- LOGIN
- MemberShip
- 2025-12-25 08:27:06
- Company
- Keytruda may make ₩300 billion with 1st-line reimb
- by Mar 02, 2022 05:55am
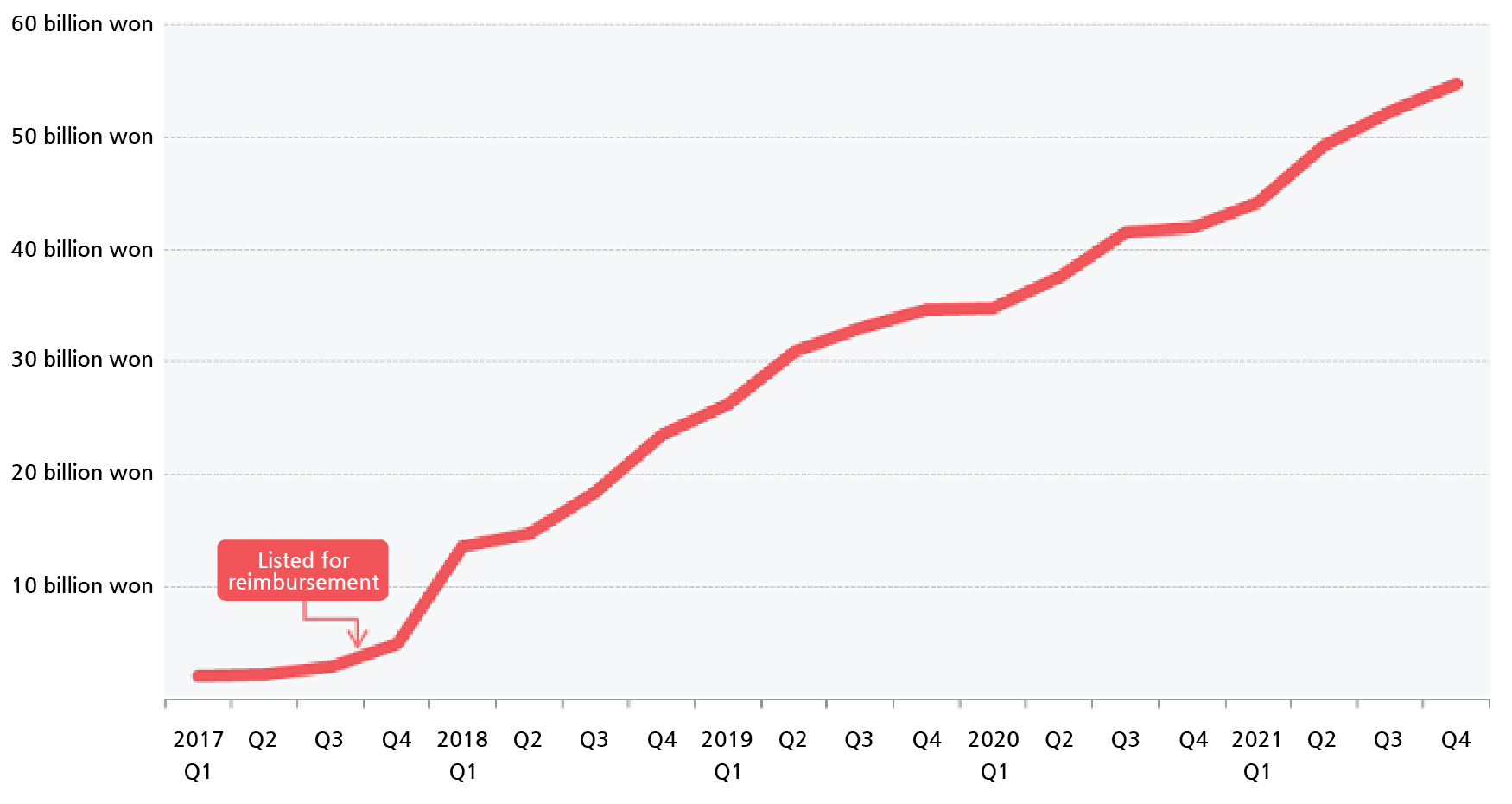
- The reimbursement expansion approval of Keytruda is expected to add wings to the sales growth of an already leading product in the domestic pharmaceutical market. It is expected that the company may achieve ₩300 billion in sales with its reimbursement expansion to the first-line, which has more patients, from the ₩200 billion sold last year. According to industry sources, the insurance benefit for MSD’s PD-1 inhibitor Keytruda (pembrolizumab) will be expanded to first-line treatment of NSCLC. This is the first time an immunotherapy drug was granted reimbursement for the first-line treatment of cancer. Also, a new reimbursement category was prepared for the drug’s use as second or higher-line monotherapy in Hodgkin’s lymphoma. Patient groups that will be newly benefitting from the reimbursement expansion are ▲ Patients with advanced (Stage IV) NSCLC (PD-L1 positive (TPS≥50%) without EGFR or ALK mutations, ▲ patients with metastatic non-squamous NSCLC without EGFR or ALK mutations (in combination with chemotherapy) ▲ metastatic non-squamous cell lung cancer without EGFR or ALK mutation (in combination with chemotherapy) ▲ adult and ages 2 or older patients who have relapsed/refractory typical Hodgkin’s lymphoma and have failed two or more therapies and failed or cannot receive autologous stem cell transplants (ASCT). The reimbursement expansion this time is expected to become a ‘catalyst’ for the explosive growth of Keytruda’s sales. Based on IQVIA, Ketyruda’s sales had surpassed ₩200 billion for the first time last year. This is a 28.5% increase from the ₩155.7 billion made in the previous year. The drug has topped the ranks with its sales for the second consecutive year. With its reimbursement expanded to first-line treatment, an area with relatively more patients, Keytruda is expected to be the first to exceed ₩300 billion in annual sales. The health authorities believe 4,000 additional metastatic NSCLC and Hodgkin lymphoma patients will receive insurance benefits with the expanded reimbursement. Simple calculation of once every three weeks (2 vials) administration at the discounted price (₩2,107,642) will bring around ₩72 million in annual sales per patient. In other words, even if only half of these potential patients are treated with Keytruda, this will bring in ₩140 billion in sales. Quarterly sales of Keytruda (Source=IQVIA) Keytruda's sales had surged when it was initially approved for reimbursement. Its sales, which stayed at ₩10 billion after its release in Korea in 2015, skyrocketed to ₩70.4 billion after it started being reimbursed as a second-line treatment for NSCLC in August 2017. In 2019, its sales exceeded ₩100 billion. It has been analyzed that the reimbursement approval played a big part in Keytruda's sales exceeding ₩200 billion only 6 years since its release. Less than 10 products including Lipitor, Avastin, Tagrisso, and Humira, had made over ₩100 billion in annual sales in the domestic prescription drug market. Among the products, Keytruday was the only product that recorded over ₩200 billion in sales as a single product. If patients start using Keytruda for first-line treatment, its annual sales may easily reach ₩400 billion. Of course, due to the expenditure-cap type RSA that was agreed upon for the reimbursement, the company’s profit may not be proportional to the increased sales. This is because the company and the authorities agreed to increase the company's share of the cost burden after undergoing 9 deliberations by the Cancer Disease Deliberation Committee. The reimbursement extension was also good news for the patients. The immune checkpoint inhibitor Keytruda treats cancer with a different mechanism of action than other existing anticancer drugs. It inhibits PD-1 (programmed death 1) proteins expressed at the surface of activated T cells, thereby inhibiting its binding to PD-L1 and activating the immune system to treat cancer. The introduction of Keytruda had changed the treatment paradigm for NSCLC, which was untreatable using targeted therapies. However, its high cost had acted as a high barrier in its access. Until now, the non-reimbursed use of Keytruda had cost nearly ₩100 million, an amount difficult to come up with unless you have private medical indemnity insurance or go to hospitals that are applied the new DRG system. However, with the reimbursement expansion, its price had fallen 25.6%, and patients will now only need to bear 5% of the cost as a copayment, which is around ₩3.5 million a year.
- Company
- Tylenol's sales amounted to ₩83.1 billion last year
- by Chon, Seung-Hyun Mar 02, 2022 05:54am
- OTC Tylenol recorded the highest sales ever. Sales more than doubled last year from the previous year due to a surge in demand for COVID-19 vaccinations. However, growth slowed in the second half of last year as demand for Tylenol was resolved. According to IQVIA, a pharmaceutical research institute, sales of the Tylenol series last year amounted to 83.1 billion won, up 118.4% from 38.1 billion won a year earlier. It is sales of four types: Tylenol, Tylenol 8-hour ER, Tylenol Cold, and Women's Tylenol. Sales of the Tylenol series did not change much from 20 billion won to 30 billion won every year, but soared suddenly last year. Sales of Tylenol and Tylenol 8-hour ER, which are used as antipyretic analgesics, surged. Sales of Tylenol and Tylenol 8-hour ER totaled 80.2 billion won last year, up 133.4% from the previous year. Tylenol's sales surged 159.4% from 24.3 billion won in 2020 to 62.9 billion won last year, while Tylenol's 8-hour ER sales rose 71.1% from the previous year to 17.3 billion won last year. It is analyzed that sales have increased significantly as COVID-19 vaccinations started purchasing Tylenol in preparation for fever and muscle pain. As COVID-19 vaccinations began in earnest in Korea from the beginning of last year, demand for Tylenol surged. Tylenol ranked first in sales among all OTCs last year. It was also pointed out that the government contributed to the surge in Tylenol's sales. The quarantine authorities informed those subject to vaccination in March last year, "If side effects such as fever occur, it is better to take Tylenol." Since then, the demand for purchasing Tylenol has soared, leading to a shortage. According to quarterly sales of acetaminophen single-drug Tylenol, it surged in the first half of last year and slowed down somewhat in the second half. Sales of Tylenol and Tylenol 8-hour ER more than tripled from the previous quarter from 10.7 billion won in the first quarter of last year to 32.9 billion won in the second quarter. In the case of Tylenol, sales more than tripled from 8.1 billion won in the first quarter of last year to 25.5 billion won in the second quarter, while Tylenol 8-hour Al also expanded from 2.6 billion won to 7.5 billion won during the same period. Sales of Tylenol and Tylenol 8-hour ER jumped sharply from the previous year to 20.4 billion won and 16.2 billion won in the third and fourth quarters of last year, respectively, but decreased from the second quarter. Sales in the fourth quarter of last year fell by half compared to the second quarter. It is analyzed that the unstable supply and demand of Tylenol has been resolved and sales of Tylenol have been on the decline through the purchase of generics. In May last year, the MFDS disclosed information that more than 60 acetaminophen single agents were on sale in addition to Tylenol, inducing the purchase of other products with the same ingredients. In June last year, the MFDS decided to cooperate with KPA, KPBMA, and KPDA in the smooth supply of Acetaminophen. KPDA also urgently implemented a plan to supply Acetaminophen drugs, which are supplied first from producers, to pharmacies nationwide.
- Company
- Pfizer's 3rd attempt to reimburse ATTR-CM drug ‘Vyndamax'
- by Eo, Yun-Ho Feb 28, 2022 05:55am
- The third attempt at reimbursement for Vyndamax, a new drug for transthyretin amyloid cardiomyopathy, has been made by its company. According to industry sources, Pfizer Korea had again applied for the insurance reimbursement of its new drug that is indicated for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM). This is the company’s third attempt. Earlier last year, Pfizer failed to first designate Vyndamax as an essential medicine. In the first half of the same year, the company made the second attempt at reimbursement using the Risk-sharing Agreement but failed again. With perseverance, the company made its third attempt in 2022. Vyndamax is virtually the only treatment option available for ATTR-CM. ATTR-CM is a fatal condition with a poor treatment outcome due to a lack of specific treatment and is often mistaken for simple heart failure If not treated properly, patients with ATTR-CM have a survival period of only 2 to 3.5 years. In this area with a dire need, Vyndamax had demonstrated a reduction of cardiovascular events in ATTR-CM patients and improvement in their functional athletic ability in the six-minute walk through the Phase III ATTR-ACT study. Based on these results, healthcare professionals in Korea are also insisting on the necessity of prescribing Vyndamax. Due to its high cost, the decision lies in the will of the government and company. As the less attention paid to reimbursement listing of rare disease treatments is emerging as an issue, whether Vyndamax will bring a different result this time remains to be seen. In the ATTR-ACT study, 441 patients were randomly assigned in a 2:1:2 ratio to receive the tafamidis 80 mg dose, tafamidis 20 mg dose, or placebo, respectively. The primary endpoint of the study was the hierarchical combination of all-cause mortality and frequency of cardiovascular-related hospitalizations. The key secondary endpoints were the change from baseline to month 30 for the 6-minute walk test and the score on the Kansas City Cardiomyopathy Questionnaire–Overall Summary (KCCQ-OS), in which higher scores indicate better health status. Study results showed that the tafamidis demonstrated a statistically significant reduction in all-cause mortality and frequency of cardiovascular-related hospitalizations compared to placebo.
- Company
- Osteoporosis should be taken care of for the rest of life
- by Feb 28, 2022 05:55am
- Ha Yongchan, chairman of The Korean Society for Bone and Mineral ResearchThe Korean Society for Bone and Mineral Research has begun to improve awareness of osteoporosis treatment. This is to enable continuous treatment by recognizing the seriousness of diseases that can lead to death from fractures and improving standards. Osteoporosis is a disease in which holes are formed in bones, and when bone strength weakens, fractures easily occur even with small shocks. It is not easy to think that a broken bone usually leads to death. However, if the hip joint connecting the upper and lower limbs is fractured, various complications such as pneumonia can occur as they cannot walk and have to lie down for a long time even if surgery is performed. In a study that analyzed the cause of short-term death of fracture patients, deaths from complications such as pulmonary embolism and pneumonia after fracture were the highest. Still, there is not a high perception that osteoporosis should be treated steadily. Ha Yong-chan, chairman of The Korean Society for Bone and Mineral Research (professor of orthopedic surgery at Chung-Ang University Hospital), said in a meeting with Dailypharm, "It is natural to fracture easily when pts get older, and I think pts only need to treat the fracture area." However, fractures caused by osteoporosis cause deformities, gait disorders, and death, so it should be taken as a concept of disease, he said. He said, "The treatment rate for osteoporosis is better than before, but the one-year treatment rate for osteoporosis patients with fractures is still less than 40%. Osteoporosis patients are lower than this, he explained. The start of osteoporosis treatment is important, but persistence is more important, Ha stressed. Usually, 5 to 10 years of treatment is required, but only 10% of all patients maintain treatment for more than 5 years. He cited ▲ lack of awareness of the disease ▲ discomfort of taking medicine ▲ limited benefit standards as the reason for the poor continuous treatment of osteoporosis. The existing drug, bisphosphonate, was difficult to take, such as taking enough water and not lying down for at least 30 minutes after taking it one to two hours before meals. Side effects of bisphosphonate formulations are also one of the factors that lowered the continuous treatment rate. Fortunately, the treatment environment has improved significantly recently with the emergence of the latest drugs that require only two shots a year and minimize complications. It is evaluated that the limited standard for new drugs is still preventing continuous treatment. Chairman Ha said, Prolia was released in Korea at the lowest drug price in the world, but the benefit standard has not been resolved. According to the current standard, if the T-score is slightly higher than -2.5 in follow-up observation a year after prolia treatment, the administration will be stopped. "To minimize the side effects of stopping treatment, the drug should be able to continue to be used even if the T-score improves." Chairman Ha cited the perception of osteoporosis treatments as the reason for the limited standards. Many people, including policymakers, think of osteoporosis treatments only as "preventive drugs." He said, "We should recognize osteoporosis treatment as a concept of lifelong management, not prevention," adding, "For example, hypertension drugs are blood pressure management drugs to prevent stroke or stroke, but they do not stop because blood pressure drops due to treatment." The treatment for osteoporosis also needs to be recognized as a drug that focuses on managing osteoporosis and preventing fractures through continuous treatment, he explained. Chairman Ha believes that the deadline for administration should be extended to five years so that he can receive treatment for at least five years. He said, "It is better to receive lifelong treatment for more than 10 years, but considering the financial burden of health insurance, we should be able to increase the benefit deadline by five years as much as much as possible. Meanwhile, various activities will be carried out to improve awareness of diseases at the academic level. It includes policy symposiums for continuous treatment, revision of medical guidelines, and strengthening post-management of osteoporosis screening in national health checkups.
- Company
- Moderna and BeiGene joined KRPIA
- by Eo, Yun-Ho Feb 25, 2022 05:57am
- According to related industries, U.S.-Moderna Korea and Chinese-BeiGene Korea recently joined as KRPIA members. As a result, the number of KRPIA member companies has increased to 46. Both Moderna and BeiGene are multinational pharmaceutical companies that established a Korean subsidiary last year, and promotional activities are expected to begin in earnest this year. Moderna, well-known as a developer of the COVID-19 vaccine, recruited Son Ji-young, former CEO of CSL Behring Korea, as the first corporate president at the end of last year. The company is currently taking three strategies: ▲ High-capacity booster shots ▲ New booster shots ▲ Omicron-specific booster shots to cope with some variations in Omicron and existing vaccines. The first alternative, the high-capacity booster shot, has doubled the existing vaccine to 100μg capacity, and has completed safety and immunogenicity tests on 306 adults. Vaccine candidate substance mRNA-1273.529 targeting Omicron is also being developed and will take about two to three months. BeiGene is a company owned by Amgen, a global pharmaceutical company. Amgen acquired about 20% of BeiGene's stake for $2.7 billion (about 3.1 trillion won) last year. The company is supplying three new drugs in China: Amgen's multiple myeloma treatment Kyprolis (Carfilzomib) and acute leukemia treatment Blincyto (Blinatumomab). PD-1 inhibitory immuno-cancer drug candidates such as Keytruda(Pembrolizumab) and Opdivo(Nivolumab) were technically exported to Celgene and obtained FDA approval from Brukinsa, a BTK inhibitory cell lymphoma treatment. Meanwhile, KRPIA includes Japanese companies such as Takeda, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Santen Pharmaceutical, Astellas, Kowa, Kyowa Kirin Korea, and multinational companies such as Galderma, Sandoz, Fresenius Kabi, Fresenius Medical Care, and Baxter.
- Company
- Shionogi COVID tx developed by Ildong has been approved
- by Kim, Jin-Gu Feb 25, 2022 05:56am
- Ildong Pharmaceutical announced on the 23rd that it has been approved by the MFDS to change the clinical trial plan of Shionogi's oral COVID-19 treatment candidate "S-217622" under development in Korea. Earlier, Ildong Pharmaceutical applied to change its clinical plan in the direction of confirming the results of each phase by modifying the existing plan to conduct phase 2b/3 clinical trials of S-217622 at once and dividing it into phase 2b and phase 3. Ildong Pharmaceutical plans to separate and check the validity and safety of Phase 2b and Phase 3 while continuing the existing clinical trials that have been conducted in Korea since the beginning of this year. This is to accelerate commercialization in line with changes in Shionogi's S-217622 development strategy and clinical plan. Shionogi is said to have recently confirmed significant results related to the COVID-19 virus suppression effect in phase 2a clinical trials. Ildong Pharmaceutical explained that it is planning to commercialize the EUA based on the interim results after completing the process up to phase 2b. The changed clinical trial plan is designed to be divided into a group (Cohort A) that conducts phase 2a, phase 2b, and phase 3 clinical trials for mild and moderate patients, respectively, and a group (Cohort B) that conducts phase 2a and phase 2b/3, respectively. The total size of the global clinical trial conducted by Shionogi is 1260 for cohort A and 600 for cohort B, of which 200 patients are eligible for clinical trials in Korea. S-217622 is a mechanism that inhibits the proliferation of viruses in the body by inhibiting the protease (3CL-protase) of the SARS-CoV-2 virus that causes COVID-19. Currently, global clinical trials are underway in Korea, Japan, Singapore, Vietnam, and Europe.
- Company
- Yuhan’s ‘Leclaza’ makes ₩4.1 billion in 6 months
- by Chon, Seung-Hyun Feb 24, 2022 05:58am
- Yuhan Corporation's new drug ‘Leclaza’ has made ₩4.1 billion in its first year of release. In only 6 months since its sales began in earnest with reimbursement listing in July, Leclaza rose to the top among homegrown anticancer drugs, raising expectations on its potential for commercial success. According to the pharmaceutical research institution IQVIA on the 22nd, Leclaza recorded ₩4.1 billion in sales last year. After raising ₩1.5 billion in Q3, sales rose to ₩2.6 billion in Q4. Leclaza is the 31st homegrown new drug that was approved for the treatment of non-small cell lung cancer in January last year. It is indicated for patients with locally advanced or metastatic non-small cell cancer who have developed resistance to the T790 mutation after using 1st or 2nd generation EGFR TKIs. Before ‘Leclaza,’ the treatment options available for prescription in Korea were first-generation EGFR-TKIs ‘Iressa (gefitinib)’ and ‘Tarceva(erlotinib),’ and second-generation EGFR-TKIs ‘Giotrif (afatinib) and ‘Vizimpro (dacomitinib),’ and third-generation EGFR-TKI ‘Tagrisso (Osimertinib).’ Yuhan Corp made its debut in the market in July last year after being listed for reimbursement. The drug received the reimbursement approval only 165 days since its authorization using the ‘approval-benefit appraisal linkage system.’ The drug was approved its reasonableness of receiving medical care benefits was recognized in the cost-effectiveness aspect, as the drug ‘is therapeutically equivalent to Tagrisso (osimertinib) while being cheaper. Leclaza’s sales exceeded expectations, making ₩4.1 billion in the first 6 months. In general, anticancer drugs used in large hospitals may only be prescribed after passing reviews of drug committees in each hospital, therefore it takes a considerable amount of time for such drugs to generate sales after their release. Leclaza rose to the ranks and took the lead among domestically developed anticancer drugs as the most sold product during the past 6 months. Other homegrown anticancer drugs approved before Leclaza in Korea include Supect, Dongwha Pharm’s Milican, Chong Kun Dang’s Camtobell, Sam Sung Pharmaceutical’s Riavax, Hanmi Pharmaceutical’s Olita. Among the drugs, Supect’s sales recorded ₩7.4 billion last year, but its sales in the second half of the year were ₩4.1 billion, similar to Leclaza. Supect is the 18th homegrown novel drug that was approved in January 2012 for the treatment of chronic myeloid leukemia. Camtobell’s only made ₩3.8 billion last year, and other products have been revoked their licenses or withdrawn from the market. Therefore, Leclaza is the first to demonstrate the possibility of commercialization among homegrown anticancer drugs. Leclaza had started being prescribed at a rapid pace after landing in large hospitals with reimbursement. Leclaza can be prescribed in the ‘Big 4’ tertiary hospitals in Korea - the Seoul National University Hospital, Sinchon Severance Hospital, Samsung Seoul Medical Center, and the Seoul Asan Medical Center - and 30 other medical institutions including the National Cancer Center, Seoul National University Bundang Hospital, Seoul St. Mary's Hospital, Chonnam National University Hwasun Hospital, Kyungpook National University Chilgok Hospital, and the Pusan National University Hospital. In Yuhan Corp’s IR data that was recently released, the company presented its mid-to-long-term goal of “rising to the ranks in the global market by becoming the first homegrown new drug to earn over $1 billion (₩1.2 trillion) in annual sales with Leclaza.”
- Company
- It was improved by changing the targeted therapy formulation
- by Eo, Yun-Ho Feb 23, 2022 05:50am
- According to related industries, Pfizer Korea's breast cancer treatment Ibrance(Palbociclib) has obtained an item license in a type of tablet not a capsule, six years after its launch in Korea. Also, Takeda is also preparing to introduce a tablet formulation of "Zejula (Niraparib toosylate monohydrate)," a treatment for ovarian cancer. It's easy to use both "Ibrance tablet" and PPI Ibrance is the first targeted anticancer drug developed among CDK4/6 inhibitors to be used as a standard treatment along with endocrine therapy in the treatment of patients with progressive or metastatic hormone receptor-positive breast cancer. In postmenopausal women, it is used in combination with "Aromatase inhibitors" for primary treatment and with Fulvestrant for secondary treatment, lowering the risk of disease progression and death in stage 4 breast cancer patients and prolonging survival. Pfizer changed the existing Ibrance capsule formulation to tablets and improved the patient's administration convenience. Ibrance tablet contains biologically the same active ingredient as conventional capsules, but can be taken regardless of food. By coating the tablet with a film, it can also be administered with a Proton Pump Inhibitor or antacids used to control gastrointestinal disorders and diarrhea, which are treatment side effects that usually appear in cancer patients. Zejula, minimize the number of pills pt takes Zejula is a PARP inhibitor and targets the BRCA gene. Lynparza of AstraZeneca Korea, a drug of the same family, has already been released in Korea. The conversion of PARP inhibitors to tablet formulations has the advantage of minimizing the number of pills. This drug has a variety of indications, including monotherapy in adult patients (including ovarian cancer or primary peritoneal cancer) who responded to ▲1st and 2nd platinum-based chemotherapy, and ▲3rd or higher chemotherapy, that is, quaternary monotherapy. Among them, benefits for primary and secondary maintenance therapy are recognized, and benefits are not applied to BRCA-negative patients. The new formulation requires a separate benefit registration procedure. Even if the indications may be different and the same indication is the same, the validity of the changed formulation should be confirmed. It remains to be seen whether anticancer drugs with new formulations will be able to quickly convert prescriptions.
- Company
- Lilly-RosVivo signed a contract to transfer diabetes txs
- by Eo, Yun-Ho Feb 23, 2022 05:49am
- Nexturn Bio announced on the 22nd that its U.S. subsidiary RosVivo Therapheutics has signed a "MTA (Material Transfer Agreement)" with Eli Lilly for commercial development of a new drug candidate for diabetes treatment, "RSVI-301. MTA is a contract concluded by the counterparty to verify the efficacy and research results of the drug through experiments. Lilly will review and acknowledge animal experiment data and reaffirm the excellence of RSVI-301 through this MTA. RosVivo's RSVI-301 is a new drug candidate that can fundamentally treat diabetes by restoring the function of beta cells that secrete insulin, the cause of diabetes, and lowering insulin resistance. In the last RSVI-301 diabetes-causing animal test, it was found to be effective in treating obesity, fatty liver, and gastrointestinal disorders. An official from RosVivo said, "The signing of an MTA with Eli Lilly, a global diabetes treatment company, proves the possibility of new drug candidates," adding, "Love calls from various global pharmaceutical companies continue."
- Company
- Endless evolution of cancer immunotherapy
- by Eo, Yun-Ho Feb 22, 2022 05:54am
- According to related industries, news of approval for the expansion of domestic indications of cancer immunotherapy Opdivo (Nivolumab) and Keytruda (Pembrolizumab) in PD-1 inhibition mechanisms continues. Although it is the same mechanism, it is competing by securing different indications. In the case of Opdivo, two postoperative adjuvant therapy and three combined therapy indications were added. Postoperative adjuvant therapy in patients with esophageal cancer or gastroesophageal junction cancer with residual pathological diseases after receiving chemotherapy (CRT) as a preoperative adjuvant therapy and Postoperative adjuvant therapy in patients with root resection were added. Combination therapy with Carboplatin, Paclitaxel, and Bevacizumab as the primary treatment for metastatic or recurrent non-small cell lung cancer patients without EGFR or ALK mutation and combination therapy with Cabozantinib as the primary treatment of advanced Neoplasmine, and Fluoropyrimidine, Oxaliplatin and combination therapy with Yervoy (Ipilimumab) was also added to the treatment of adult patients with metastatic direct bowel cancer with microsatellite instability-high (MSI-H) or dMMR that recurred after Irinotecan treatment. As a result, Opdivo can be used alone or with other treatments such as melanoma, non-small cell lung cancer, malignant pleural mesothelioma, renal cell cancer, typical Hodgkin lymphoma, head and neck squamous cell cancer, urinary epithelial cell cancer, gastric cancer, gastroesophageal adenocarcinoma, esophageal cancer, and direct bowel cancer. In the case of Keytruda, it entered the field of renal and endometrial cancer through Lenvima combination therapy. The permission for the first-line treatment indication for renal cell cancer was based on data from the CLEAR study (KEYNOTE-581/Study 307), a phase 3 clinical trial. In the CLEAR study, Keytruda-Lenvima combination therapy demonstrated statistically significant Progression-Free Survival (PFS) and Overall Survival (OS) improvements over the existing treatments Sunitinib. Keytruda-Lenvima combination therapy reduced the risk of disease progression or death by 61% and the risk of death by 34% compared to Sunitinib.