the second KRAS inhibitor receiving approval, following Amgen's 'Lumakras (sotorasib),' which was approved in 2021. The development of KRAS-targeted anti-cancer drugs has come approximately 40 years after the initial discovery of the oncogene. Amgen and BMS are engaged in fierce competition to dominate this new market. Lumakras and Krazati share many similarities, including their target mutation and indications. Both target the KRAS G12C mutation, and their initial approved indication is NSCLC. Both companies are also conducting clinical trials in combination with compounds with different mechanisms of action developed in-house or through collaborations. Meanwhile, Krazati's initial approval was based on cohorts from the KRYSTAL-1 study who are eligible for Phase 2 trials. Last year, the primary analysis results of the confirmatory Phase 3 study were disclosed. This study compared Krazati with docetaxel in 301 patients with previously treated KRAS G12C-mutated locally advanced or metastatic NSCLC. These patients had previously received platinum-based chemotherapy and anti-PD-1/PD-L1 immunotherapy. They were randomized 1:1 to either the Krazati treatment group or the docetaxel treatment group. The primary endpoint was progression-free survival (PFS) as assessed by blinded independent central review (BICR). After 9.4 months of follow-up, the median PFS for Krazati was 5.49 months, which met the primary endpoint by reducing the risk of disease progression or death by 42% compared to 3.84 months for the docetaxel group.
- Company
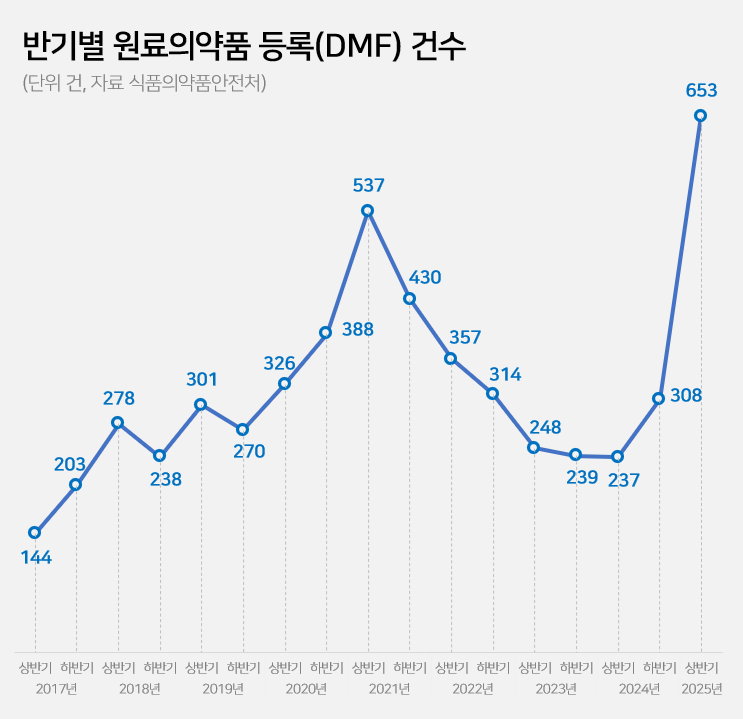
- Drug Master File for API 237→653…'easing of regulation'
- by Kim, Jin-Gu Jun 26, 2025 06:07am
- The number of Drug Master File, DMF, cases in the first half of this year surged by 2.8 times compared to the same period last year. This is the highest for a half-year period. Analysis suggests that this is due to the easing of Active Pharmaceutical Ingredient (API) registration requirements at the beginning of the year. The government had previously eased regulations to allow GMP evaluation to be replaced by GMP certificates for API registration starting this year. 'Record-High' DMF Registrations of 653 Cases in the first half of 2025...Up 2.8x YoY According to the Ministry of Food and Drug Safety (MFDS) on June 26, the number of API registrations by Korean pharmaceutical and biotech companies in the first half of this year reached 653 cases. Compared to 256 cases in the first half of last year, this year's marks a 2.8-fold increase in one year. It has already surpassed the total number of API registrations for the entire 2024 (545 cases). In terms of half-yearly API registrations, it has exceeded the 537 cases in the first half of 2021, reaching an all-time high. Half Yearly Cases of Drug Master Files (DMFs) for API (unit: number of cases, source: MFDS) The robust increase in DMF cases is attributed to the government's easing of regulations. Earlier this year, MFDS reformed the DMF system to replace on-site inspections with GMP certificates. Previously, DMF applications required on-site inspections, along with manufacturing facility data, production country manufacturing certificates, and 11 types of GMP documents. From this year, on-site inspections have been abolished. Additionally, documents can now be replaced by GMP certificates issued by the government agency of the production country or a PIC/S member country. The administrative processing period has also been shortened from 120 days to 20 days. An MFDS official explained, "Previously, to register API, the applying company had to undergo a GMP on-site inspection, but from this year, it can be substituted with a certificate," and added, "It seems that nearly 1,000 piled-up DMF applications were processed in large numbers this year, leading to a surge in DMF cases." Concerns over API quality verification...MFDS states "On-site inspections maintained for high-risk Items" Regarding this deregulation, some in the pharmaceutical industry express concerns that API quality control could become lax. Critics argue that, with registration now possible solely based on GMP certificates, it will be challenging to identify quality issues beforehand through document-based evaluations. This easing of regulation is a complete reversal from MFDS's previous stance. Since the introduction of the DMF system in 2002, MFDS has consistently strengthened quality control. In 2014, GMP evaluation standards were reinforced with PIC/S membership. At this time, 11 types of GMP documents and on-site inspection standards were introduced. In 2019, DMF registration became mandatory not only for new items but also for previously approved items. In 2021, the on-site inspection system was further strength with a focussing on high-risk items. During a briefing last year, MFDS explained that they adjusted the evaluation system in response to the administrative bottleneck caused by a surge in DMF applications, which also delayed the review of finished pharmaceutical products (FPP). Overall, MFDS's policy is to shift its GMP approach to be 'FPP-centric.' Regarding concerns about API quality, MFDS states that on-site inspections are exceptionally maintained for high-risk items, and GMP certificate requirements have been strictly set in line with international standards. Indeed, for high-risk items such as biopharmaceuticals and sterile APIs, on-site inspections and submission of evaluation data are still required. Furthermore, on-site inspections are maintained as before for drug approval and suitability judgments. They also plan to introduce the concept of a 'Site Master File' to understand the quality management system of manufacturing sites comprehensively. Up and down of cases based on regulatory changes...Decline after 2021 peak→ rebounding This Year The number of DMF registrations by Korean pharmaceutical and bio-companies has fluctuated significantly each year due to system changes and policy factors. Over the past eight years, DMF cases have exhibited fluctuating trends: ▲347 in 2017 ▲516 in 2018 ▲571 in 2019 ▲714 in 2020 ▲967 in 2021 ▲671 in 2022 ▲487 in 2023 ▲545 in 2024. With 653 cases in the first half of this year alone, there is a possibility of exceeding 1,000 cases by year-end. The surge in DMF cases in 2021 coincided with the implementation of a policy that made API registration mandatory, even for previously approved items. In 2019, MFDS expanded the scope of DMF to include 'previously approved items' from the original 'newly approved items.' It is analyzed that commercial drugs were required to complete registration by 2021, leading to a concentrated influx of DMF applications. A reform of the drug pricing system around the same time also influenced the increase in DMFs. In July 2019, the government introduced a 'step-wise drug pricing system.' Generics that did not meet the highest price criteria could maintain their previous drug prices if they submitted data from bioequivalence tests and demonstrated the use of registered APIs. This led to a surge in DMF applications from pharmaceutical and biotech companies seeking to maintain drug prices. After 2023, the situation changed. In February 2023, the submission of DMF documents for drug price maintenance concluded. With most DMFs for previously approved items also finalized, the number of registrations began to decline. Indeed, DMFs, which had reached 967 cases in 2021, nearly halved to 487 cases by 2023. However, with the lowering of DMF hurdles this year, the number of registrations is showing a rebounding trend.
- Company
- Vemlidy indication extended to children aged 6 and older
- by Whang, byung-woo Jun 25, 2025 06:01am
- Pic of Vemlidy Gilead Sciences Korea announced on the 24th that its hepatitis B treatment Vemlidy (tenofovir alafenamide, TAF) has been approved by the Ministry of Food and Drug Safety for the treatment of chronic hepatitis B in children aged 6 years and older. Vemlidy offers improved renal and bone safety compared to existing chronic hepatitis B treatments. The long-term efficacy and safety profile for hepatitis B, which requires prolonged treatment like chronic diseases, has been demonstrated through various clinical studies, including 8-year clinical data. With this extended indication, Vemlidy has become the only treatment option available in Korea for patients as young as 6 years of age, the lowest age among tenofovir formulations currently available in the country. Previously, treatment options for chronic hepatitis B were limited to ▲entecavir formulations (for patients aged 2 years and older) ▲TDF drugs such as Viread (for patients aged 12 years and older) ▲TAF drugs such as Vemlidy (for adults). However, with the approval, pediatric patients aged 6 years and older weighing at least 25 kg may now access a treatment that has high viral suppression rates confirmed by long-term data and improved outcomes in terms of kidney function and bone density. This indication expansion applies only to Vemlidy and will not apply to its generic version for 4 years from the date of approval. The dosage is the same as for adults: one tablet once daily, regardless of food intake. This indication expansion approval was based on the results of a global clinical trial in pediatric and adolescent patients with chronic hepatitis B (CHB). The study was a randomized, double-blind, placebo-controlled clinical trial involving 88 patients aged 6 to 18 years with chronic hepatitis B. The trial was converted to an open-label design after 24 weeks and continued for 96 weeks. Results showed that the viral suppression rate (HBV DNA
- Company
- K-Bio successfully signs multiple technology transfer deals
- by Son, Hyung Min Jun 25, 2025 06:01am
- In the first half of this year, the Korean pharmaceutical and biotech industry has successfully achieved technology transfers in various areas, including changed formulations for immunotherapy, new obesity drug candidates, and new drug discovery platforms. Companies, such as LigaChem Biosciences and Onconic Therapeutics, have successfully received additional milestones from clinical achievements of previously exported new drug candidates. Technology transport deals continue to yield accomplishments this year According to the Financial Supervisory Service (FSS)'s electronic disclosure system on June 24, Abion posted that it has recently signed a technology transfer agreement with an overseas pharmaceutical company for its 'ABN501,' a new protein antibody drug candidate. The contracting party remains undisclosed. Through this agreement, Abion will conduct non-clinical research on five protein targets, including Claudin 3 (CLDN3), targeted by ABN501, while the contracting party will handle other research, development, and commercialization. The license is valid worldwide. The upfront payment is set at $25 million, with $5 million per targeted antibody. The total contract value amounts to $1.315 billion (approximately KRW 1.8 trillion), including $290 million in development-stage milestones and $1 billion in commercialization-stage milestones. Claudins are a family of protein that regulates the exchange of cellular molecules and maintains cell junctions. While these proteins are typically limited in healthy tissues, they are overexpressed in certain types of solid tumors. Currently, Astellas' Vyloy, which targets Claudin18.2, has been commercialized, drawing significant attention from the domestic and international pharmaceutical and biotech industries to this biomarker. Among them, CLDN3 is known to be overexpressed in small-cell lung cancer, breast cancer, ovarian cancer, and prostate cancer. Abion is currently conducting preclinical studies for ABN501. Clinical results disclosed to date have confirmed the safety of ABN501 through toxicity evaluations, and improved therapeutic effects have also been secured when ABN501 is combined with chemotherapy in preclinical models. Autotelic Bio signed a technology transfer agreement this month with the Brazilian pharmaceutical company Ache for its hybrid fixed dose combination new drug, 'ATB-101'. Autotelic Bio's entry into the Brazilian market follows its signing of an exclusive supply agreement with Chinoin of Mexico in June 2024. ATB-101 is being developed as a combination of olmesartan, used for treating hypertension, and dapagliflozin, used for treating diabetes. Currently, Phase 3 clinical trials are underway in approximately 33 institutions, including Seoul National University Bundang Hospital, for patients with essential hypertension and type 2 diabetes. The composition of ATB-101 has been patented not only in Korea but also in the United States, Japan, Mexico, Brazil, and Russia, allowing the company to exercise exclusive rights upon market entry in these countries. AriBio has successfully achieved the technology transfer of its Alzheimer's disease new drug candidate, AR1001 , for the second consecutive year. This month, the company signed a technology export agreement with Arcera, a global pharmaceutical company established by Abu Dhabi Developmental Holding (ADQ), a sovereign wealth fund of the United Arab Emirates (UAE). The total contract value is $600 million (approximately KRW 820 billion), with the upfront payment remaining undisclosed. AriBio also achieved the technology transfer of 'AR1001' in March of last year. At that time, AriBio received an upfront payment of KRW 120 billion. AR1001 is a multi-target drug directed to the causes of Alzheimer's disease, including PDE5 and toxic proteins. This new drug candidate is an oral treatment that improves Mirodenafil (Mvix), an erectile dysfunction drug similar to Viagra. The basis of AR1001's mechanism is being strengthened, with recent announcements indicating the efficacy of phosphodiesterase-5 (PDE5) inhibitors, such as Viagra and Cialis, in preventing Alzheimer's disease. AR1001's Phase 3 clinical trial is progressing smoothly. AriBio is conducting global Phase 3 clinical studies in the United States, where the first patient was dosed in December 2022, as well as in Korea, the UK, Germany, France, Spain, Italy, Denmark, the Netherlands, the Czech Republic, and China. In April, ABL Bio signed a technology export agreement with GlaxoSmithKline (GSK) for its blood-brain barrier (BBB) shuttle platform, 'Grabody-B,' for the development of new neurodegenerative disease treatments. Through this agreement, ABL Bio will receive a non-refundable upfront payment of 38.5 million pounds (approximately KRW 73.9 billion) and a short-term milestone of 38.6 million pounds. The total contract value amounts to 2.1401 billion pounds(approximately KRW 4 trillion). Under the terms of the agreement, GSK acquires exclusive rights related to the development and commercialization of multiple new target candidates applying ABL Bio's Grabody-B. ABL Bio will transfer its Grabody-B related technology and know-how to GSK, while GSK will be responsible for preclinical and clinical development, manufacturing, and commercialization. The agreement aims to develop multi-target-based therapies using various modalities, including oligonucleotides (such as siRNA and ASO) and polynucleotides, as well as antibodies. ABL Bio is also planning additional technology transfers. The company holds the largest number of bispecific antibody candidates among domestic companies. It currently possesses over seven pipelines, including ABL001 (VEGFxDLL4), ABL111 (Claudin18.2x4-1BB), and ABL503 (PD-L1x4-1BB). Olix·Alteogen have successfully achieved technology transfers Olix and Alteogen have successfully achieved technology transfers for their new drug candidates targeting metabolic dysfunction-associated steatohepatitis (MASH)·obesity, as well as formulation change technology for anti-cancer drugs, respectively. Eli Lilly has challenged the development of new MASH drugs by in-licensing a new drug candidate from the Korean company Olix Pharmaceuticals. Olix announced in February that it had signed a co-development and technology export agreement with Lilly for its MASH and obesity treatment candidate 'OLX75016 (OLX702A)'. The total contract value is $630 million (approximately KRW 911.7 billion). This amount comprises a non-refundable upfront payment, as well as development and commercialization milestones contingent upon clinical progress. Specific details, such as the proportion of the upfront payment, were not disclosed. OLX75016 is a MASH and obesity treatment candidate based on siRNA technology, a type of short double-stranded RNA genetic material within RNA interference technology. OLX75016 is being developed as a subcutaneous (SC) formulation for the treatment of obesity, administered once every three months. Olix is currently conducting a Phase 1 clinical trial of OLX75016 in Australia. The company is also investigating the possibility of combining OLX75016 with new drugs targeting GLP-1 and glucagon. As most MASH treatments target GLP-1, Olix plans to develop a treatment with a differentiated mechanism to enhance synergistic effects with GLP-1 agents. Alteogen signed two exclusive license agreements in March with MedImmune, AstraZeneca's bio R&D subsidiary, for its human hyaluronidase-based subcutaneous (SC) formulation change platform, 'ALT-B4'. The non-refundable upfront payment for the contract signed between Alteogen and MedImmune is KRW 36.4 billion. The development and commercialization milestones amount to KRW 1.0547 trillion. The upfront payment for the contract signed with MedImmune is KRW 29.1 billion, with milestones totaling KRW 843.8 billion. Both agreements include separate sales royalties. Alteogen's proprietary human hyaluronidase-based technology works by increasing the permeability of subcutaneous tissue, allowing for the rapid dispersion of drugs within the tissue and their subsequent absorption into the bloodstream. While drug delivery to subcutaneous tissue has historically been challenging due to the protection of hyaluronan, human hyaluronidase technology enables the degradation of hyaluronan. Existing anti-cancer drugs are primarily administered intravenously (IV), a method criticized for requiring over an hour of administration time. The development of C formulations for anti-cancer drugs is expected to shorten administration time and enhance patient convenience. Currently, the SC formulation of the cancer immunotherapy Keytruda, utilizing Alteogen's technology, has shown non-inferiority compared to the existing IV formulation in its Phase 3 clinical trial. Alteogen is also developing an anti-cancer SC injection formulation with Daiichi Sankyo, a developer of antibody-drug conjugates (ADC). More milestone payment recipients In the first half of the year, many more companies received additional milestone payments from technology transfers. Genomictree recently received a KRW 1 billion milestone payment from 'Shandong Lukang Hao Li You' following the completion of clinical trials for its colorectal cancer diagnostic product 'EarlyTect-C' in China. Genomictree signed a technology transfer agreement in May 2021 with Shandong Lukang Hao Li You, a joint venture established by Orion Holdings and China's Lukang Pharma for the commercialization of its colorectal cancer in vitro diagnostic product in the Chinese market. The total contract value is KRW 6 billion, and Genomictree received an upfront payment (contract payment) of 2 billion KRW in 2021. Shandong Lukang Hao Li You subsequently established production infrastructure for the colorectal cancer in vitro diagnostic product in China. The company commenced clinical trials in 2023 and completed them in January of this year. Based on the recent clinical results, they have applied to the China's 'National Medical Products Administration (NMPA)' for manufacturing approval of the colorectal cancer diagnostic product. LigaChem Biosciences announced this month the receipt of a short-term milestone payment for 'LCB97', an ADC-based new anti-cancer drug out-licensed to the Japanese pharmaceutical company Ono Pharmaceutical. While the exact amount received was not disclosed, the company announced it received more than one-tenth of last year's revenue of KRW 125.9 billion. LigaChem Biosciences has successfully received its third milestone payment from Ono Pharmaceutical, following additional milestone payments in November last year and March this year. LCB97, for which the milestone was received, is an ADC discovered and developed based on LigaChem Biosciences' proprietary ADC platform technology. It targets L1CAM, known to be overexpressed in various solid tumors. The company states that LCB97 has shown excellent anti-cancer efficacy in various tumor mouse models conducted to date. LigaChem Biosciences signed two technology transfer agreements related to ADCs with Ono Pharmaceutical in October last year. These agreements included ▲a contract transferring the exclusive global development and commercialization rights for LCB97 ▲a contract transferring ADC platform technology for multiple targets. The specific contract amount was not disclosed by mutual agreement between the two companies, and the total value of the two contracts exceeds KRW 943.5 billion. Zacubo, a treatment for gastroesophageal reflux disease Onconic Therapeutics completed the technology transfer for the production of Zacubo to its Chinese partner, Livzon Pharmaceutical Group, in March. Through this, Onconic Therapeutics claimed a milestone payment of $1.5 million (KRW 2.2 billion). Onconic Therapeutics signed a technology export agreement for Zacubo with the Chinese pharmaceutical company Livzon Pharmaceutical Group in March 2023. The contract value is up to $127.5 million. Onconic Therapeutics initially received a non-refundable upfront payment of $15 million and is set to receive up to $112.5 million in technology fees based on development, approval, and commercialization milestones. Zacubo is a P-CAB (Potassium-Competitive Acid Blocker)-based treatment for gastroesophageal reflux disease. It was approved as the 37th domestically developed new drug in April last year. Onconic Therapeutics received a milestone payment of $3 million last month following the first patient dosing in Zacubo's Phase 3 clinical trial in China. With the completion of CMC (Chemistry, Manufacturing, and Controls) technology transfer for production, the company is expected to receive further milestone payments.
- Company
- CSL Seqirus partners with Samjin...seeks NIP in Korea
- by Whang, byung-woo Jun 24, 2025 06:02am
- CSL Seqirus Korea and Samjin Pharmaceutical have signed a strategic sales partnership agreement for the domestic distribution of influenza vaccines, sparking attention over whether the two companies can achieve synergistic effects. Samjin Pharmaceutical and CSL Seqirus Korea sign strategic sales partnership agreementOn the 18th, the two companies announced that they had signed a strategic sales partnership agreement for the domestic distribution of the adjuvanted influenza vaccine ‘Fluad Quad Prefilled Syringe’ and the cell-cultured influenza vaccine ‘Flucelvax Quad Prefilled Syringe.” Samjin Pharmaceutical will be responsible for marketing and promoting Fluad Quad and Flucelvax Quad, while domestic distribution will be jointly conducted with CSL Seqirus Korea, which also handles vaccine imports. Through the agreement, Samjin Pharmaceutical and CSL Seqirus aim to achieve synergistic effects in entering the vaccine distribution business and expanding its position in the domestic market. Through the agreement, Samjin Pharmaceutical is focusing on expanding its portfolio from chronic diseases to the vaccine sector. At the signing ceremony, Sang-Jin Kim, President of Samjin Pharmaceutical, said, “Through this collaboration, Samjin Pharmaceutical will be able to expand its business beyond the treatment-oriented business to the prevention-oriented vaccine field.” Currently, Samjin Pharmaceutical owns a lineup of specialty drugs for chronic diseases and pediatric drugs, such as Trestan, and expects the agreement to bring synergy to its existing sales capabilities. A Samjin Pharmaceutical representative said, “For a long time, the company has been striving to provide effective treatments for patients with chronic diseases, who are at high risk for influenza. With our product portfolio focused on chronic disease treatments, we aim to make Fluad Quad, an immune-boosting influenza vaccine, available to the same chronic disease patients we have been focusing on.” Ahead of the flu vaccine season, Samjin Pharmaceutical plans to strengthen education and communication on its vaccine through specialized channels, such as conferences, symposiums, and online webinars for medical professionals. In addition, the company will launch advertisements and campaigns targeting the general public to raise awareness of the importance of vaccination and CSL Seqirus Korea's premium vaccines. A company official emphasized, “We plan to carry out various evidence-based marketing programs to ensure that children's hospitals, pediatricians, and ENT specialists are well aware of the unique features of Flucelvax Quad, a vaccine that can be administered to a wide range of patients from children to the elderly.” CSL Seqirus is targeting the market for influenza vaccines for seniors aged 65 and older In addition, the companies made the strategic decision to target the elderly influenza vaccine market through the partnership. Korea has a National Immunization Program (NIP) in place that provides free influenza vaccines to seniors aged 65 and older, but most of the vaccines currently used in the NIP are quadrivalent vaccines. For this influenza vaccination season, trivalent vaccines are scheduled to be used as part of the NIP. Adjuvant vaccines and high-dose vaccines, which are considered to provide higher protection for the elderly, are not yet included in the NIP. In the case of overseas, the US CDC Advisory Committee on Immunization Practices (ACIP) recommends high-dose adjuvanted vaccines for people aged 65 and older, and there is a trend in developed countries to administer vaccines with improved efficacy compared to standard vaccines for the elderly. In Korea, the need for vaccines tailored to the elderly is emerging due to the aging population and increased risk of influenza, and the pharmaceutical industry is closely eyeing the possibility of including such premium vaccines in the NIP in the future. CSL Seqirus’ decision to partner with Samjin Pharmaceutical is interpreted as a strategic move to secure business synergy with an eye on entering the NIP in the long term. Although it had previously sought to expand into Korea’s domestic market through partnerships with Ilsung Pharmaceuticals and other companies, the company is seeking greater synergy this time through collaboration with Samjin Pharmaceutical, which has solid sales capabilities and a doctors’ network in primary care facilities such as hospitals and clinics. This is because in the long term, increasing the vaccination rate of immune-enhancing vaccines among the elderly and accumulating supporting evidence can ultimately strengthen the case for government adoption. (From left) Photos of CSL Seqirus From CSL Seqirus’ perspective, last year, Sanofi launched a high-dose quadrivalent vaccine, creating a two-way competition between adjuvanted and high-dose vaccines in the domestic influenza vaccine market for the elderly, making it impossible to ignore the partner company’s domestic sales capabilities. While overseas real-world studies have shown that adjuvanted vaccines reduce influenza-related medical utilization more effectively than high-dose vaccines in high-risk elderly populations, their higher prices compared to standard vaccines remain a hurdle to their domestic access, necessitating further accumulation of long-term efficacy data. For Samjin Pharmaceutical, the partnership aligns with its strategic interests, as entering the vaccine business could serve as an opportunity to diversify its portfolio and create new revenue streams. GeeSeung Yoo, Country Head of CSL Seqirus Korea, stated, “We are very excited to partner with Samjin Pharmaceutical, a company with outstanding sales and marketing capabilities. Through this agreement, CSL Seqirus Korea expects to be able to provide its differentiated influenza vaccines, Fluad and Flucelvax, to a broader population.”
- Company
- Nubeqa’s indication expanded to msHPC in combo with ADT
- by Whang, byung-woo Jun 24, 2025 06:00am
- Pic of Nubeqa Bayer Korea announced on the 23rd that its oral androgen receptor inhibitor (ARi) Nubeqa (darolutamide) has been approved by the Ministry of Food and Drug Safety as part of a two-drug regimen in combination with androgen deprivation therapy (ADT) for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC). With this expanded indication, Nubeqa can now be used not only as part of a three-drug regimen with ADT and the chemotherapy drug docetaxel but also as part of a two-drug regimen with ADT. This enables more flexible treatment approaches tailored to the individual condition and treatment goals of mHSPC patients. The approval was based on the results of the ARANOTE study, a global Phase III clinical trial evaluating the efficacy and safety of the two-drug regimen combining Nubeqa and ADT in 669 patients with mHSPC. Study results showed that the group treated with Nubeqa+ADT had a 46% statistically significant reduction in the risk of radiographic progression or death compared to the placebo group. This improvement in radiographic progression-free survival (rPFS) was consistent across all groups, including high-risk and low-risk mHSPC patients. Also, analysis of overall survival (OS), the secondary endpoint, showed that the Nubeqa combination therapy group demonstrated potential survival benefits compared to the placebo group, with significant delays in PSA progression, deterioration in quality of life, and progression of pain, thereby demonstrating clinically significant improvements in quality of life. The incidence of treatment-emergent adverse events (TEAEs) was low, with most events occurring at Grade 1 or 2, and no significant differences were observed between treatment groups. Nubeqa is already approved in Korea for use in combination with ADT and docetaxel for the treatment of mHSPC patients and for use in combination with ADT for the treatment of high-risk non-metastatic castration-resistant prostate cancer (nmCRPC). With this third indication, Nubeqa is now available for elderly patients and patients who are not suitable to receive chemotherapy. Professor Jae-Young Joung, Director of the Urologic Cancer Center at the National Cancer Center, said, "In prostate cancer, treatment strategy is critically important and needs to be selected based on the stage of diagnosis, disease stage, and the patient's overall condition. Nubeqa is the only treatment approved as both a three-drug regimen with docetaxel+ADT and as a two-drug regimen with ADT in patients with hormone-sensitive metastatic prostate cancer, so the approval will offer more flexible treatment options tailored to the patient's individual circumstances." He added, “Given its favorable tolerability profile, which reduces treatment burden and may positively impact long-term outcomes, we anticipate that it will rapidly improve treatment access for patients in Korea.” Meanwhile, Nubeqa has been approved as a treatment for mHSPC and nmCRPC in over 85 countries worldwide and has surpassed annual sales of approximately KRW 2.4 trillion as of 2024, rising as a blockbuster therapy.
- Company
- 'Fabhalta' closer to being reimbursed, shaking up inj market
- by Moon, sung-ho Jun 24, 2025 06:00am
- Next month, new oral agent will be introduced to the market for paroxysmal nocturnal hemoglobinuria (PNH). Injectables, such as Soliris and Ultomiris, currently dominate the PNH market. Attention is drawn to whether this oral agent will enhance patient satisfaction, as it offers the convenience of administration compared to the previous treatments. Product photo of FabhaltaAccording to pharmaceutical industry sources, the Ministry of Health and Welfare (MOHW) has recently given an administrative announcement on the revision to the "The detailed measure on the application standards and methods of medical reimbursements," which affects the reimbursement status of the Novartis Factor B inhibitor 'Fabhalta (iptacopan).' If there are no special circumstances, Fabhalta can be approved for reimbursement in July. PNH is currently known as a disease with no fundamental cure. However, the approach to treatment is changing with the development of therapies that inhibit the activity of the complement system. The complement system is a core component of innate immunity, serving as a powerful defense mechanism that directly attacks and destroys pathogens. The complement system includes several pathways, including C3 and C5, and ultimately functions to destroy red blood cells by forming the 'Membrane Attack Complex (MAC).' Previous treatments primarily target the terminal component within the complement system's alternative pathway. Treatment settings have been improved with the introduction of Soliris (eculizumab),' a once every 2 weeks injection, and then, 'Ultomiris (eculizumab),' which can be administered via injection at 8-week intervals. These treatments are still commonly used in clinical practices. Fabhalta works by inhibiting Factor B, a crucial component in the activation of C3. In addition to this novel mechanism, Fabhalta is also the first and only single oral agent for PNH treatment in South Korea, offering the advantage of twice-daily dosing. The efficacy of Fabhalta was confirmed in the Phase 3 APPLY-PNH clinical study, which enrolled 97 adult PNH patients (18 years or older) who had received C5 inhibitors for at least 6 months but still experienced anemia (with average hemoglobin levels below 10 g/dL). In a randomized assignment, 35 out of 97 patients continued C5 inhibitor treatment, while the remaining 62 switched to Fabhalta. The impact on treatment outcomes was assessed over 24 weeks. Clinical results showed that patients who switched to Fabhalta experienced normalized hemoglobin levels starting from week 4, with the effect sustained until week 24. Hemoglobin normalization was observed in approximately two-thirds of patients. Furthermore, four out of five patients exhibited a clinically significant increase in hemoglobin levels, and 95% of patients overcame transfusion dependence. Based on these findings, the MOHW plans to apply reimbursement starting next month for PNH patients meeting specific criteria: ▲adults aged 18 or older with a PNH granulocyte clone size of 10% or more (as measured by flow cytometry) with lactate dehydrogenase (LDH) levels at least 1.5 times the upper limit of normal, and for whom C5 inhibitors (Soliris or Ultomiris injections) cannot be administered due to medical reasons. Additionally, ▲reimbursement will apply if patients have received C5 inhibitors for more than 6 months under reimbursement criteria but have hemoglobin (Hb) levels below 10 g/dL or if there is a need for drug switching due to side effects. As a result, clinical practices expect Fabhalta to be able to resolve unmet medical needs that could not be addressed with conventional treatments. Currently, some PNH patients experience unmet needs such as persistent fatigue, insufficient symptom improvement, and transfusion dependency. Even with C5 inhibition, upstream C3 activation continues, often leading to repeated situations where red blood cells are prematurely removed from the bloodstream in the liver·spleen, necessitating transfusions. As a result, Novartis is expected to establish a dedicated team and focus on addressing these unmet medical needs. Dr. Jun Ho Jang, a professor at Samsung Medical Center Seoul, said, "Factor B is a proximal factor that acts as a gate upstream of C5 in the alternative complement pathway. In other words, inhibiting Factor B affects not only C5 but also C3, significantly improving hemolysis that occurs both intravascularly and extravascularly." Dr. Jang added, "Based on these mechanistic characteristics, the efficacy and safety profile of Fabhalta has not only been confirmed in clinical trials for patients without prior C5 inhibitor treatment experience but also showed superior results in patients who switched from anti-C5 therapy to Fabhalta compared to those who continued anti-C5 therapy." Dr. Jang emphasized, "Fabhalta as a single oral formulation offers high convenience for taking medication. It is favorable that we have an effective treatment option with a new mechanism that did not exist before. A paradigm shift in PNH treatment is expected." Meanwhile, PNH is a chronic, complement-mediated blood disorder, a severe rare disease that can be life-threatening. PNH patients have acquired mutations in some hematopoietic stem cells, leading to the production of red blood cells prone to premature destruction, resulting in intravascular hemolysis (IVH) and extravascular hemolysis (EVH). PNH can cause complications such as thrombosis, renal failure, and pulmonary hypertension, potentially leading to death, with a 5-year mortality rate of 35% and a 10-year mortality rate of approximately 50%. It can also impact the quality of life due to symptoms such as anemia and weakness.