- LOGIN
- MemberShip
- 2025-12-22 15:49:07
- Company
- Samsung Bioepis launches Stelara biosimilar Pyzchiva in EU
- by Hwang, Byung-woo Jul 30, 2024 05:52am
- Samsung Bioepis has launched Pyzchiva, its biosimilar version of Stelara (ustekinumab), in the European market. Pic of PyzchivaPyzchiva was approved in Europe and Korea in April and was launched into the Korean market as Epyztek. The drug was approved in the U.S. in June. The company’s marketing partner, Sandoz, will take charge of its sales in Europe. Samsung Bioepis signed a partnership agreement with Sandoz in September last year to sell Pyzchiva in North America and Europe Stelara is a treatment for autoimmune diseases such as plaque psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis that was developed by Janssen. It posts annual global sales of approximately KRW 14 trillion (USD 10.858 billion). With the launch of Pyzchiva, Samsung Bioepis has now launched 8 biosimilars in Europe. In addition to the 3 tumor necrosis factor-alpha (TNF-α) inhibitors, Samsung Bioepis has expanded its autoimmune disease treatment portfolio in Europe by adding an interleukin inhibitor. "Our goal is to ensure that patients across Europe have access to life-changing medicines, and Pyzchiva marks an important milestone as one of the first ustekinumab biosimilars released in Europe," said Rebecca Guntern, Region President of Sandoz Europe. Meanwhile, Samsung Bioepis has been directly selling Epyztek in Korea. The company’s strategy was to strengthen its marketing capabilities and increase profitability by adding Epyztek to the list of autoimmune disease treatments it has been selling directly since March. According to the 'Drug Reimbursement List and Ceiling Price Table' as of July 1 by the Health Insurance Review and Assessment Service, Epyztek’s drug price was set at KRW 1,298,290 for the 45mg/0.5ml prefilled Inj. This is about 40% lower than the price of the original drug. Stelara’s price is also set to be reduced following the launch of the biosimilar, but Epyztek offers a price benefit even with the original drug’s price discount.
- Company
- First patient dosing with Pluvicto imminent in Korea
- by Moon, sunh-ho Jul 30, 2024 05:52am
- Novartis Korea's prostate cancer drug Pluvicto, which has been in the spotlight since its approval by the Ministry of Food and Drug Safety, is gaining further attention as the company prepares to administer the first dose to patients in Korea. Pluvicto is a blockbuster drug that generated more than KRW 1 trillion in global sales last year and is considered to have ushered in the era of so-called ‘radiopharmaceutical missiles’ in oncology. # Unlike Bayer's Xofigo, which was licensed as a radioactive therapeutic agent in 2014 but was ignored by the market, Pluvicto opened a new era making noteworthy performance. As a result, global pharmaceutical companies are rushing to develop radiopharmaceuticals after witnessing the success, intensifying competition among domestic pharmaceutical and biotech companies. According to the medical and pharmaceutical industry on the 27th, the first patient is expected to be treated at one of the largest general hospitals in Korea in late August, after the Ministry of Food and Drug Safety’s approval of Novartis Korea's prostate cancer drug Pluvicto (lutetium Lu 177 vipivotide tetraxetan) in late May. Pluvicto is a radioligand therapy that binds the radioactive isotope lutetium (177Lu) to prostate-specific membrane antigen (PSMA), which is overexpressed in prostate cancer, to kill cancer cells. The treatment was acquired by Novartis through its acquisition of US-based Endocyte in 2018. Radioligands are therapeutic agents that combine a therapeutic radioisotope with a ligand (which targets specific cancer cells). When the radioligand binds to the target cell, it releases the therapeutic radioisotope, inhibiting cancer cell proliferation. Its May approval was based on the Phase III VISION trial. The trial evaluated the efficacy and safety of Pluvicto versus standard-of-care monotherapy in 831 patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). Results showed that the the primary endpoint of radiologic progression-free survival (rPFS) was 8.7 months in the Pluvicto arm, which was longer compared to 3.4 months in the control arm. Median overall survival (OS) was 15.3 months in the Pluvicto arm and 11.3 months in the control arm. The risk of radiographic progression or death was reduced by 60% with the use of Pluvicto. Since the drug was approved by the MFDS, Novartis Korea has been working to start treating patients at the largest hospitals in Korea. In order for medical institutions to introduce the radiopharmaceutical Pluvicto, the institutions need to have a PSMA PET-CT dedicated to prostate cancer and have a separate room for dispensing, quality control, and patient administration of radioactive therapeutic agents. Currently, 15 medical institutions nationwide, including the largest hospitals in Korea, are equipped with PSMA PET-CT for testing. Novartis is currently in discussions with 11 of these medical institutions, including Asan Medical Center in Seoul, to provide Pluvicto to prostate cancer patients. With the first patient expected to be treated without reimbursement as early as August, Novartis Korea is considering introducing a patient program to address the high price of the drug. For reference, the recommended dose of Pluvicto is 7.4 GBq (200 mCi), administered intravenously up to 6 times every 6 weeks (±1 week), and it is expected that tens of millions of won will be spent per dose without reimbursement in clinical sites. "From the HCP’s point of view, we believe the introduction of Pluvicto will have a positive impact in that it increases the number of weapons available for prostate cancer, and is a proven treatment with no significant side effects," said Dr. In-Keun Park, Professor of Oncology at Asan Medical Center. "The problem is that it is expensive and there are only a limited number of institutions that can perform PSMA PET-CT, rendering its administration equally limited." "Because it is a radioactive therapeutic agent, it requires a separate space for its administration rather than a general hospital room. Like Kymriah, Pluvicto is also produced through pre-orders," said a representative from Novartis Korea, "So it takes a considerable process to produce the drug in Europe and deliver it to patients in Korea." "We are reviewing the possibility of introducing a patient program. However, due to the nature of radioactive therapeutic agents, it is quite expensive. This is because it utilizes lutetium, a radioactive isotope," the representative added, "We are also discussing the possibility of applying for insurance reimbursement." With the use of Pluvicto nearing on-site, the competition among domestic pharmaceutical and biotech companies to develop radiopharmaceuticals is also heating up. Currently, FutureChem is at the forefront in the area. In mid-May, FutureChem began dosing its first patient in the U.S. for a Phase IIa clinical trial of FC705, a prostate cancer drug for castration-resistant metastatic patients. FC705 is a radiopharmaceutical that targets PSMA, which is overexpressed on the surface of prostate cancer cells. It kills cancer cells by introducing a therapeutic isotope into a peptide that binds to the PSMA protein. In a Phase I clinical trial, an objective response rate (ORR) and disease control rate (DCR) were confirmed in all patients who were administered FC705. In addition to the U.S. clinical trial, FutureChem is also conducting Phase II in Korea, including at Seoul St. Mary's Hospital, and is reportedly discussing technology transfer negotiations with China. In addition, AbTis, a subsidiary of Dong-A ST, is working with CellBion to develop a new radiopharmaceutical. The two companies signed a joint development agreement last month and will utilize AbTis’ linker platform technology AbClick and CellBion's radiopharmaceutical lab linker technology to develop a new Antibody-Radionuclide Conjugate (ARC) drug targeting stomach and pancreatic cancer. Recently, SK Biopharmaceuticalsentered into a license-in technology transfer agreement with Full-Life Technologies to acquire global development and commercialization rights to FL-091, a radiopharmaceutical candidate targeting neurotensin receptor 1 (NTSR1), from Full-Life Technologies. FL-091 small-molecule radioligand vector designed to deliver actinium-225 (225Ac), a next-generation radioisotope capable of killing cancer cells by selectively binding to NTSR1, a receptor protein, which is selectively overexpressed in various types of solid tumors, including colorectal cancer, prostate cancer, and pancreatic cancer. SK Biopharm has been discussing introducing radiopharmaceuticals into its pipeline since last year as the next step after its epilepsy drug cenobamate (U.S. brand name: Xcopri). The company acquired global-level Targeted Protein Degradation (TPD) technology through the acquisition of ProteoVant Sciences last year. The TPD technology seeks to overcome the limitations of existing therapeutics by degrading and removing target proteins and solving the causes of diseases. "This license agreement is the most concrete achievement we have made since the announcement of our entry into the field of radiopharmaceutical therapeutics last year," said Dong-hoon Lee, CEO of SK Biopharmaceuticals. "We plan to unveil a more specific business plan for the entire radiopharmaceutical business this year and accelerate clinical development and commercialization."
- Company
- Pfizer's MM drug Elerexfio seeks reimb in KOR
- by Eo, Yun-Ho Jul 29, 2024 05:48am
- Pfizer is attempting reimbursement listing of its new multiple myeloma drug ‘Elerexfio’ in Korea. According to industry sources, Pfizer Korea recently submitted a reimbursement application for its relapsed-refractory multiple myeloma (RRMM) treatment q (elranatamab). The company promptly started its listing process after receiving approval on May 30. Elerexfio previously received approval as the fourth drug to be designated as a Global Innovative products on Fast Track (GIFT) by the MFDS. GIFT is an accelerated review support system operated by the MFDS to promptly introduce highly innovative medicines, such as treatments for life-threatening or serious diseases, to the market and patients. Elerexfio is a subcutaneously delivered B-cell maturation antigen (BCMA)-CD3-directed bispecific antibody (BsAb) immunotherapy used to treat patients with relapsed or refractory multiple myeloma (RRMM). BCMA is commonly expressed in multiple myeloma patients, selectively expressed in plasma cells, and overexpressed in myeloma cells. Elerexfio binds to BCMA on myeloma cells and CD3 on T-cells to enhance the immune response. The treatment is administered weekly from Week 2 through Week 24 of treatment following a step-up dosing schedule, with patients who achieve a response after at least 24 weeks of treatment switching to a 2-week interval beginning at Week 25. It is available in a single-dose vial for immediate outpatient administration and is administered by subcutaneous injection only. The approval was based on the global Phase II MagnetisMM-3 study, which included 123 patients who had never received a BCMA-targeted therapy. In patients with multiple myeloma who have already received at least 3 treatment regimens (including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody) and have no prior treatment experience with a BCMA-targeted therapy, Elerexfio achieved a primary endpoint objective response rate (ORR) of 61.0% in patients, with 56.1% of responders achieving a very good partial response (VGPR) or better. In responders, the median time to response (TTR) was 1.2 months, and the median time to complete response (CR) was 6.1 months. In addition, the probability of maintaining ≥CR at 9 months was 89.0%, with a progression-free survival of 50.9% at 15 months. Meanwhile, according to the World Health Organization's ‘Global Cancer Observatory’ report that was published in 2022, approximately 187,000 new cases of multiple myeloma were newly diagnosed worldwide. Multiple myeloma is the second most common type of blood cancer, with a 5-year relative survival rate of 50.1% in Korea in 2021. According to the annual report of the National Cancer Registry, 2018 cases were diagnosed in Korea in 2021.
- Company
- Eylea biosimilar 'Afilivu' will be prescribed
- by Eo, Yun-Ho Jul 29, 2024 05:48am
- Product photo of Samsung Bioepis Samsung Bioepis' Eylea biosimilar 'Afilivu' is becoming available for prescription at general hospitals. Industry sources said that Afilivu (aflibercept), a macular degeneration treatment, has passed the drug committees (DC) of medical centers, including Seoul National University Hospital and Seoul National University Bundang Hospital. Afilivu is a biosimilar referencing Eylea, a blockbuster product generating KRW 12 trillion in global sales. In February, it obtained approval from the Ministry of Food and Drug Safety (MFDS), and in May, it received approval from the FDA under the product name of 'Opuviz.' Afilivu's active ingredient, aflibercept, works by inhibiting vascular endothelial growth factor (VEGF), preventing abnormal blood vessel growth in the eye. By blocking VEGF, Aflibercept is used to treat macular maculation, slowing macular damage and preserving vision. Macular degeneration is an eye disease that occurs due to aging and inflammation of the macula, located in the central region of the retina. Samil Pharmaceutical, which signed an exclusive domestic distribution and sales agreement with Samsung Bioepis, officially launched 'Afilivu' on May 1st. Within the first month of its launch, the drug reached sales of KRW 1 billion, achieving significant success. Meanwhile, Samsung Bioepis conducted phase 3 trials for Eylea. From June 2020 to March 2022, the trial enrolled 449 patients with Neovascular Age-related Macular Degeneration (nAMD) from 10 countries, including the United States and South Korea. The final data from the phase 3 trial were presented at the Association for Research in Vision and Ophthalmology (ARVO) conference in April last year. The trial confirmed the clinical equivalence of the drug to the original medicine, including its efficacy and safety.
- Company
- GC accelerates global entry…targets China and Vietnam
- by Her, sung-kyu Jul 29, 2024 05:48am
- The GC group accelerating its global market entry after exporting Alyglo to the U.S., seeking to expand its business to China and Vietnam. In particular, it chose to cooperate with China's CR Pharmaceutical Group after selling its Chinese subsidiary, and the company is also seeking to enter Southeast Asia by collaborating with local companies in Vietnam. #On the 19th, GC (Green Cross Holdings) announced that it would enter the Vietnamese healthcare market in cooperation with Phenikaa Group. The plan is for GC Labs to build a diagnostic laboratory using the organization's long-standing know-how, and GC iMED, a comprehensive health checkup agency, to establish a premium health checkup center for the Vietnamese middle-and-upper class. In addition, GC plans to expand into the Southeast Asian market after entering the Vietnamese healthcare market. The entry into the Vietnamese market is particularly noteworthy because it demonstrates the company's plan for rapid global expansion following its entry into the U.S. market. Along with the U.S. market, GC Biopharma is accelerating its expansion into markets such as China and Vietnam in cooperation with local companies. The company obtained U.S. FDA's approval for its blood product Alyglo late last year. Alyglo is a 10% immunoglobulin liquid for intravenous administration used for primary humoral immunodeficiency (PI), also known as congenital immunodeficiency. In 2020, the company conducted a Phase III clinical trial in North America in patients with primary immunodeficiency and met the efficacy and safety endpoints in compliance with FDA guidelines, but due to delays in on-site inspections among others due to COVID-19, the company obtained approval late last year. Since then, GC Biopharma has continued its efforts with its U.S. subsidiary to register the drug on the formulary and made its first shipment to the U.S. on the 8th of this month. Along with this entry into the U.S. blood product market, GC has been expanding its global reach to China and other countries. First of all, the GC Group sold its Chinese subsidiaries, including the Hong Kong subsidiary, and is seeking to enter the Chinese market in cooperation with local companies. In fact, the company signed a stock purchase agreement (SPA) with CR Boya, a subsidiary of China’s state-owned CR Pharmaceutical Group, to sell all shares of its Hong Kong subsidiary. In addition, the company entered into a separate Distribution Agreement with CR to distribute GC Biopharma-GC Wellbeing's main products in China. In the process, GC secured approximately KRW 350 billion through the sale of 6 companies, including GC China, its wholly-owned subsidiary in China. Through the agreements, GC plans to enhance its financial health and utilize the funds to make strategic investments for future businesses. In addition, GC Biopharma is seeking to maximize the efficiency of its blood product production by exporting immunoglobulin, one of the main derivatives produced in the process of manufacturing its blood products, to the U.S., and albumin to China. The company’s strategy is interpreted as an attempt to reorganize its business and focus on its strengths. In other words, in addition to selling different products by country, the company is organizing its underperforming subsidiaries to increase efficiency while addressing concerns about the relationship between the U.S. and China. Currently, the US is in the process of enacting the Biosecure Act, which aims to restrict Chinese biotech companies from operating in the US. In this context, GC will continue to pursue business in the US through its subsidiaries, while targeting the Chinese markets through cooperation with local groups rather than entering the market on its own. In addition, the company’s plan to expand cooperation with local companies in Vietnam is also interpreted as a strategy to reduce its risk of entering the Southeast Asian market.
- Company
- Samsung Bioepis’ 3mth operating profit exceeds last year's
- by Chon, Seung-Hyun Jul 26, 2024 05:47am
- Samsung Bioepis posted the largest Q2 revenue in its history. The company recorded twice as much revenue as in its previous record, and its operating income surpassed what it earned in the entire year last year. This is due to the large influx of milestones, receiving a series of approvals for biosimilars in the U.S. and Europe. According to Samsung BioLogics on the 25th, Samsung Bioepis posted revenue of KRW 529.9 billion in Q2, up 107.1% from the KRW 259.9 billion a year earlier, and an operating profit of KRW 257.1 billion, up more than six times from the KRW 41.9 billion a year earlier. Both revenue and operating profit are the largest ever. Samsung Bioepis' Q2 revenue was 89.2% higher than the company’s previous record of KRW 288.9 billion it had made in Q4 last year. Operating profit more than doubled from the KRW 101.5 billion it had recorded in Q3 2021. In just 3 months, the company posted more operating profit than the KRW 205.4 billion it earned in the entire year last year. As a result, the company’s operating profit margin in Q2 reached 48.5%. Samsung Bioepis Smooth sales of biosimilars overseas, and new biosimilar approvals in the U.S. and Europe brought in large milestones. Samsung Bioepis received approval for a total of 3 biosimilars in Europe and the U.S. in Q2 this year. Last month, Samsung Bioepis received marketing authorization from the European Commission for the Stelara biosimilar Pyzchiva. Stelara is an autoimmune disease treatment developed by Janssen that is prescribed for plaque psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis. It inhibits the activity of interleukin (IL)-12,23, a class of inflammatory cytokines involved in the immune response. The drug has made annual global sales of KRW 14 trillion. Samsung Bioepis received marketing authorization from the U.S. Food and Drug Administration (FDA) to sell Pyzchiva in the U.S. In May, Samsung Bioepis received approval from the U.S. Food and Drug Administration (FDA) for Opuviz, a biosimilar of the macular degeneration treatment Eylea. Eylea, which was developed by Regeneron, has indications for wet (neovascular) age-related macular degeneration. Eylea generated global sales of approximately KRW 13 trillion last year. Sandoz has been marketing the newly approved Stelara biosimilar in the U.S. and Europe. The Eylea biosimilar is marketed by Biogen. The new approvals in Europe and the U.S. have led to an influx of milestone payments from its partners. Considering how Samsung Bioepis' Q2 sales and operating income increased by KRW 249.8 billion and KRW 219 billion from the previous quarter, respectively, the milestone inflow in Q2 is expected to have been around KRW 200 billion. View of the Samsung Bioepis building Sales of Samsung Bioepis' existing biosimilar products also continued to grow steadily. Samsung Bioepis has received approval for 8 biosimilars each in Europe and the U.S. In Europe, Samsung Bioepis has biosimilars of Enbrel, Remicade, Humira, Herceptin, Avastin, Lucentis, Soliris, Stelara. In the U.S., the company has neared commercialization of its Remicade, Herceptin, Enbrel, Humira, Lucentis, Eylea, Stelara, and Soliris biosimilars. Samsung Bioepis' licensed biosimilars are marketed by Biogen, Organon, and Sandoz in the global market. Biogen is in charge of 3 biosimilars of autoimmune disease treatments in Europe, including Enbrel, Remicade, and Humira. Biogen also markets Samsung Bioepis' Lucentis biosimilar in the U.S. market. Organon markets 3 biosimilars of Enbrel, Remicade, and Humira in the global market outside of Europe and South Korea. Organon also markets 3 autoimmune disease treatments in the U.S. and 2 anticancer drugs, Herceptin and Avastin biosimilars, outside of Europe and Korea.
- Company
- "Active viral hepatitis treatment can prevent carcinoma"
- by Son, Hyung-Min Jul 26, 2024 05:47am
- Maria Buti, Chair of Public Health at the European Association for the Study of the Liver (EASL) "Most individuals affected by viral hepatitis do not have symptoms. They may not be aware of their infection. When individuals realize that they have abnormalities in their body, liver disease has already progressed. It is important to identify and treat patients early in their disease progression to prevent complications related to hepatocellular carcinoma and liver disease." During a meeting with Daily Pharm, Maria Buti, Chair of Public Health at the European Association for the Study of the Liver (EASL), emphasized the importance of early treatment for hepatitis B and hepatitis C. Hepatitis B is caused by hepatitis B virus (HBV). It can cause infection, advanced liver injury, and chronic liver disease. Hepatitis B is implicated in the cause of hepatocellular carcinoma by over 60%. Recently, there has been a discussion about maximizing the preventative effect of hepatocellular carcinoma by early treatment of hepatitis B. Vemlidy is indicated for treatment. Compared to the conventional use of TDF (tenofovir disoproxil fumarate), such as Viread, Vemlidy has been shown to reduce the occurrence of hepatocellular carcinoma by half. Hepatitis C is identified as contributing to the occurrence of hepatocellular carcinoma in 10-15% of the patients. However, hepatitis C is now curable with the introduction of Epclusa. There are various genotypes of hepatitis C, and Epclusa is highly effective in most patients, regardless of their genotypes. Buti emphasized the timely use of treatments to prevent hepatocellular carcinoma now that effective new drugs are available. Vemlidy has been shown to be effective in preventing hepatocellular carcinoma Because Hepatitis B is incurable, individuals must take medicines their whole lives, but treatments for managing the virus are available in the market. Vemlidy, a type of TAF (tenofovir alafenamide), Vemlidy, and Baraclude are used to treat hepattiis B. Currently, the drugs used in clinical practice are known to suppress viral load significantly. These treatments can suppress the virus to an undetectable level in tests, even though the virus continues to attempt replication. "Antiviral drugs such as Vemlidy, Viread, and Baraclude can help prevent liver decompensation in patients," Buti said. "However, ensuring the safety of drugs is increasingly important due to their long-term use." Vemlidy is also suitable for use in pregnant women and has the advantage of not requiring dose adjustment based on the patient's renal function status. The 8-year follow-up clinical trials, 'Studies 108 & 110,' for Vemlidy conducted on both treatment-naive and treatment-experienced patients, confirmed 5-year viral suppression rates and liver cancer prevention effects. Out of the 1,298 patients involved in the study, there were no instances of decompensated hepatocirrhosis reported in the Vemlidy-treated group. Moreover, during the 8-year period, there were 21 cases (1.6%) of hepatocellular carcinoma in the Vemlidy-treated group. There were also no documented cases of drug resistance among patients treated with Vemlidy. In a study evaluating the safety of Vemlidy in patients with severe kidney dysfunction and kidney diseases, no additional adverse reactions were observed. "If active treatment with drugs like Vemlidy is initiated early during hepatitis B infection, favorable outcomes can be achieved," Buti said. "Additionally, it has been shown that Vemlidy can prevent the occurrence of hepatocellular carcinoma and other complications. Currently, treatment trends focus on the early use of TAF-based drugs such as Vemlidy." Buti added, "Korean treatment guidelines, such as those from the Korean Association for the Study of the Liver, have stricter restrictions on the use of drugs compared to European guidelines. In Europe, the criteria for initiating treatment, such as HBV DNA levels or liver function tests, are relatively lower, allowing for earlier treatment initiation." Buti said, "To effectively treat hepatitis B, starting treatment as early as possible for as many patients as possible is crucial. It is necessary to compare different guidelines to broaden the scope of treatment." Hepatitis C, without vaccines…drugs can be used for prevention Hepatitis C has a lower risk of hepatocellular carcinoma compared to hepatitis B, but if left untreated, it can lead to liver cancer or severe liver disease. Hepatitis C is considered curable if detected early and treated with drugs on time. Epclusa has made it possible to treat hepatitis C regardless of genotype or liver cirrhosis status. Buti emphasized, "Hepatitis C has various genotypes, but Epclusa is effective regardless of genotype. Epclusa can be administered orally once daily, making treatment easier for patients." Patient screening is important for Hepatitis B since it's curable, according to Buti. "Spain was the first country in the world to achieve hepatitis C elimination. A key factor in this success was the micro-elimination strategy. While definitions of high-risk groups may vary by country, in Spain, high-risk groups with high hepatitis C prevalence, such as injection drug users and patients with mental illnesses, were required to undergo screening whenever they visited the emergency room," Buti said. In Spain, individuals have participated in screening for hepatitis C, which has led to significant achievements. "Since there is no vaccine for hepatitis C, treatment is used as a preventive measure. From a public health perspective, this is a critical concept," Buti said. "Treating infected individuals to block further virus spread is a good approach to prevention." "When a patient is diagnosed with hepatitis C through screening, it is crucial to provide timely information about appropriate treatments, such as antiviral drugs, and ensure that the treatment is pursued. A diagnosis without follow-up treatment is meaningless. Therefore, linking diagnosis to treatment is crucial for achieving hepatitis C elimination," Buti added. "To prevent viral liver infection from progressing and patients suffering from side effects, early diagnosis of patients is crucial. The only way is to do a screening. This applies to both hepatitis B and hepatitis C," Buti emphasized.
- Company
- Mitsubishi accepts DREC results for Uplizna...nears reimb
- by Eo, Yun-Ho Jul 26, 2024 05:47am
- Mitsubishi Tanabe Pharma’s twice-yearly neuromyelitis optica spectrum disorder (NMOSD) drug Uplizna is headed for reimbursement listing in Korea. According to industry sources, Mitsubishi Tanabe Pharma’s Korea accepted the ‘below the evaluated amount’ condition set by the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee for the reimbursement of Uplizna (inebilizumab), a treatment used to treat adult patients with for neuromyelitis optica spectrum disorder (NMOSD) who are positive for anti-Aquaporin-4 (AQP4) antibodies. As a result, the company will enter pricing negotiations with the National Health Insurance Service in the near future. Uplizna is administered at an initial 300 mg dose, followed by an additional 300 mg dose 2 weeks later, and then every 6 months thereafter from the date of the initial dose. NMOSD occurs when AQP4 autoantibodies, a disease-specific biomarker produced by B cells, bind to AQP4, a target antigen present on glial cells in the central nervous system, and activate the immune responses, causing nerve damage. Uplizna is an anti-CD19 human monoclonal antibody that selectively binds to CD19, a B cell-specific surface antigen, depleting B cells that produce AQP4 antibodies, thereby preventing disease relapse. The safety and efficacy of Uplizna were demonstrated in the N-MOmentum study, which evaluated the use of Uplizna monotherapy in 230 patients without the use of concomitant immunosuppressive agents. Study results showed that 89% of patients treated with Uplizna did not experience a relapse during 197 days of follow-up, resulting in a 77.3% reduction in the risk of relapse compared to placebo. Safety evaluations Uplizna also showed comparable rates of adverse events to the placebo group. Also, in an extension study, Uplizna continued to reduce the risk of relapse for at least 4 years, with an 87.7% relapse-free rate. In terms of long-term safety profile, Uplizna was generally well tolerated, with no increase in infection rates due to B-cell depletion. NMOSD is a serious autoimmune disease in which most patients experience persistent relapses and incomplete recovery, resulting in accumulated nerve damage that can cause vision loss, gait disturbances, and even death from respiratory failure.
- Company
- Guidelines for new obesity drugs must be established
- by Moon, sung-ho Jul 26, 2024 05:46am
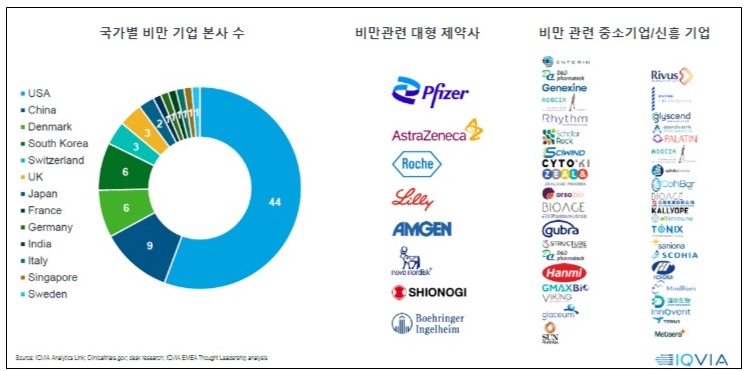
- Obesity is projected to impact around 2 billion individuals by 2035. Including overweight, about 4 billion individuals will be affected by obesity, making it a significant global healthcare issue. Following this trend, pharmaceutical and biotech industry is focusing on developing next-generation treatments for obesity. Glucagon-like peptide 1 (GLP-1) drugs, including Saxenda, Wegovy, and Zepbound, showed outstanding weight loss effects in clinical trials. As a result, companies are developing GLP-1-based therapeutics. The number of companies developing obesity drugs by country; big pharmas developing obesity drugs; small businesses and new companies developing obesity drugs. Recently, these drugs have been found to provide cardiovascular benefits and aid weight loss effects. As a result, their expanded use has been gathering attention in clinical settings. The analysis suggests that it's time to consider using these drugs in clinical settings in South Korea. According to drug market research company IQVIA on July 20th, there are 79 obesity drug pipelines worldwide, from preclinical to launched products. Pharmaceutical and biotech companies have developed over 148 products. GLP-1 drugs account for 39% of the pipelines. This suggests that pharmaceutical and biotech companies have begun developing drugs in this class as latecomers after witnessing the success of Saxenda, Wegovy, and Zepbound. 79 obesity drug pipelines worldwide, from preclinical to launched products, and 148 products from pharmaceutical and biotech companies have been developed. The analysis indicates that major companies that have begun developing obesity drugs employ two major development strategies. They either develop a drug as 'monotherapy' based on their differentiation strategy, or consider potential expansion for treating obesity, type 2 diabetes, cardiovascular diseases, and metabolic dysfunction-associated steatohepatitis (MASH) as part of their portfolio strategy. As part of differentiation therapy, monotherapy is being developed to achieve the ▲Highest weight loss rate, ▲Improved safety, and ▲Chronic disease management with oral formulation. For instance, Pfizer and Viking Therapeutics are developing candidate products. Under the portfolio strategy, the market leaders, such as Novo Nordisk (Wegovy) and Lily (Zepbound), and latecomers, aim to expand indications. Lately, the development of 'oral formulation drugs' has gained attention. These drugs are expected to shift the paradigm of a market dominated by injectables. Global companies, Pfizer and Viking Therapeutics, and domestic companies, including Ildong Pharmaceutical and D&D Pharmatech, have started to develop them. Novo Nordisk and Lily, the market-leading companies that already have injectables, have proprietary pipelines. However, Wegovy and Zepbound, which dominate the global market for obesity, have unresolved issue of weight loss rebound. IQVIA Korea's Marketing & Sales Director Kang-Bok Lee said, "Most obesity pipelines at the clinical stage are being developed as oral formulations." Lee added, "However, there are still discussions about oral obesity drugs. Along with the convenience, we must consider whether these drugs are suitable for chronic and maintenance management, and whether their cost and supply network outweigh any remaining issue." Lee added that "There are questions about whether it can have similar efficacy compared to injectables, as well as concerns about tolerability." Lee expressed optimism about oral drug development, saying, "Recently, Viking's oral drug, VK2735, had no clinically significant gastrointestinal side effects compared to placebo, with most being mild." Reflecting on the global trend for obesity drug development, new GLP-1 drugs, such as Wegovy and Zepbound, will likely be introduced to South Korea. According to IQVIA, the market for obesity drugs is rapidly growing after the launch of Wegovy. The worldwide market size in 2023 totaled US$11 billion (about KRW 15.3 trillion), driven by Wegovy. Wegovy contributed 72% of the total US$11 billion-worth market in 2023. In contrast, in South Korea, the release of Wegovy has been delayed due to an issue with 'securing stock' after obtaining marketing approval. As a result, Saxenda (Novo Nordisk) and Qsymia (Alvogen Korea) have taken 60% of the market share, dominating the market. Sources said that the release of Wegovy is set to be released in the Korean market, making it the ninth country globally. A professor from the Department of Endocrinology at an unnamed University Hospital, who is also an executive member of the Korean Society for the Study of Obesity, said, "Following Japan, China has also approved Wegovy. Since an official launch date has not yet been set, it is difficult to guarantee the timing of the release." He analyzed, "This appears to reflect the position of the domestic market within the global market." "Even if it is released, it seems that obesity drugs will be used entirely as non-reimbursable in the domestic market," He added. "While there has been some progress in recognizing obesity as a disease, reimbursement coverage, especially concerning domestic insurance finances, will not be easy." As a result, the pharmaceutical and biotech industries have proposed that, considering the potential of the drug market due to the rising prevalence of obesity, there is a need to accelerate discussions on disease recognition, enhancements in social awareness, and the establishment of clinical practice guidelines and insurance. At the same time, domestic pharmaceutical and biotech companies developing treatments may need to focus on differentiation strategies from competing products, improving efficacy such as preventing weight regain after discontinuation and establishing a stable supply chain to meet global demand. IQVIA Korea's Director Kang-Bok Lee said, "In the past two years, global spending on obesity has increased rapidly with new drugs, and by 2030, more than 15 new items are expected to enter the market, making the next-generation obesity treatment market much more competitive." Lee also said, "Improving educational programs for healthcare professionals to raise awareness of obesity treatment and integrating it into chronic disease management would be an ideal approach." Lee also added, "Currently, even though obesity treatments are approved by the Ministry of Food and Drug Safety (MFDS), there are no cases where these drugs are covered by reimbursement. In the U.S., Medicare (Part D) will now cover Wegovy for some patients with a history of heart disease, as announced by the Centers for Medicare & Medicaid Services (CMS)." Lee added, "In the future, obesity drugs will be divided into reimbursed and out-of-pocket markets, so it is necessary to consider establishing reimbursement criteria for patients with severe obesity or accompanying diseases. We must develop and distribute comprehensive obesity treatment guidelines through collaboration with the medical community and organizations."
- Company
- Antibiotic prescriptions had surged with the pandemic
- by Kim, Jin-Gu Jul 25, 2024 05:51am
- The amount of outpatient antibiotic prescriptions in Korea had changed dramatically during the COVID-19 pandemic. In 2020 and 2021, early stages of the pandemic, antibiotic prescriptions dropped sharply but then surged in 2022. In contrast, the proportion of cephalosporin and quinolone antibiotic prescriptions, which are more powerful than other antibiotics, increased in 2020 and 2021 and then decreased in 2022. The Ministry of Health and Welfare released the "Healthcare Quality Statistics" that contained the above findings on the 24th. The MOHW releases this data annually for comparative statistics among OECD countries. One of the statistical items examines outpatient antibiotic prescriptions in primary care clinics. According to the statistics, in 2022, outpatient prescriptions of local antibiotics, not systemic antibiotics amounted to 21.3DDD per 1000 inhabitants in primary healthcare centers in Korea. Defined Daily Dosage (DDD) is a unit used to measure drug consumption and refers to the average maintenance dose that an adult weighing 70 kg should take per day. Total outpatient antibiotics prescribed for systemic use (Source: Healthcare Quality Statistics 2022) The number of antibiotic prescriptions in Korea has been steadily decreasing since 2016 when it reached 26.9 DDD. Especially during the pandemic, the number dropped below 20.0 DDD in 2020-2021. It is analyzed that as people refrained from outdoor activities due to social distancing measures, respiratory infections decreased, reducing antibiotic prescriptions. In 2022, when the social distancing measures were eased, antibiotic prescriptions made a rebound. From 16.0 DDDs in 2021 to 21.3 DDDs in 2022, the amount increased 33% in 1 year. In contrast, prescriptions for cephalosporin and quinolone antibiotics, which are broader and more potent than other antibiotics, increased significantly early in the pandemic and then decreased near the endemic. Among all outpatient antibiotic prescriptions, the percentage of cephalosporin and quinolone antibiotics increased from 39.5% in 2019 to 43.6% in 2020, then to 44.8% in 2021. In 2022, their share decreased slightly to 43.1%. Percentage of cephalosporin and quinolone antibiotics prescribed (Healthcare Quality Statistics 2022) The prescription and use of antibiotics require management due to resistance issues and is considered one of the important areas to monitor through national antimicrobial resistance management policies. The OECD Healthcare Quality Statistics also includes two indicators related to antibiotics: the total number of outpatient antibiotics prescribed for systemic use and the proportion of cephalosporin/quinolone antibiotics prescribed. The MOHW explained, "The total volume of outpatient antibiotic prescriptions decreased by 34% in 10 years since 2011 due to the strengthening of antibiotic management policies and increased public awareness. However, the proportion of cephalosporin/quinolone antibiotics prescribed for systemic use has continued to increase over the decade and remains high at 43% as of 2022."