- LOGIN
- MemberShip
- 2025-12-23 11:38:06
- Company
- Janssen ‘will continue endeavors to the Korean society’
- by Son, Hyung-Min Oct 25, 2023 11:08am
- Janssen Korea held a press conference celebrating its 40th year of establishment at the Seoul Plaza Hotel on the 23rd Janssen, which has succeeded in developing treatments in various areas including mental health, autoimmune diseases, and oncology over the past 40 years, emphasized that it will continue to contribute to Korean society by providing innovative medicines and fostering talent. On the 23rd, the company held a press conference celebrating its 40th year of establishment at the Seoul Plaza Hotel. At the event, the company introduced the major achievements it had made in the past 40 years and their significance in each of its therapeutic areas. Managing Director of Janssen Korea Cherry Huang, as well as Chris Hourigan, the Company Group Chairman, Pharmaceuticals, Asia Pacific, Jung-Hee Lee, Chairman of the Board at Yuhan Corp., Professor Sang-Yeol Lee, President of the Korean College of Neuropsychopharmacology, and Young-Shin Lee, CEO of the Korean Research-based Pharmaceutical Industry Association, attended the event. During the four decades, Janssen Korea has provided its innovative drugs to patients in various fields in Korea, including oncology, autoimmune diseases, mental health, and pulmonary hypertension, According to data disclosed by the company, its market share of prescription drugs for autoimmune diseases that are used to treat plaque psoriasis, ulcerative colitis, and Crohn’s disease, has occupied, and is leading the ETC market for autoimmune diseases in Korea. Janssen also strengthened its position in the field of neurology. Its Invega (paliperidone), a treatment for schizophrenia, and Concerta (methylphenidate), a treatment for severe attention deficit hyperactivity disorder (ADHD), were also successfully launched in Korea. In oncology, the company announced its market entry by receiving domestic approval for its CAR-T treatment Carvykti (ciltacabtagene autoleucel) and lung cancer treatment Rybrevant (amivantamab). Janssen Korea is recognized as a global pharmaceutical company that has successfully introduced open innovation to Korea. Janssen purchased the global distribution rights to Yuhan Corporation’s Leclaza (lasertinib) in 2018. Leclaza was developed by Genosco, an American subsidiary of Oscotec, whose technology Yuhan Corp imported to Korea. Since then, Janssen has been evaluating the effectiveness of Leclaza in combination with Rybrevant in lung cancer treatment. The two-drug combination is likely to become a first-line treatment option for non-small cell lung cancer in the future. Jung-Hee Lee, Chairman of Yuhan Corp, who participated that day, highly evaluated Janssen. Yuhan also took the company’s value into consideration when deciding to select Janssen as its global partner and licensed out Leclaza’s technology. Lee said, “I have high respect for Paul Janssen, who founded the company. “Janssen’s philosophy of ‘respect for humanity’ was greatly taken into consideration when choosing a global partner for Leclaza.” Janssen pointed to activities such as fostering domestic talent and providing innovative medicines as measures it will pursue to coexist with Korean society in the future. For this, Huang expressed intentions to cooperate closely with the Korean government, HCPs, and industry officials. Cherry Huang, Managing Director of Janssen Korea, said, “Janssen Korea and will work together with Korea to envision and depict a future in which innovative medicines can be provided to more domestic patients and a future in which we can foster talents that can contribute to the Korean society. I hope we can go beyond the 40 years we have spent together and record another 10 years of history, even 100 years.” Chris Hourigan, the Company Group Chairman, Pharmaceuticals, Asia Pacific, said, “We have been investing in the development of treatments with high unmet needs and for the underprivileged in our society, We are also working closely with the Korean government, HCPs, and industry partners to lead the wave of innovation that can fundamentally tackle the way we deal with disease.”
- Company
- JW Pharma terminates contract after 5 yrs of atopy new drug
- by Chon, Seung-Hyun Oct 25, 2023 05:25am
- View of JW Gwacheon office building JW Pharma announced on the 20th that the technology transfer contract for 'JW1601', a treatment for atopic dermatitis, signed with Danish pharmaceutical company Leopharma, has been terminated. The company explained, “We have received a notice of termination of the contract from the Leopharma side and have agreed to return all rights.” JW1601 was transferred to Leopharma in the preclinical phase by JW Pharmaceutical in August 2018. The contract size is $42 million. JW Pharmaceuticals is a condition of receiving a non-refundable down payment of $17 million from Leopharma and up to $385 million sequential milestones for clinical development, licensing, commercialization, and sales. With this termination of the contract, the down payment of $17 million received by JW Pharmaceutical does not need to be returned. Leopharma began clinical phase 2a/b in December 2021 after the introduction of JW1601, and the trial ended last July. JW Pharmaceutical explained that Leopharma has not met the primary evaluation indicators in the initial key results of global clinical phase 2a/b. JW Pharmaceutical said, "Tolerability has been confirmed in all drug dosing groups, and no new safety issues or concerns have been identified. Safety has also been confirmed in the results of clinical trials related to heart safety that were conducted separately." JW1601 has a dual mechanism of action, which selectively acts on the histamine H4' receptor, blocks the activity and movement of immune cells that cause atopic dermatitis, and inhibits the signaling of histamine, which causes itching. Histamine H4 is a receptor that regulates the movement and activity of inflammatory cells and regulates itching signaling, and is evaluated as a target that can fundamentally solve the pathological causes of atopic dermatitis. JW Pharmaceutical emphasized, “Based on the drug's properties that selectively act on the H4 receptor of histamine and the safety region that has been confirmed in clinical practice, we plan to review the future development direction, including the possibility of new indications,” JW Pharmaceuticals.
- Company
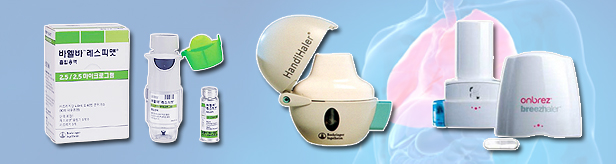
- Combo drugs strong in the COPD treatment market
- by Son, Hyung-Min Oct 25, 2023 05:24am
- The growth of the combination drugs was notable in the chronic obstructive pulmonary disease (COPD) treatment market in Q3 this year. With major COPD treatment guidelines recommending a long-acting muscarinic antagonist (LAMA) and long-acting beta2 agonist (LABA), the prescription trend appears to have shifted toward combination drugs. In particular, the growth of the LAMA+LABA combination drugs owned by GlaxoSmithKline (GSK) and Boehringer Ingelheim (BI) had stood out. Presciptions of LAMA+LABA combos (Source: UBIST). According to the market research institution UBIST on the 23rd, the amount of outpatient prescriptions for LAMA+LABA combination drugs in Q3 amounted to KRW 10.3 billion. This is a 10.8% YoY increase from last year. The bronchodilators LAMA and LABA are used to improve lung function in COPD patients. Anoro, a LAMA+LABA combination drug developed by GSK, recorded KRW 5.5 billion in prescriptions in Q3 this year, a 17.0% YoY increase compared to the same period of last year. Anoro’s sales have been steadily increasing since 2018, recording over KRW 10 billion every year. Anoro is a LAMA+LABA combination drug that was first released on the market in 2015. Boehringer Ingelheim's Vahelva's prescriptions rose 11.5% YoY to record KRW 2.9 billion, contributing to the rise in prescriptions in the LAMA+LABA combination drug market. Prescriptions of major LAMA+LABA COPD treatments (Source: UBIST) On the other hand, single-agent drug prescriptions had slowed down. Onbrez, a LABA single-agent drug developed by Novartis, was prescribed only worth KRW 130 million in Q3 this year. Onbrez’s sales have not exceeded KRW 1 billion in annual sales ever since 2021. Sales of LAMA single-agent drugs also showed a downward trend. Prescriptions of Boehringer Ingelheim's Spiriva increased to KRW 2.7 billion in Q3 this year. This is a 28.9% decrease from the KRW 3.8 billion recorded in the same period last year. GSK's Incruise also recorded KRW 970 million in Q3, down 30.7% YoY. Prescriptions of LAMA+LABA combinations are on the rise because of their effectiveness. In clinical trials that were conducted on COPD patients, combination drugs improved lung function by more than 2 times compared to single-agent drugs. Favorable results were also observed in major indicators such as lung function and shortness of breath. Analysts also have suggested that the COPD treatment guidelines have also contributed to the increase in prescriptions for LAMA+LABA combination drugs. The updated 2023 COPD treatment guidelines by the Global Initiative for Chronic Obstructive Lung Disease (GOLD), prioritized the use of LAMA+LABA combinations. GOLD recommended LABA+LAMA combination therapy or combination drugs for COPD treatment rather than LABA or LAMA monotherapy. The ICS + LABA combination therapy, which was previously recommended for treatment when the blood eosinophil count was over 300, was replaced by the LABA + LAMA + ICS combination therapy. The domestic COPD treatment guidelines also recommend prior use of LABA+LAMA combination drugs.
- Company
- Hanmi’s P3T for ’Korean GLP-1 obesity drug’ is approved
- by Lee, Seok-Jun Oct 25, 2023 05:24am
- The development of an ‘GLP-1 obesity treatment optimized for Koreans' is gaining momentum. Hanmi Pharmaceutical announced on the 23rd that the Ministry of Food and Drug Safety approved the Phase III clinical trial for its ‘efpeglenatide,’ a glucagon-like peptide 1 (GLP-1) receptor agonist developed by the company. Epeglenatide is a GLP-1 receptor agonist that acts as a GLP-1 hormone analogue that helps secrete insulin and suppress appetite in the body. After being licensed out to the global pharmaceutical company Sanofi in 2015, its efficacy in weight loss and blood sugar control was confirmed through a large-scale global Phase III clinical trial. In addition, it significantly reduced the incidence of major cardiovascular and kidney diseases, and the results were published in a number of academic journals including the world-renowned New England Journal of Medicine (NEJM). As the innovativeness of efpeglenatide has been confirmed through large-scale global clinical trials, Hanmi Pharmaceutical plans to conduct clinical trials to commercialize the drug in Korea within 3 years. Efpeglenatide will be produced at Hanmi Pharmaceutical's state-of-the-art biopharmaceutical plant, 'Pyeongtaek Smart Plant'. This is because the domestic production of the drug will ensure stable supply at an economical cost, greatly increasing the accessibility and sustainability of drugs for obese patients in Korea. Hanmi Pharmaceutical recently launched the 'H.O.P Project’ that seeks a treatment approach that encompasses the whole cycle of obesity, from its treatment, and management, to prevention. The company seeks to accelerate the development of efpeglenatide as the first commercial model. Na-young Kim, Senior Managing Director at Hanmi Pharmaceutical (Head of new product development), said “Starting with the first new GLP-1 obesity drug developed solely by a Korean pharmaceutical company with its proprietary technology, we strive to bring innovative results through the simultaneous development of drugs through H.O.P. project.”
- Company
- Celltrion’s Remsima SC was approved in the US
- by Chon, Seung-Hyun Oct 24, 2023 05:22am
- Pic of ZYMFENTRA On the 23rd, Celltrion announced that it had received marketing authorization for Zymfentra, which is a subcutaneous formulation of its antibody biosimilar Remsima. Remsima is a Remicade biosimilar. Zymfentra has been approved in Europe under the product name Remsima SC. Celltrion said, “Zymfentra is the world’s only infliximab SC formulation treatment developed by changing the formulation of Remsima, an existing intravenous formulation drug, to a subcutaneous injection to secure strong competitiveness in the TNF-alpha inhibitor market. Remsima SC has obtained marketing authorization in approximately 50 countries, including Europe and Canada, and is rapidly expanding its share in the market. Zymfentra is Celltrion's first product approved as a new drug in the US market. According to Celltrion, the FDA recognized the product's differentiated value from the discussion stage and recommended it take the new drug approval process. To obtain approval as a new drug, Celltrion conducted two new global Phase III clinical trials. Based on the safety and efficacy results demonstrated through the clinical trials, Celltrion submitted an application for its approval in December of last year in accordance with the FDA's new drug approval process and obtained authorization in 10 months. In the new Phase III trials that were conducted on 343 Crohn's disease patients and 438 patients with ulcerative colitis for 54 weeks, Zymfentra demonstrated statistically superior efficacy and comparable safety with placebo as maintenance therapy in all endpoints including the primary endpoint of clinical remission and endoscopic response rate, as well as the other major secondary endpoints. The company said, “We expect to receive patent protection until 2040 through patents on the SC formulation and administration method that we have already filed patents for." This patent is a barrier patent designed to protect against the market entry of not only Zymfentra but also other infliximab subcutaneous injection biosimilars. If the company successfully receives the patent, Zymfentra will be able to enjoy its exclusive status until patent expiry. Through this, Celltrion expects that it will be able to set a higher selling price for Zymfentra than existing biosimilars, which will serve as a stable mid- to long-term profit base for the company. According to IQVIA, a pharmaceutical market research firm, the size of the U.S. TNF-alpha inhibitor market which includes infliximab, was USD 47.736 billion (approximately KRW 62 trillion) as of last year. Celltrion believes it would be possible to achieve annual sales that exceed KRW 600 billion and KRW 3 trillion in sales within 3 years. An official from Celltrion said, “Unlike existing new drugs, Zymfentra has already demonstrated its convenience and efficacy in major global markets including Europe, so it is expected to also show great success in the world’s largest pharmaceutical market. the United States, Given the relatively poor medical environment in the United States, where patients lack economic and physical access to medical facilities, the inherent convenience of the SC formulation, which allows patients to administer the medication at home, will serve as a significant competitive advantage.”
- Company
- CMV treatment Livtencity can be prescribed at general hosp
- by Eo, Yun-Ho Oct 24, 2023 05:21am
- The cytomegalovirus treatment ‘Livtencity’ can now be prescribed at general hospitals in Korea. According to industry sources, Takeda Pharmaceuticals Korea’s Livtencity has passed the Drug Committees (DCs) of tertiary hospitals in Korea including Samsung Medical Center, Saint Mary's Hospital, Asan Medical Center, and Sinchon Severance Hospital, as well as other medical institutions such as the Kyungpook National University Hospital, Jeonbuk National University Hospital, and Chonnam National University Hwasun Hospital. Cytomegalovirus (CMV) is a type of herpes virus that's extremely common worldwide. Over 60% of all adults are infected with CMV within their lifetime and typically develops in patients who use immunosuppressants after hematopoietic stem cell transplantation (HSCT). Around 30-70% of HSCT patients experience CMV viremia. In HSCT patients, CMV causes multisystemic diseases such as pneumonia, hepatitis, gastroenteritis, retinitis, and encephalitis. Among these, pneumonia’s mortality rate is near 60%. Because CMV in immunocompromised patients is fatal, patients had generally received preemptive treatment mainly with ganciclovir, valganciclovir, foscarnet, and cidofovir, and hospitalization had been essential. Additionally, because these drugs have similar mechanisms of action if resistance to one drug develops, it is highly likely that the patient will not respond to other treatments as well. However, the introduction of Livtencity brings hope to these patients for secondary treatment. Livtencity has almost no side effects compared to existing drugs and offers an alternative if resistance to the existing treatments develops. Livtencity’s antiviral activity inhibits CMV multiplication and migration through a differentiated multi-modal mechanism of action that inhibits the protein kinase of the HCMV enzyme UL97. It not only inhibits DNA from coming out of cells, but also interferes with viral DNA replication, encapsidation, and nuclear egress. Meanwhile, Livtencity was first approved in November 2021 by the US FDA as the first treatment for patients with post-transplant CMV infection/disease and was approved in Korea in December last year.
- Company
- Next-generation antibiotic Zavicefta can be prescribed
- by Eo, Yun-Ho Oct 23, 2023 05:14am
- The new antibiotic drug Zabicefta is entering the prescription range of general hospitals. According to related industries, Pfizer Pharmaceuticals Korea's Zavicefta is being used in tertiary general hospitals such as SMC, Seoul St. Mary's Hospital, Asan Medical Center in Seoul, and Sinchon Severance Hospital, as well as Gangnam Severance Hospital, Korea University Guro Hospital, Korea University Ansan Hospital, National Cancer Center, and Ewha Womans University Mokdong Hospital. It has passed the Drug Committee (DC) of 39 medical institutions across the country. Zavicefta was developed to respond to the urgent need for a new antibiotic for serious infections where drug resistance is a serious problem, such as multidrug-resistant Pseudomonas aeruginosa, carbapenem-resistant Gram-negative pathogens, and ESBL-producing enteric bacteria. This drug is administered intravenously and is intended for adult patients suffering from clAI patients, cUTI, ventilator HAP, and aerobic Gram-negative infections with limited treatment options. Zavicefta is a drug originally developed by AstraZeneca and was acquired by Pfizer through the acquisition of its antibiotics division in 2016. Meanwhile, securing new treatment alternatives for carbapenems is a global health issue announced by the World Health Organization. Multidrug-resistant Gram-negative bacteria are increasing worldwide and have recently become a serious problem in healthcare-related infections. In particular, the World Health Organization has designated carbapenem-resistant Pseudomonas aeruginosa as one of the highest-priority pathogens requiring research and development of new antibiotics. The domestic Pseudomonas aeruginosa resistance rate to carbapenems was 30.6%, the second highest among the surveyed countries after Greece, and ESBL (extended-spectrum beta-lactamases)-producing enteric bacteria are also showing resistance to cephalosporin antibiotics, which are effective against a wide range of gram-negative bacteria. Antibiotics currently available in Korea include MSD's antibacterial drug 'Zerbaxa' and Pfizer's antifungal drug Cresemba, but only Zerbaxa is covered.
- Company
- New anemia tablets offer increased options
- by Nho, Byung Chul Oct 23, 2023 05:14am
- Whether the introduction of tablet formulations that offer improved convenience in intake in the KRW 100 billion renal anemia treatment market will shift the market paradigm and offer new options for anemia patients is gaining attention. Until now, erythropoietin stimulating agents were the mainstream treatment for anemia caused by chronic kidney disease, but in 2021, 2022, 2023, AstraZeneca·JW Pharmaceutical·Mitsubishi Tanabe Pharma Korea each received approval for their Evrenzo Tab(Roxadustat)· Enaroy Tab(Enarodustat)· Vadanem Tab(Vadadustat) from the Ministry of Food and Drug Safety, respectively, and showed the new potential held by hypoxia-inducible factor-prolyl hydroxylase inhibitors (HIF-PHI). According to drug distribution data, the size of Korea’s domestic renal anemia treatment market is around KRW 100 billion, and the same market is worth KRW 10 trillion in overseas markets where HIF-PHI is also prescribed Evrenzo, which was approved in Korea in 2021, can be administered to patients regardless of dialysis status to treat symptomatic anemia in patients with chronic kidney disease. Patients can benefit from its use as switching from ESA agents is allowed. Evrenzo activates the hypoxia-inducible factor (HIF), which regulates gene expression by regulating the formation of red blood cells. It targets the HIF and reversibly inhibits HIF-proline hydroxylase (HIF-PH). This stimulates the natural response that normally occurs when oxygen levels are low, including the production of erythropoietin and hemoglobin, reducing the symptoms of anemia. Its high efficacy has been confirmed in three clinical trials (ANDES, OLYMPUS, ALPS), and the trials confirmed that the drug can maintain hemoglobin concentration in patients who switch from ESA therapy. The drug, which was co-developed by AstraZeneca and FibroGen, was first approved in China in December 2018 and was approved in Japan the following year. JW Pharmaceutical’s Vadanem which was approved in 2022 is indicated for the treatment of adult patients with chronic kidney disease who are receiving hemodialysis. It is a HIF-PH inhibitor that stimulates ‘erythropoietin,’ a hormone that promotes the production of red blood cells while reducing ‘hepcidin,’ which regulates iron metabolism to improve the patient’s hemoglobin level. Unlike existing injections, it was developed as an oral formulation and is offered in 3 doses-1 mg, 2 mg, and 4 mg - improving the convenience of intake for the patients. In 2016, JW Pharmaceutical signed a licensing agreement with the Japanese company Japan Tobacco for the domestic development and distribution rights of 'JTZ-951', a new drug candidate for anemia of CKD. Afterward, the efficacy and safety of JTZ-951 were proven through Phase III bridging trials at 28 domestic hospitals. The trial confirmed the candidate’s non-inferiority to the existing treatment. ‘darbepoetin alfa.’ Mitsubishi Tanabe Pharma Korea’s new anemia treatment for patients with chronic kidney disease, Vadanem, was also approved by the Ministry of Food and Drug Safety in March this year. This drug is also indicated as a treatment for adult patients with chronic kidney disease who are receiving hemodialysis. It promotes red blood cell production by inhibiting proline hydroxylase, which degrades hypoxia-inducible factor (HIF). At the time of its approval, the Ministry of Food and Drug Safety, said, “The MFDS will continue being committed to the rapid provision of safe and effective treatment for the Korean people based on its expertise in regulatory science.” Evrenzo Tab, Enaroy, and Vadanem are all tablet (pill) formulations that offer significantly improved convenience over existing injection formulations. They are evaluated to be highly cost-effective in treating patients. Meanwhile, renal anemia is a common complication in patients with kidney disease that is caused by abnormal kidney function. The kidneys secrete erythropoietin (EPO), a hormone that promotes the production and maturation of red blood cells and helps the bone marrow that facilitate the production of red blood cells. However, when EPO production capacity decreases along with kidney function decline, hematopoiesis decreases, leading to anemia. Renal anemia reduces the patient's activity as it reduces energy production in organs due to a lack of red blood cells and lack of oxygen supply. It is also accompanied by fatigue, loss of appetite, decreased exercise ability, insomnia, and depression, lowering the quality of life and also affecting the patient's mortality rate. Currently, there are more than 700 million people with chronic kidney disease around the world, and 1 in 7 are known to suffer from anemia. Since it takes a long time for the symptoms of renal anemia to appear, many patients are known to lack awareness of their condition.
- Company
- Forxiga generics take over 30% of market in 6 mths
- by Kim, Jin-Gu Oct 23, 2023 05:14am
- Generic versions of ‘Forxiga (dapagliflozin)’ have increased their share in the market to 30% in half a year since the original Forxiga’s patent expiry. Since April of last year, 63 companies have been fiercely competing in the market after concurrently releasing their respective generic versions, and Boryung Pharmaceutical, Hanmi Pharm, Aju Pharm, Kyung Dong Pharma, and Daewon Pharmaceutical have recorded cumulative prescriptions worth more than KRW 1 billion in Q2 and Q3. On the other hand, 50 (79%) of the 63 companies that released generics posted cumulative prescriptions of less than KRW 300 million. Forxiga market increases from KRW 24.9 bil to KRW 35.8 bil…share of generic drugs increase to 30% According to the market research institution UBIST on the 21st, the volume of outpatient prescriptions for dapagliflozin single-agent drug and metformin combinations as of Q3 amounted to KRW 35.8 billion. This is a 44% increase in one year compared to KRW 24.9 billion it had made in Q3 last year. The analysis is that the large number of generics that joined the market drove the market expansion. The combined prescription performance of Forxiga and Xigduo generics in Q3 amounted to KRW 10.6 billion. Their share reached 30%. In just half a year after the generic drugs entered in bulk, their market share has increased to 30%. The generic companies have poured out products since Foxiga’s patent expiry in April. A total of 90 companies have received approval for their generic versions of Forxiga and Xigduo, 63 of which have released their products. The generic drugs have rapidly expanded their share in the market following their release. In Q2 last year, about 60 companies accounted for 18% of the market share with a combined prescription amount of KRW 5.9 billion. The companies then increased their market share to 30% in 3 months. Quarterly presriptions of Forxiga and Xigduo In particular, the penetration of generic drugs in the single-agent market is fast. In Q3, the original Forxiga recorded prescription sales of KRW 13.7 billion and generic drugs of KRW 6.8 billion in cumulative sales. The market share is around 33%. Generics accounted for one-third of the market within half a year of launch. In the case of combination drugs, original drugs recorded KRW 11.6 billion and generic drugs recorded KRW 3.8 billion. The proportion of market share is 75% to 25%. #SB Boryung and Hanmi’s cumulative prescriptions account for over KRW 2 billion... 50 out of 63 places earn less than KRW 300 million Amid fierce competition between generic companies, Boryung and Hanmi Pharmaceuticals achieved cumulative sales of more than KRW 2 billion. Boryung recorded KRW 2.3 billion in Q2 and Q3 with its single-agent drug Trudapa and its Trudapa+metformin combination TrudapaM. Hanmi Pharm recorded prescription sales of KRW 2.1 billion with its Dapalon and Dapalon Duo. Aju Pharm, Kyung Dong Pharma, and Daewon Pharmaceutical also recorded prescriptions of over KRW 1 billion in Q2 and Q3 combined. Aju posted sales of KRW 1.4 billion, Kyung Dong Pharma KRW 1.3 billion, and Daewon Pharmaceutical KRW 1.1 billion. However, with so many companies entering the competition at the same time, most companies showed performance that was below expectations. Among the 64 companies that released their respective products, 50 companies' cumulative prescription performance in Q2 and Q3 was less than KRW 300 million. This means that 4 out of 5 (79%) distributors of Forxiga generics are having difficulties in this market. The average prescription amount per generic company in Q2 and Q3 only amounted to KRW 260 million. Original Forxiga and Xigduo fare well despite generic entry…prescriptions rise 2% YoY The original Forxiga and Xigduo have also achieved not bad results. Rather, the total prescriptions for the two products combined amounted to KRW 25.3 billion, up 2% YoY. Forxiga recorded prescription sales of KRW 13.7 billion in Q3. Compared to Q3 last year (KRW 13.1 billion), prescriptions increased by 4%. In the case of Xigduo, prescriptions amounted to KRW 11.6 billion. No significant change has been made compared to Q3 last year (KRW 11.7 billion). Industry evaluation is that the original drugs are faring well considering the aggressive expansion of generic drugs in the market. AstraZeneca, the company that manufactured the original drug, had postponed the drug price reduction that was applied following the release of generics through administrative litigation. AstraZeneca filed an administrative suit against the Ministry of Health and Welfare's ex officio adjustment of insurance ceiling prices because Forxiga’s indications are not only for diabetes but also for heart failure. At the same time, an application for suspension of execution was filed to postpone the disposition until the conclusion of the main lawsuit. The court accepted this request, and the drug price cut for Forxiga and Xigduo was postponed until February of next year.
- Company
- Reimb pricing negotiations for Luxturna start in KOR
- by Eo, Yun-Ho Oct 23, 2023 05:14am
- The one-shot retinal dystrophy treatment ‘Luxturna’ has entered its last stage to reimbrursement in Korea. According to industry sources, Novartis Korea recently entered drug pricing negotiations for its Inherited Retinal Dystrophy (IRD) treatment Luxturna (voretigene neparvovec) with the National Health Insurance Service. Luxturna passed the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee review on September 7th. The company had applied for reimbursement of its drug in September 2021, but no progress had been made at the time. In March, Luxturna failed to pass the Drug Reimbursement Evaluation Committee review and set reimbursement standards. The company had supplemented and reapplied for the drug’s reimbursement, thanks to which the company was able to start reimbursement negotiations. The government and company had been unable to reach an agreement in Luxturna's evaluation process due to differences in opinion regarding the terms of the Risk Sharing Agreement (RSA) (refund type, etc.). In this situation, Novartis has submitted supplementary data to apply again for reimbursement, and both the government and pharmaceutical companies have shown a strong will to reimburse the drug in Korea. Therefore, it remains to be seen whether the drug price negotiations can be completed. By replacing the defective or defective RPE65 gene - one of the causes of IRD - with a normal gene, Luxturna restores the visual function of an IRD patient with a single administration. In other words, the drug provides a fundamental cure for IRD. In the US, the drug was granted a Breakthrough Therapy Designation by the FDA in 2014, the drug was approved as an orphan drug in 2016, then was granted Priority Review and a Fast-Track designation in 2017. Meanwhile, the efficacy of Luxturna was demonstrated through a Phase III trial that was conducted on IRD patients with confirmed biallelic RPE65 mutations. Study results showed that the group of patients that received Luxturna demonstrated statistically significant improvements in their functional vision compared to the control group at one year of treatment. Using the mean score of the multi-luminance mobility test (MLMT), which evaluates the ability to complete the obstacle course at low light levels by recreating the daily walking environment, as the primary endpoint at one year of treatment, the MLMT score change in the Luxturna treatment group was 1.8 points, 1.6 points higher than the 0.2 points in the control group.