- LOGIN
- MemberShip
- 2025-12-25 10:12:34
- Keytruda may make ₩300 billion with 1st-line reimb
- by | translator Alice Kang | 2022-03-02 05:55:29

It is expected that the company may achieve ₩300 billion in sales with its reimbursement expansion to the first-line, which has more patients, from the ₩200 billion sold last year.
According to industry sources, the insurance benefit for MSD’s PD-1 inhibitor Keytruda (pembrolizumab) will be expanded to first-line treatment of NSCLC.
This is the first time an immunotherapy drug was granted reimbursement for the first-line treatment of cancer.
Also, a new reimbursement category was prepared for the drug’s use as second or higher-line monotherapy in Hodgkin’s lymphoma.
Patient groups that will be newly benefitting from the reimbursement expansion are ▲ Patients with advanced (Stage IV) NSCLC (PD-L1 positive (TPS≥50%) without EGFR or ALK mutations, ▲ patients with metastatic non-squamous NSCLC without EGFR or ALK mutations (in combination with chemotherapy) ▲ metastatic non-squamous cell lung cancer without EGFR or ALK mutation (in combination with chemotherapy) ▲ adult and ages 2 or older patients who have relapsed/refractory typical Hodgkin’s lymphoma and have failed two or more therapies and failed or cannot receive autologous stem cell transplants (ASCT).
The reimbursement expansion this time is expected to become a ‘catalyst’ for the explosive growth of Keytruda’s sales.
Based on IQVIA, Ketyruda’s sales had surpassed ₩200 billion for the first time last year.
This is a 28.5% increase from the ₩155.7 billion made in the previous year.
The drug has topped the ranks with its sales for the second consecutive year.
With its reimbursement expanded to first-line treatment, an area with relatively more patients, Keytruda is expected to be the first to exceed ₩300 billion in annual sales.
The health authorities believe 4,000 additional metastatic NSCLC and Hodgkin lymphoma patients will receive insurance benefits with the expanded reimbursement.
Simple calculation of once every three weeks (2 vials) administration at the discounted price (₩2,107,642) will bring around ₩72 million in annual sales per patient.
In other words, even if only half of these potential patients are treated with Keytruda, this will bring in ₩140 billion in sales.
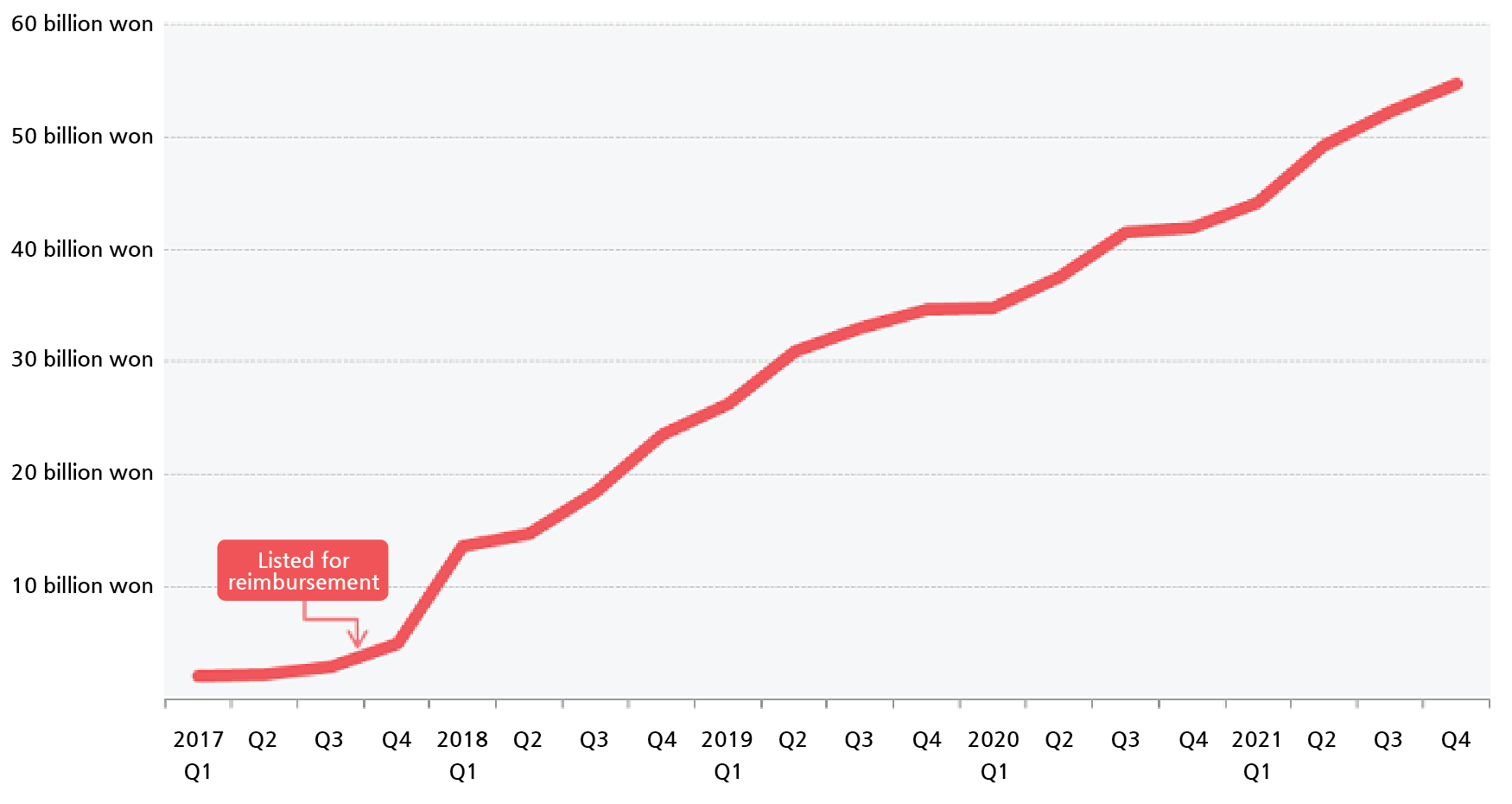
Its sales, which stayed at ₩10 billion after its release in Korea in 2015, skyrocketed to ₩70.4 billion after it started being reimbursed as a second-line treatment for NSCLC in August 2017.
In 2019, its sales exceeded ₩100 billion.
It has been analyzed that the reimbursement approval played a big part in Keytruda's sales exceeding ₩200 billion only 6 years since its release.
Less than 10 products including Lipitor, Avastin, Tagrisso, and Humira, had made over ₩100 billion in annual sales in the domestic prescription drug market.
Among the products, Keytruday was the only product that recorded over ₩200 billion in sales as a single product.
If patients start using Keytruda for first-line treatment, its annual sales may easily reach ₩400 billion.
Of course, due to the expenditure-cap type RSA that was agreed upon for the reimbursement, the company’s profit may not be proportional to the increased sales.
This is because the company and the authorities agreed to increase the company's share of the cost burden after undergoing 9 deliberations by the Cancer Disease Deliberation Committee.
The reimbursement extension was also good news for the patients.
The immune checkpoint inhibitor Keytruda treats cancer with a different mechanism of action than other existing anticancer drugs.
It inhibits PD-1 (programmed death 1) proteins expressed at the surface of activated T cells, thereby inhibiting its binding to PD-L1 and activating the immune system to treat cancer.
The introduction of Keytruda had changed the treatment paradigm for NSCLC, which was untreatable using targeted therapies.
However, its high cost had acted as a high barrier in its access.
Until now, the non-reimbursed use of Keytruda had cost nearly ₩100 million, an amount difficult to come up with unless you have private medical indemnity insurance or go to hospitals that are applied the new DRG system.
However, with the reimbursement expansion, its price had fallen 25.6%, and patients will now only need to bear 5% of the cost as a copayment, which is around ₩3.5 million a year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









