- LOGIN
- MemberShip
- 2025-12-23 13:23:06
- Forxiga generics take over 30% of market in 6 mths
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2023-10-23 05:14:26

Since April of last year, 63 companies have been fiercely competing in the market after concurrently releasing their respective generic versions, and Boryung Pharmaceutical, Hanmi Pharm, Aju Pharm, Kyung Dong Pharma, and Daewon Pharmaceutical have recorded cumulative prescriptions worth more than KRW 1 billion in Q2 and Q3.
On the other hand, 50 (79%) of the 63 companies that released generics posted cumulative prescriptions of less than KRW 300 million.
Forxiga market increases from KRW 24.9 bil to KRW 35.8 bil…share of generic drugs increase to 30% According to the market research institution UBIST on the 21st, the volume of outpatient prescriptions for dapagliflozin single-agent drug and metformin combinations as of Q3 amounted to KRW 35.8 billion.
This is a 44% increase in one year compared to KRW 24.9 billion it had made in Q3 last year.
The analysis is that the large number of generics that joined the market drove the market expansion.
The combined prescription performance of Forxiga and Xigduo generics in Q3 amounted to KRW 10.6 billion.
Their share reached 30%.
In just half a year after the generic drugs entered in bulk, their market share has increased to 30%.
The generic companies have poured out products since Foxiga’s patent expiry in April.
A total of 90 companies have received approval for their generic versions of Forxiga and Xigduo, 63 of which have released their products.
The generic drugs have rapidly expanded their share in the market following their release.
In Q2 last year, about 60 companies accounted for 18% of the market share with a combined prescription amount of KRW 5.9 billion.
The companies then increased their market share to 30% in 3 months.
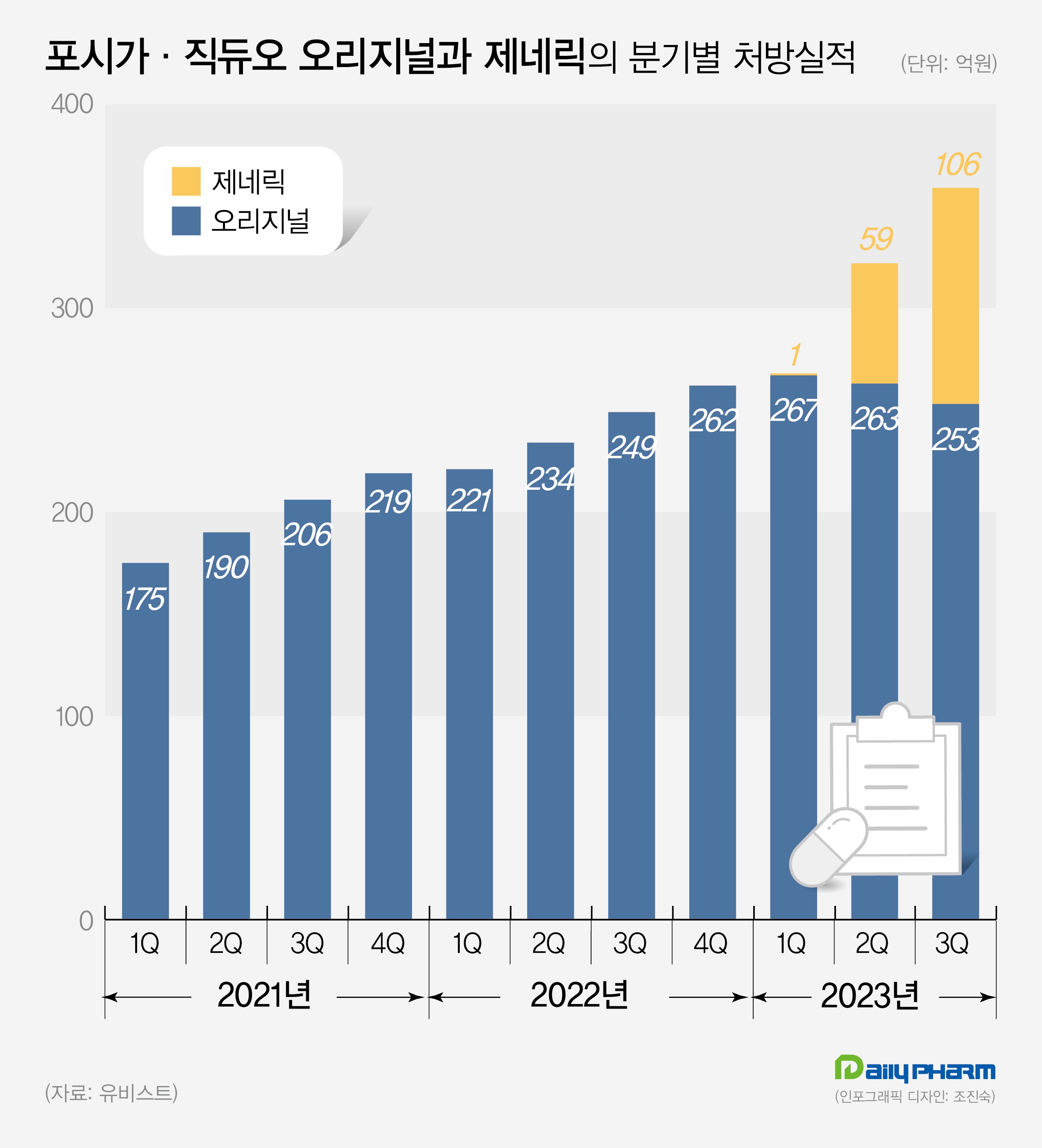
In Q3, the original Forxiga recorded prescription sales of KRW 13.7 billion and generic drugs of KRW 6.8 billion in cumulative sales.
The market share is around 33%.
Generics accounted for one-third of the market within half a year of launch.
In the case of combination drugs, original drugs recorded KRW 11.6 billion and generic drugs recorded KRW 3.8 billion.
The proportion of market share is 75% to 25%.
#SB Boryung and Hanmi’s cumulative prescriptions account for over KRW 2 billion...
50 out of 63 places earn less than KRW 300 million Amid fierce competition between generic companies, Boryung and Hanmi Pharmaceuticals achieved cumulative sales of more than KRW 2 billion.
Boryung recorded KRW 2.3 billion in Q2 and Q3 with its single-agent drug Trudapa and its Trudapa+metformin combination TrudapaM.
Hanmi Pharm recorded prescription sales of KRW 2.1 billion with its Dapalon and Dapalon Duo.
Aju Pharm, Kyung Dong Pharma, and Daewon Pharmaceutical also recorded prescriptions of over KRW 1 billion in Q2 and Q3 combined.
Aju posted sales of KRW 1.4 billion, Kyung Dong Pharma KRW 1.3 billion, and Daewon Pharmaceutical KRW 1.1 billion.
However, with so many companies entering the competition at the same time, most companies showed performance that was below expectations.
Among the 64 companies that released their respective products, 50 companies' cumulative prescription performance in Q2 and Q3 was less than KRW 300 million.
This means that 4 out of 5 (79%) distributors of Forxiga generics are having difficulties in this market.
The average prescription amount per generic company in Q2 and Q3 only amounted to KRW 260 million.
Original Forxiga and Xigduo fare well despite generic entry…prescriptions rise 2% YoY The original Forxiga and Xigduo have also achieved not bad results.
Rather, the total prescriptions for the two products combined amounted to KRW 25.3 billion, up 2% YoY.
Forxiga recorded prescription sales of KRW 13.7 billion in Q3.
Compared to Q3 last year (KRW 13.1 billion), prescriptions increased by 4%.
In the case of Xigduo, prescriptions amounted to KRW 11.6 billion.
No significant change has been made compared to Q3 last year (KRW 11.7 billion).
Industry evaluation is that the original drugs are faring well considering the aggressive expansion of generic drugs in the market.
AstraZeneca, the company that manufactured the original drug, had postponed the drug price reduction that was applied following the release of generics through administrative litigation.
AstraZeneca filed an administrative suit against the Ministry of Health and Welfare's ex officio adjustment of insurance ceiling prices because Forxiga’s indications are not only for diabetes but also for heart failure.
At the same time, an application for suspension of execution was filed to postpone the disposition until the conclusion of the main lawsuit.
The court accepted this request, and the drug price cut for Forxiga and Xigduo was postponed until February of next year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









