- LOGIN
- MemberShip
- 2025-12-22 21:27:17
- Company
- Clinical trial failures…novel PD drugs face challenges
- by Son, Hyung-Min May 23, 2024 05:48am
- Novel candidates for Parkinson’s disease that have gathered the industry’s attention are repeatedly failing to prove effectiveness in clinical trials. Bukwang Pharmaceutical was developing novel drugs for Parkinson’s disease but failed to prove its efficacy in Europe phase 2 clinical trials. D&D Pharmatech and Peptron also failed in phase 2 clinical trials. U.S. Amneal Pharmaceuticals and AbbVie also faced hurdles from the U.S. FDA. Despite of these circumstances, pharmaceutical companies continue to develop novel drugs for Parkinson’s disease and work to turn around failing history. Contera Pharma, Bukwang Pharmaceutical’s subsidiary According to industry sources on the 22nd, Denmark’s Contera Pharma, Bukwang Pharmaceutical’s subsidiary, announced that the phase 2 trial to confirm the efficacy of JM-010, a novel candidate for Parkinson’s disease, failed to meet the primary endpoint. Contera Pharma specializes in developing neurological disease treatments and was acquired by Bukwang Pharmaceutical in 2014. JM-010 targets Parkinson’s disease’s dyskinesia treatment. It has been developed to lessen involuntary movements of arms, legs, and face in Parkinson’s disease patients. This clinical trial, named Astoria, evaluated the efficacy and safety of JM-010 by administering either JM-010 or placebo in patients with Parkinson’s disease experiencing dyskinesia. The study was conducted at 38 clinical sites across Europe and Asia, including Germany, France, Italy, Spain, Slovakia, and South Korea, and involved 38 patients with Parkinson's disease. The clinical trial included patients who experienced dyskinesia as a side effect of levodopa, which is approved for the treatment of Parkinson's disease. The primary endpoint was the result of dyskinesia reduction, which was evaluated by the Unified Dyskinesia Rating Scale (UDysRS) at 12 weeks. The clinical results demonstrated that the total scores of UDysRS in the low-dose and high-dose groups of JM-010 were reduced by 0.3 points and 4.2 points, respectively, but the values were not statistically significant. For the safety profile, two doses of JM-010 have shown good tolerability, and no adverse reactions have been found in the clinical trial. Contera Pharma is also conducting additional analysis of Astoria’s complete clinical data, including secondary endpoints. The company will disclose the complete clinical results at a major conference. D&D Pharmatech·Peptron fail at phase 2 trials…AbbVie rejected by FDA Clinical trials for Parkinson's disease treatment have failed before. Korean biotech ventures like D&D Pharmatech and Peptron have also experienced challenges in proving efficacy in clinical trials. D&D Pharmatech's NLY01, a glucagon-like peptide-1 (GLP-1) agonist mechanism, failed to demonstrate efficacy in a phase 2 clinical trial in 2020. After 36 weeks of administration, it did not show statistically significant improvement in symptom alleviation compared to the placebo group which was set as the primary endpoint parameter. In detail, at 24 weeks of administration, a significant difference was found between the NLY01 treatment group and the placebo group. However, between 24-36 weeks, the placebo group’s symptoms showed more improvements than those of the NLY01 group. Peptron also failed to secure the efficacy of ‘PT320,’ a Parkinson’s disease candidate drug, in a phase 2 trial in South Korea, which was disclosed in 2022. The PT320 2.0 mg group’s UPDRS part 3 score, which evaluated Parkinson’s disease movement symptoms and was used as the primary endpoint, was not different from the placebo group’s. The PT320 2.5㎎ treatment group showed symptom improvements but failed to demonstrate statistical significance. AbbVie failed to receive FDA approval last year. ABBV-951, under development by AbbVie, has shown significant improvement in the onset time of efficacy compared to Duodopa (active ingredients: carbidopa·levodopa) used as a treatment for Parkinson's disease in phase 3 clinical trials, without exhibiting persistent dyskinesia. When taken once daily, this medication's effects can last 24 hours. However, the FDA has requested further clarification on using the adjunctive exercise device with ABBV-951 and has thus rejected approval. Novel drugs for Parkinson’s disease have failed multiple times in the past…development continues Despite past failures, global pharmaceutical and pharmaceutical and biotech companies in South Korea continue to conduct clinical trials. Amneal Pharmaceuticals’s IPX203 is an oral formulation of levodopa and carbidopa extended-release capsules. Levodopa addresses dopamine shortage, which has been pointed out as the primary cause of Parkinson’s disease. Carbidopa is used as a combination therapy because it can inhibit the conversion of levodopa to dopamine in peripheral nerves. Last year, the FDA found scientific evidence of levodopa’s safety based on pharmacokinetics research last year. However, the FDA requested additional information on Carbidopa due to inadequate proof. The company plans to develop IPX203, which offers longer treatment effects with a lower dose than the conventional formulation. AbbVie plans to receive approval through an additional novel drug candidate in addition to ABB-951. AbbVie is conducting a phase 3 trial through Cerevel Therapeutics, which recently disclosed the top-line results of Tavapadon’s phase 3 trials. Tavapadon is a novel drug candidate for Parkinson’s disease. It is designed as a dopamine D1/D5 receptor partial agonist taken orally once daily to balance motor control activity and drug efficacy. The clinical trial evaluated the efficacy and safety of Tavapadon as a levodopa adjuvant therapy in 507 Parkinson’s patients. Clinical results showed that during the 27-week trial period, the Tavapadon treatment group had a longer duration of Parkinson’s disease remission than the placebo group. AbbVie plans to share additional data from monotherapy trials of TEMPO-1 and TEMPO-2 by the end of this year. The novel drug development for Parkinson's disease continues in the pharmaceutical and biotech industry in South Korea. S.Biomedics is working on stem cell therapy TED-A9, Mthera Pharma is developing the natural drug MT-101, and Kainos Medicine is targeting the Fas Associated Factor 1 (FAF1) protein with KM-819. Additionally, ABL Bio is working on a dual antibody, ABL-301. Pipelines of pharmaceutical and biotech industry in Korea: Bukwang Pharmaceutical, S.Biomedics, Mthera Pharma, Kainos Medicine, ABL Bio, Hanall Biopharma, and Ildong Pharmaceutical.
- Company
- Leclaza’s sales soar with reimbursement expansion
- by Chon, Seung-Hyun May 23, 2024 05:48am
- The sales of Yuhan Corp’s new anti-cancer drug 'Leclaza' have jumped significantly. Sales have more than tripled in one year since the drug has been granted reimbursement as a first-line treatment for lung cancer this year. Leclaza’s sales quickly reached the KRW 20 billion mark in quarterly sales, paving the way for the drug to achieve the KRW 100 billion mark in annual sales. According to the drug research institution IQVIA on Wednesday, Leclaza reported sales of KRW 18.9 billion in Q1, up 269.9% from the KRW 5.1 billion in Q1 last year. Leclaza’s first-quarter sales more than tripled from the previous quarter. Quarterly Leclaza sales (Unit: KRW 100 million, Source: IQVIA) Leclaza is a treatment for non-small cell lung cancer that was approved in January 2021 as the 31st new drug developed in Korea. It entered the prescription market in July 2021 with its reimbursement listing. Leclaza’s quarterly sales last year were in the KRW 5 billion to 6 billion range, but its sales have more than tripled this year. The surge in prescriptions is likely due to Leclaza’s reimbursement expansion as a first-line treatment. Leclaza was first approved as a second-line treatment for patients with locally advanced or metastatic non-small-cell lung cancer (NSCLC) who developed a resistance to a specific gene (T790M) after being previously treated with 1st generation or 2nd generation EGFR-TKIs. In June last year, the Ministry of Food and Drug Safety approved Leclaza’s indication expansion to ‘first-line treatment of NSCLC.’ Yuhan Corp applied for the reimbursement of Leclaza as a first-line treatment to health authorities in Korea, and the Ministry of Health and Welfare approved its reimbursement expansion to first-line treatment last month. Starting this year, Leclaza will be reimbursed by health insurance as a ‘first-line treatment of locally advanced or metastatic NSCLC with certain gene mutations.’ The Ministry of Health and Welfare analyzed that Leclaza’s first-line reimbursement will cost the country an additional KRW 88.1 billion. Combined with Leclaza’s Q1 sales and the reimbursement expansion, this means that Leclaza’s sales could exceed 100 billion won this year. Leclaza is the first homegrown drug to exceed KRW 10 billion in quarterly sales. Other homegrown new anticancer drugs that were approved before Leclaza include Il-Yang Pharmaceuticals’ Supect, Dongwha Pharm’s Milican, Chong Kun Dang’s Camtobell, Sam Sung Pharmaceutical’s Riavax, Hanmi Pharmaceutical’s Olita. None of the products posted annual sales that exceeded KRW 10 billion. Sales of Leclaza surged in Q1 this year, bringing its cumulative sales since its launch up to KRW 61.7 billion. Leclaza is also on track to enter the U.S. market. Late last year, Johnson & Johnson (J&J) submitted an Investigational New Drug (IND) application and a supplemental biologics license application to the U.S. Food and Drug Administration (FDA) for a combination of its EGFR-positive non-small cell lung cancer drug Rybrevant and Leclaza. In November 2018, Yuhan Corp licensed out its technology for Leclaza to Janssen Biotech for a non-refundable upfront payment of USD 50 million. Johnson & Johnson's application is based on the results of the Phase III MARIPOSA trial, which evaluated the efficacy of the combination of Rybrevant plus Leclaza. The interim analysis data from the trial were presented at the European Society for Medical Oncology Annual Congress (ESMO Congress 2023) in October. Trial results showed that the combination of Rybrevant plus Leclaza improved progression-free survival (PFS), the primary endpoint, compared to Tagrisso (osimertinib) alone. Median PFS was 23.7 months for the Rybrevant plus Leclaza combination arm versus 16.6 months for Tagrisso alone. The Rybrevant plus Leclaza combination was associated with a 30% lower risk of disease progression and death compared with Tagrisso alone.
- Company
- Oral renal anemia drug meets reimb listing terms
- by Nho, Byung Chul May 22, 2024 05:47am
- Mitsubishi Tanabe Pharma and JW Pharmaceutical have filed an application to the Health Insurance Review and Assessment Service (HIRA) for drug pricing of licensed renal anemia drugs, Vadanem and Enaroy. An application for drug pricing has been filed for two items of renal anemia drugs that are equivalent in efficacy to conventional EPO injections but cost much less. New treatment options are expected to become available. According to industry sources, Mitsubishi Tanabe Pharma and JW Pharmaceutical have filed an application to the Health Insurance Review and Assessment Service (HIRA) for drug pricing of licensed renal anemia drugs, Vadanem and Enaroy. The insurance pricing of these drugs is about KRW 1.2 million, which is about KRW 300,000-500,000 lower annually than EPO injections. It is expected to significantly save the finances of the National Health Insurance. Furthermore, the efficacy of these drugs demonstrated equivalence (non-inferiority) to a comparator drug through clinical trials. The adverse reactions are similar to or less than those of the existing drugs. It is considered to be the new treatment option. If these two drugs were to secure indications for nondialysis patients, it was expected that they would quickly gain market share due to the improvements in administration convenience compared to injectables. However, it is a disappointing that they were only approved for indications for dialysis patients. However, for over the past 30 years, the market has been virtually monopolized by three first-generation erythropoiesis-stimulating agents (ESAs), which are Epoetin alfa, Darbepoetin alfa, and Methoxy polyethylene glycol, and epoetin beta. These have seen annual growth rates exceeding 10%. Consequently, the introduction of an oral formulation is seen as a response to the demands of the current era. Under these circumstances, AstraZeneca voluntarily withdrew its Evrenzo Tab (roxadustat), approved by the Ministry of Food and Drug Safety (MFDS) in 2021, due to various complications, including drug pricing issues. Anemia is caused by decreased kidney function and EPO production capacity, followed by lowered hematopoiesis. Renal anemia is accompanied by oxygen depletion due to decreased red blood cells, and it is commonly accompanied by in tiredness, decreased appetite, insomnia, and depression. It reduces the quality of life and impacts the mortality rate of patients. There are over 700 million patients with chronic renal kidney disease worldwide, and 1 out of 7 patients experience anemic symptoms. The remaining available drugs are Enaroy Tab (Enarodustat) and Vadanem Tab (vadadustat), which were approved in Korea in 2022 and 2023, respectively. If these drugs fail to be listed for reimbursement, Korean patients who suffer from renal anemia and doctors will permanently have no options for oral treatments. Considering that these drugs are priced 30% less than injectable formulations and have improved convenience of administration, with equivalent effectiveness, it would be a major loss as a nation. According to the National Health Insurance Service (NHIS) data, the number of patients with chronic kidney disease in Korea increased 36.9%, from 206,061 patients in 2017 to 282,169 patients in 2021. The increase was particularly steep in patients in their 80s, up 82.8%. Dialysis patients are also on the rise exponentially. Listing of competitive and new drugs may be justified as the National Health Insurance finance spent on approximately 100,000 patients amounts to KRW 3 trillion. “We expect the progress will be made soon because the initiation of negotiations related to listing was centered on whether Mitsubishi Tanabe Pharma’s Vadanem will be granted U.S. FDA approval and the direction of insurance pricing of the drug in European regions,” an industry expert said. Vadanem received FDA approval in March and is expected to receive a pricing decision in Europe between May and June. In August last year, Vadanem made a second attempt for the U.S. FDA approval for indication in 'anemia treatment (hemodialysis plus peritoneal dialysis) in adult patients with chronic kidney disease under hemodialysis,' and the FDA granted approval based on the absence of safety issues. Vadanem and Enaroy are hypoxia-inducible factor (HIF) prolyl hydroxylase (PH) enzyme (HIF-PH) inhibitors. These inhibitors have mechanisms of improving hemoglobin levels by activating 'erythropoietin (EPO)' and reducing 'hepcidin,' a hormone responsible for iron metabolism. Meanwhile, the EPO formulation, developed 30 years ago, is the only available treatment for renal anemia. Third generation injectables with extended intervals of administration have been launched recently. However, many patients do not respond to these treatments, experience changes in blood pressure, and have adverse reactions, such as nausea and vomiting. Thefore, there is a need for treatments with new mechanism.
- Company
- MSD applies for Welireg’s reimbursement in Korea
- by Eo, Yun-Ho May 22, 2024 05:47am
- The rare anti-cancer drug ‘Welireg’is seeking insurance coverage in Korea. According to industry sources, MSD Korea recently submitted an application for the reimbursement of its oral hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor Welireg (belzutifan). The drug is beginning its reimbursement voyage a year after its approval in Korea. Welireg was designated an orphan drug in Korea for the treatment of Von Hippel-Lindau disease in January last year, then formally approved in May of the same year. Specifically, the drug is indicated for the treatment of adult patients with VHL disease who require therapy for associated renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas, or pancreatic neuroendocrine tumors (pNET), not requiring immediate surgery. As a HIF-2α inhibitor, Welireg reduces transcription and expression of HIF-2α target genes associated with cellular proliferation, angiogenesis, and tumor growth. The drug’s efficacy was demonstrated in the open-label Study 004 trial, which investigated 61 patients with VHL-associated RCC who were diagnosed with at least one measurable solid tumor localized to the kidney. Patients enrolled in the trial had other VHL-associated tumors including CNS hemangioblastomas and pNET. The major efficacy endpoint of the clinical trial was the overall response rate (ORR) in patients with VHL-associated RCC as measured by radiology assessment using RECIST v1.1 as assessed by an independent review committee (IRC). Additional efficacy endpoints included duration of response (DoR) and time to response (TTR). In the study, Welireg showed an ORR of 49% in patients with VHL-associated RCC. All responses were partial responses. The median DoR had not yet been reached, and the DoR among responders who were still responding after at least 12 months was 56%. Median TTR was 8 months. Also, in patients with VHL-associated CNS hemangioblastomas, Welireg showed an ORR of 63%, with a complete response rate of 4% and a partial response rate of 58%. Recently, Welireg was additionally approved for a kidney cancer indication in the US. Its efficacy for the indication was confirmed in LITESPARK-005, a trial conducted on patients with unresectable locally advanced or metastatic clear cell renal cell carcinoma (RCC) that had progressed following both a PD-1 or PD-L1 checkpoint inhibitor and a VEGF-TKI. Results showed that Welireg improved progression-free survival (PFS) and reduced the risk of disease progression or death by 25% compared to everolimus in patients with advanced renal cell carcinoma who received a PD-1 or PD-L1 immune checkpoint inhibitor and a VEGF receptor-targeted therapy, either sequentially or in combination. Although it is yet to be reimbursed, Welireg is being approved and prescribed at various medical institutions in Korea, passing drug committee reviews at the National Cancer Center, Samsung Medical Center, and Asan Medical Center. The first prescription was issued at Samsung Medical Center in December last year.
- Company
- FDA approves Samsung and Biocon’s Eylea biosimilars
- by Son, Hyung-Min May 22, 2024 05:47am
- Samsung Bioepis’s Opuviz became the first Eylea biosimilar to be approved in the US. In particular, due to its interchangeability designation, Opuviz can be administered interchangeably with Eylea, increasing its chances of gaining an advantage in the market over its competitors. According to KoreaBIO on the 21st, the US FDA approved Samsung Bioepis’s Opuviz and the Indian Biocon Biologics's Yesafili as interchangeable biosimilars that can substitute the original macular degeneration treatment Eylea. The approval will allow pharmacies in the US to make substitutions of Eylea at the pharmacy level without an additional physician’s prescription. The FDA began designating interchangeable biosimilars in July 2021. This was the FDA's recognition that a biosimilar can be prescribed to any patient and produce the same clinical outcomes as the original drug. Unlike generic drugs, which must demonstrate bioequivalence, biosimilars demonstrate biosimilarity at the time of approval. This is because the cells used in manufacturing, the manufacturing process, among others cannot be the same as the original drug. As a result, ensuring interchangeability is considered a key to expanding sales. In fact, in the Humira biosimilar market, Samsung Bioepis and Celltrion, in addition to Boehringer Ingelheim and Pfizer, are conducting Phase IV clinical trials to confirm the possibility of its biosimilar’s interchangeability. #SBBiosimilars and new drugs heat up market competition#EB #I2The competition in the macular degeneration treatment market is expected to intensify with the entry of Eylea biosimilars. Currently, not only Samsung Bioepis and Biocon, but also later entrants such as Sam Chun Dang Pharm and Celltrion have jumped into biosimilar development. Eylea is a vascular endothelial growth factor-A (VEGF-A) inhibitor for macular degeneration that was developed by Bayer and Regeneron. The drug, which remains a strong market leader since its approval, generated global sales of USD 9.148 billion (KRW 12.58 trillion) last year. It works by inhibiting vascular endothelial growth factor (VEGF) to prevent abnormal blood vessel growth in the eye. By blocking VEGF, Eylea can help slow or reduce retinal damage and preserve vision. Eylea has an advantage in terms of dosing interval over its competitors. Eylea can be dosed every 2 months compared to Novartis' Lucentis (once a month), demonstrating its longer duration of effect. Eylea was also shown to be superior to Lucentis in diabetic macular degeneration, which results in a severe form of vision loss. In preparation for the inflow of Eylea biosimilars, Bayer and Regeneron developed a high-dose version of Eylea to defend the market. Their plan was to launch a higher-dose formulation and further increase dosing intervals. Bayer and Regeneron are aiming to get approval for Eylea high-dose for all of the indications they have secured, including diabetic macular edema, age-related macular degeneration, and retinal vein occlusion. The variable is Roche’s Vabysmo, which emerged as a formidable competitor to Eylea. Vabysmo is a macular degeneration treatment that was developed by Roche. The drug not only inhibits VEGF but also blocks the angiopoietin-2 (Ang-2) pathway to inhibit neovascularization. Blocking both pathways independently has been shown to be more effective than blocking VEGF alone in reducing inflammation, leakage, and abnormal blood vessel growth. Vabysmo improved visual acuity at a level non-inferior to that of Eylea in the TENAYA and LUCERNE trials that compared its safety and efficacy with Eylea. Its duration of response lasted 24 months. In other words, Vabysmo achieved comparable efficacy to other treatments with once every 4 months dosing compared to the once every 1-2 month dosing required for other treatments.
- Company
- FDA approval delayed and retry…what’s 'Rivoceranib'?
- by Son, Hyung-Min May 21, 2024 05:56am
- HLB’s rivoceranib, which was expected to be an FDA-approved new drug candidate, failed to receive final approval. Although HLB confirmed that Rivoceranib combined with immunotherapy camrelizumab extended overall survival in the first-line treatment for liver cancer, they received request for supplementary documents. The current request for supplementation is not related to the drug’s effectiveness but rather to an issue concerning the monitoring of Jiangsu Hengrui Pharmaceuticals’ Chemistry, Manufacturing and Controls (CMC) process. Therefore, HLB plans to cooperate and reapply for approval soon. HLB announced on the 17th that they received a complete response letter (CRL) request from the US FDA regarding the application for Rivoceranib plus camrelizumab combination therapy as the first-line treatment for liver cancer. HLB aims to work on the supplementary documentation and reapply for approval promptly. FDA must notify the approval state within the 6 months of receiving a developer’s CRL. “We cannot be certain, but there were some points during the CMC monitoring process of Jiangsu Hengrui Pharmaceuticals. In response to the request, we provided supplementary documents, but it seems our reply may not have been adequate,” HLB Chairman Jin Yang-gon said. “In my opinion, Jiangsu Hengrui Pharmaceuticals, which owns 17 pharmaceutical items, can solve any problem,” He added. “We will closely cooperate with Jiangsu Hengrui Pharmaceuticals and will try to get FDA approval soon. We will also speed up for global phase 3 clinical trials for the next indication.” Rivoceranib, acquired from Bukwang Pharm, closer to be a new FDA-approved drug Rivoceranib is an oral anticancer drug that selectively inhibits vascular endothelial growth factor receptor-2 (VEGFR2), which is involved in angiogenesis. The development of Rivoceranib started after Elevar Therapeutics of the US acquired global licensing rights of the drug from Advenchen Laboratories. Bukwang Pharm recognized Rivoceranib’s potential and secured licensing rights of Korea, Europe, and Japan from Elevar Therapeutics in 2009. Subsequently, HLB paid KRW 40 billion to acquire Rivoceranib’s development right from Bukwang Pharm in 2018. Then, HLB incorporated Elevar Therapeutics as its subsidiary and secured Rivoceranib’s global rights, excluding China. HLB also acquired the substance patent of Rivoceranib and made it its asset. HLB and Jiangsu Hengrui Pharmaceuticals have been developing Rivoceranib plus camrelizumab combination therapy for the treatment of liver cancer and stomach cancer. Jiangsu Hengrui Pharmaceuticals’ camrelizumab inhibits PD-1 protein expressed on the surface of immune cells (T cells), preventing the binding to PD-L1 receptors on cancer cell surfaces, thereby activating immune cells. HLB obtained positive results for the combination therapy in the first-line treatment of liver cancer and submitted a New Drug Application (NDA) to the FDA in May last year. The approval was based on the results of the CARES-310 Phase 3 trials, disclosed at 2022 ESMO by HLB and Jiangsu Hengrui Pharmaceuticals. The clinical trials compared the efficacy and safety of Rivoceranib plus camrelizumab to Bayer’s Nexabar, which is used as a standard therapy for liver cancer. In clinical trials, Rivoceranib plus camrelizumab recorded a median overall survival (OS) of 22.1 months, demonstrating an improvement compared to Nexabar’s 15.4 months. This result was more extended than the OS of 19.2 months recorded by Roche’s combination therapy of Tecentriq, an immunotherapy for cancer, plus Avastin, a targeted anticancer agent. Furthermore, it is also extended than the OS of 16.4 months of AstraZeneca’s Imfinzi plus Imjudo combination therapy. These drugs were compared with Nexabar alone. Furthermore, Rivoceranib plus camrelizumab secured statistical significance, with a progression-free survival (PFS) of 5.6 months and an overall response rate (ORR) of 33.1%. Currently, HLB and Jiangsu Hengrui Pharmaceuticals are also conducting clinical trials of the drug for stomach cancer, in addition to liver cancer. At the 2023 ESMO of last year, Jiangsu Hengrui Pharmaceuticals disclosed the DRAGON IV Phase 3 clinical trial’s first interim results of triple combination therapy of Rivoceranib plus camrelizumab plus chemotherapy in localized, resectable stomach or gastroesophageal adenocarcinoma. The clinical results have shown that the combination therapy recorded a pathophysiological complete remission rate of 18.3%, which is an improvement compared to the chemotherapy group’s 13.7%.
- Company
- Flu drug market enjoys rise for 6 consecutive quarters
- by Chon, Seung-Hyun May 21, 2024 05:56am
- The outpatient prescription market for flu drugs has recovered. Their prescription market, which had remained almost nonexistent for 3 years after the COVID-19 pandemic, has expanded significantly since then. With the flu epidemic lasting more than a year, the market for flu treatments has returned to pre-pandemic levels. According to the market research institution UBIST, the outpatient prescription market for influenza (flu) totaled KRW 6.8 billion in Q1, up 34.8% YoY. The prescription market for flu medications has grown more than 160 times in 2 years from just KRW 40 million in Q1 2022. The flu drug market has undergone significant changes throughout the COVID-19 pandemic and endemic. After recording KRW 8.2 billion in prescriptions in Q1 2020, the flu treatment market was virtually nonexistent until Q3 2022. Its prescription market was below KRW 100 million for 10 consecutive quarters since Q2 2020. This is due to the significant decline in other infectious disease outbreaks since the COVID-19 pandemic began in earnest, due to increased personal hygiene practices such as handwashing and mask-wearing. According to the Korea Disease Control and Prevention Agency, the number of suspected influenza patients per 1,000 outpatients never exceeded 5 until August 2022, since reaching 6.3 in the first week of March 2020, week 9. That means the flu had not even once spread and become an epidemic for 2 and a half years. The prescription market for flu treatments soared to KRW 10.4 billion in Q4 from just KRW 70 million in Q3 2022. On September 16, 2022, a flu epidemic warning was issued for the first time in 2 years and 6 months and then lasted for 2 years and 7 months until April this year. After the COVID-19 pandemic ended last year, the flu epidemic period has been lengthening due to fully lifted social distancing measures and an increase in outdoor activities. In Q4 last year, the amount of outpatient prescriptions for flu drugs exceeded KRW 20 billion. Number of suspected influenza patients per 1000 outpatients (Unit: people, Source: KDCA) In April, the number of suspected flu patients per 1,000 outpatients remained steady at around 10. The prescription market for flu medicines in Q1 of this year is similar to the KRW 6.7 billion in Q1 2019 before the pandemic. The prolonged flu pandemic has brought the market back to pre-pandemic levels. Oseltamivir accounts for most of the outpatient prescriptions in the flu treatment market. Oseltamivir is the main active ingredient in Tamiflu. The prescription market for oseltamivir was not able to surpass KRW 100 million until Q3 2022, after reaching KRW 8.2 billion in Q1 2020. However, it rebounded to KRW 10.4 billion in Q4 2022 and has continued to grow since then. In Q1 this year, oseltamivir-based prescriptions amounted to KRW 6.8 billion, up 34.8% year-on-year.
- Company
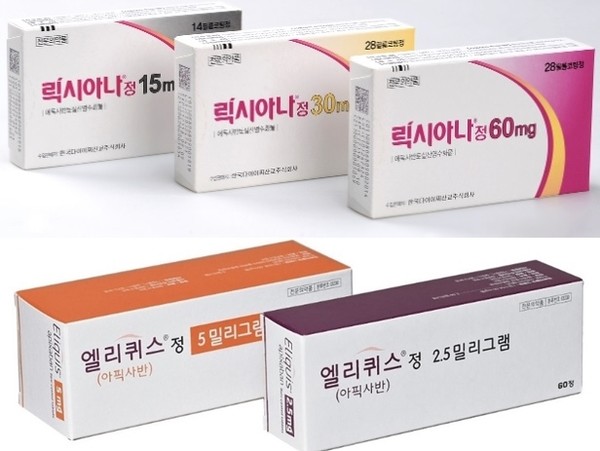
- Series of drug patent expiries change ₩250B DOAC mkt
- by Moon, sung-ho May 21, 2024 05:55am
- The direct oral anti-coagulant (DOAC) market is changing shape at an accelerated pace. This is due to the series of patent expiries of original drugs that had recorded high sales, mainly in the cardiology departments of university hospitals and clinic-level medical centers. #In particular, with the accelerated entry of generic drugs (generics), whether companies of original drugs will be restructuring their business units is also attracting attention. According to industry sources on the 18th, generic versions of original DOACs from major global pharmaceutical companies have recently been launched or announced one after another upon the originals’ patent expiry. DOACs that are currently prescribed in internal medicine hospitals and clinics and form the major market in Korea are Bayer’s Xarelto (rivaroxaban), Boehringer Ingelheim’s Pradaxa (dabigatran), BMS’s Eliquis (apixaban), and Daiichi Sankyo’s Lixiana (edoxaban). Among these, Xarelto’s decline in the prescription market has become more pronounced in recent years with its patent expiry in 2H 2022, and the bulk of domestic generics versions that followed. According to the drug research institution UBIST, Xarelto’s prescriptions plummeted 37% from KRW 49.4 billion in 2022 to KRW 31 billion in 2023. The downward trend has continued in Q1 this year, recording sales of KRW 7.6 billion. In addition, another blockbuster, BMS’s Eliquis, is also set to go off patent in 2H this year. As a result, generic versions of this drug are also expected to flood the market once the patent expires in September, which is expected to affect prescriptions. For reference, Eliquis has continued to dominate the clinical scene, with prescriptions totaling KRW 77.3 billion last year. In Q1 this year, its sales amounted to KRW 19.3 billion, but the general prospect is that this sales flow will change after the launch of its generics. In fact, major domestic companies are already preparing to launch generic versions of Eliquis. For example, Dongkook Pharmaceutical recently received approval from the Ministry of Food and Drug Safety for “Apigaban,” an apixaban generic. These changes are expected to solidify the sole lead of Daiichi Sankyo's Lixiana, which has been strengthening its market dominance in the field. Lixiana, which is marketed by Daewoong Pharmaceutical, has recently surpassed Eliquis in sales and solidified its dominance in the field. Last year, Lixiana's sales in Korea reached KRW 105.3 billion, and in Q1 this year, the drug posted 27.7 billion won in sales, continuing on its upward trend. "The sales of DOACs do not fluctuate much due to their need in clinical practice," said a professor of cardiology at A University Hospital, who requested anonymity. "But if generic versions are released, prescriptions will naturally be dispersed due to drug prices.” He added, "Due to rising interest in its utility after its reimbursement approval following transcatheter aortic valve implantation (TAVI) procedure, and due to the need for its continued use, pharmaceutical companies will essentially seek to own one product. It will be interesting to see how the global pharmaceutical companies that own the original products respond." The industry is watching BMS's moves following the patent expiration of Eliquis. BMS has recently undergone a major restructuring at its headquarters level. Last month, BMS reported Q1 earnings that beat expectations, with sales of multiple myeloma drugs ‘Revlimid’ and ‘Eliquis’ coming in higher than expected. However, one-time costs related to recently closed M&A deals turned the quarter into a loss, and the company said it would focus its resources on R&D programs that will provide the biggest return on investment going forward. In the process, the company announced that it would lay off 2,200 employees this year, discontinue some development programs, consolidate operations, and reduce management layers. In fact, after the BMS headquarters’ announcement, restructuring started in Japan and elsewhere. This is why there is a lot of industry speculation on whether the announcement will affect its Korean subsidiary as well. The series of generic Eliquis drugs that are set to be released upon the original drug’s expiry is also adding strength to the speculation. AstraZeneca also recently withdrew its diabetes drug Forxiga (dapagliflozin) from the Korean market after the launch of its generic versions, closed down the division, and offered voluntary retirement programs. In other words, some worry that global pharmaceutical companies will again launch early retirement programs following the patent expiry of their original drug and the launch of generics. However, BMS Korea denied any such plan. BMS Korea said, "We have no plans set following Eliquis’s patent expiry. We are alternating activities in the domestic market with Pfizer. Our plans at the headquarters level were implemented to strengthen our portfolio through recent M&A and intensive investment in R&D, and are independent of the Korean situation. At this time, we do not have any plans related to Eliquis’s patent expiry."
- Company
- Rolvedon's cumulative US sales surpasses ₩100B
- by Son, Hyung-Min May 21, 2024 05:55am
- Hanmi Pharmaceutical's neutropenia drug Rolvedon is cruising in the US market. Rolvedon has surpassed KRW 100 billion in cumulative sales in the US market. Hanmi Pharmaceutical's U.S. partner Assertio plans to further increase the drug’s market share by securing a same-day dosing indication. According to Assertio on the 18th, Rolvedon generated USD 14.5 million (about KRW 19.7 billion) in sales in Q1 this year, up 31.8% from the USD 11 million it generated in Q4 last year. Since its launch in the US in Q4 2022, Rolvedon has generated USD 80.2 million (KRW 110 billion) in cumulative sales. Rolvedon (Korean brand name: Rolontis) is a neutropenia treatment developed by Hanmi Pharmaceutical. In March 2021, it was approved as the 33rd homegrown new drug in Korea. Hanmi Pharmaceutical and its U.S. partner Spectrum (now Assertio) succeeded in obtaining U.S. Food and Drug Administration (FDA) approval for Rolvedon in September 2022. Quarterly Rolvedon Sales Hanmi Pharmaceutical licensed out Rolvedon to Spectrum Pharmaceuticals in 2012. Assertio acquired Spectrum in April last year and acquired the rights to market and develop Rolvedon and the lung cancer drug poziotinib. Assertio is a pharmaceutical company specializing in inflammation treatments like the non-steroidal anti-inflammatory drug Indocin and the orally disintegrating film Sympazan. After acquiring Rolvedon, Assertio braved the anticancer drug market. Since its release in October 2022, Rolvedon’s sales in the U.S. reached USD 10 million in Q4 sales. In December of the same year, Rolvedon was included in the National Comprehensive Cancer Network (NCCN) guidelines for prevention and treatment options for febrile neutropenia. Since then, Rolvedon's sales have continued to grow, to USD 15.6 million in Q1 and USD 21 million in Q2 last year. However, Rolvedon's growth has slowed since Assertio took over its sales. Rolvedon reported USD 8 million in revenue in Q3 last year, a 62.0% decrease from the previous quarter. Rolvedon has become reimbursable through the US’s public health insurance since April last year, but the terms are reportedly less favorable than the reimbursement system it had in place on its launch. Rolvedon’s sales rebounded in Q4 last year, recording KRW 10 million in revenue. Sales continued to grow in Q1 this year to KRW 14.5 million. Assertio attributed the rebound in sales to the new accounts it had signed using an updated commercialization strategy. Assertio has high hopes for its same-day dosing trial. Rolvedon is indicated for the treatment or prevention of neutropenia in cancer patients who receive myelosuppressive chemotherapy. As a granulocyte colony-stimulating factor (G-CSF) class that stimulates the granulocyte to increase neutrophil production, the drug has a similar mechanism of action with Amgen’s blockbuster drug ‘Neulasta (pegfilgrastim).’ However, neutropenia treatments like Neulasta cannot be administered until 24 hours after chemotherapy, which increases the number of hospitalization days for the patient hospitalized. Assertio is currently enrolling patients in a Phase I, same-day dosing trial with Rolvedon. Assertio CEO Heather Mason said, “Our goal is to have clinical data readout by the end of this year. We expect the new dosing regimen to serve as a differentiated strategy compared with competitors.”
- Company
- Dong-A, Ildong join hands…R&D synergy and resources
- by Chon, Seung-Hyun May 21, 2024 05:55am
- Dong-A ST and Idience sign a strategic equity investment and joint development agreement. Officials, (from left) Ildong Pharmaceutical President Lee Chae-joon, Idience CEO Lee Won-sik, Dong-A ST R&D President Park Jae-hong, and Dong-A ST President Kim Min-youn, taking photos. Dong-A ST and Idience join hands to develop novel anticancer drugs. Dong-A ST invested KRW 25 billion in Ildong’s subsidiary, Idience, and will co-develop novel anticancer drugs. Consequently, Dong-A ST has secured a pipeline of potential novel anticancer drugs, and Idience successfully contracted investment for its novel drug development. Dong-A ST invested KRW 25 billion in Idience…strengthens its novel oncology pipeline According to industry sources on the 20th, Dong-A ST signed a strategic equity investment and joint development agreement with Idience, Ildong’s company specializing in new drug development. Idience is delivering a third-party allocation capital increase of KRW 25 billion by issuing 19,142,420 new shares to Dong-A ST. Prior to the capital increase, Idience had 11,492,538 common shares and 10,985,074 preferred shares outstanding. Dong-A ST is expected to become Idience's second-largest shareholder after the capital increase. Founded in May 2019 as a subsidiary of Ildong Holdings, Ildong Pharmaceutical's holding company, Idience is a biopharmaceutical company specializing in new drug development. Idience is a development-centric (NRDO, No Research Development Only) biotech venture, focusing solely on drug development rather than direct drug discovery. Idience is under development of ‘Venadaparib.‘ Venadaparib is a novel targeted anticancer candidate with a mechanism focused on selective inhibition of Poly ADP-ribose polymerase (PARP), which is involved in cancer generation. Ildong Pharmaceutical developed Venadaparib as an in-house product and then transferred the rights to Idience. It is under development as an oral, targeted anticancer therapy for the treatment of gastric cancer, breast cancer, and ovarian cancer. The analysis indicates that Dong-A ST has strengthened the competitiveness of its oncology pipeline through this investment, obtaining rights for the use of Venadaparib in combination therapies. Dong-A ST continues to build its oncology pipeline by making R&D investments. In November last year, immunotherapy DA-4505 was approved for phase 1/2a trials in South Korea. Furthermore, the company presented preclinical results of SHP1(Src homology phosphatase-1) inhibitor ‘DA-4511’ at the AACR annual meeting 2024, held in April, and proved the candidate product’s potential for immunotherapy for cancer. In December, Dong-A ST invested KRW 31.4 billion in acquiring AbTis, a company specializing in antibody-drug conjugates (ADC). AbTis developed a third-generation ADC linker technology called ‘AbClick,‘ which site-selectively conjugates drugs to antibodies without antibody modification. In the second half of the year, AbTis plans to file an IND for AT-211, a Claudin 18.2 ADC candidate for gastric cancer and pancreatic cancer, in the U.S. and Korea. ”We plan to seek various measures to expand in a global market through this strategic collaboration. We will develop innovative novel anticancer drugs by joining Dong-A ST and Idience technology and products,” Dong-A ST President Kim Min-young said. Idience, secures financial resources for novel drug development…secured KRW 90 billion since its inception Idience has secured an additional financial resource for its novel drug development through Dong-A ST’s investment. Since its inception, Idience has received a total of KRW 65 billion in investment. When Idience was established, Ildong Holdings initially invested KRW 500 million and later added another KRW 4.5 billion. In 2021, Idience successfully secured an investment of KRW 40 billion. It also received a total investment of KRW 40 billion from various firms, including Yuanta Investment, TS Investment, Mirae Asset Capital, and Seoul Investment Partners. In 2022, Idience raised additional investment through a third-party allocation capital increase of KRW 15 billion from Ildong Holdings and others. With the completion of Dong-A ST's capital increase, Idience is set to secure a total of KRW 90 billion in investment capital since its inception. Dong-A ST's cash and cash equivalents in the first quarter amounted to KRW 267.3 billion. With this ample funding, Dong-A ST aims to secure a new drug pipeline while Idience secures funding for drug development. Idience is currently working on the commercialization of Venadaparib, including clinical development for targeting various cancer types, such as gastric cancer, breast cancer, and PARP inhibitor-resistant cancer. In gastric cancer, Venadaparib received orphan drug designation from the U.S. FDA in 2022 and is currently undergoing clinical trials. Earlier this year, at the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium, interim results from Phase 1 clinical trials were presented. These results demonstrated a broad range of applications and superior therapeutic effects compared to standard treatments, showcasing Venadaparib's competitive edge. In addition to their previous relationship in the Korean market, Dong-A ST and Ildong Pharmaceutical also built a collaborative partnership for developing novel drugs. Dong-A ST and Ildong Pharmaceutical are co-marketing a natural medicine Motilitone since 2019. Motilitone is a product utilizing natural substances extracted from the seeds of the morning glory flower and the stems of the fucus brown algae. Since 2019, Dong-A ST has been selling the antiulcer drug Gaster, produced by Ildong Pharmaceutical. Gaster is indicated for various conditions, such as gastric and duodenal ulcers, upper gastrointestinal bleeding, reflux esophagitis, Zollinger-Ellison syndrome, acute gastritis, and improvement of gastric mucosal lesions due to acute exacerbation of chronic gastritis. “It is significant to have partnered with Dong-A ST and successfully received investment. Idience’s R&D capacity and pipeline values are recognized through this deal,” Idience CEO Lee Won-sik said.