- LOGIN
- MemberShip
- 2025-12-19 12:11:15
- Policy
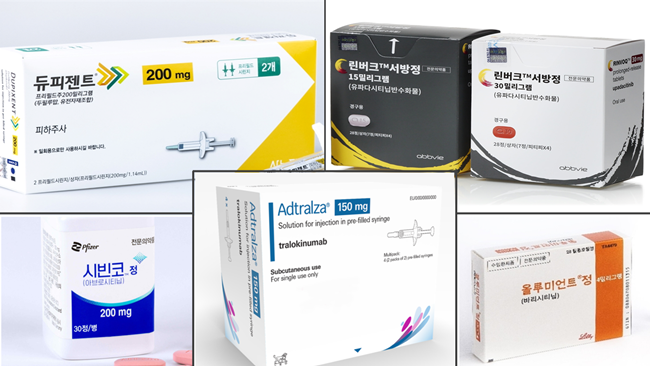
- Drug switching for atopic dermatitis measure submitted
- by Lee, Tak-Sun Nov 07, 2024 05:47am
- Pharmaceutical companies have submitted their measures for voluntary drug price reduction as part of financial allotment following expanded reimbursement. Discussions about allowing drug switching between biological agents and JAK inhibitors used to treat severe atopic dermatitis are speeding up. The Health Insurance Review and Assessment Service (HIRA) has already prepared the reimbursement criteria, and pharmaceutical companies have submitted their measures for voluntary drug price reduction as part of financial allotment following expanded reimbursement. Attention is drawn to whether it will pass the Drug Reimbursement Evaluation Committee (DREC) review, the final stage for reviewing reimbursement appropriateness. According to industry sources on November 6th, pharmaceutical companies Pfizer for Cibinqo and Lily for Olumiant submitted their financial impact analysis to the HIRA. The drug pricing reduction system for expanded scope of use requires the submission of financial impact analysis reports. The government excludes drug price reduction when the estimated additional claim amount following the expanded scope of use is below KRW 1.5 billion. The drug price reduction is applied for products over KRW 1.5 billion and below KRW 10 billion. In this case, pharmaceutical companies are required to submit a financial impact analysis, including measures to voluntarily lower prices, to the HIRA. In mid-October, the HIRA required each pharmaceutical company to submit financial impact analysis. Earlier this month, pharmaceutical companies have started to submit their reports. The issue of allowing drug switching for the treatment of severe atopic dermatitis was dealt with in the parliamentary audit held last month, so the health and welfare authority appears to be rushing the measure. The HIRA has been discussing with experts since September about whether to allow drug switching between biological agents and JAK inhibitors. The reimbursement criteria have been prepared, reflecting the latest evidence and clinical application. The remaining step is to minimize the financial impact following the expanded scope of use. Pharmaceutical companies' percentage of voluntary drug price reduction is the key. As pharmaceutical companies have submitted their measures for voluntary price reduction, the the Drug Reimbursement Evaluation Committee (DREC) review of the HIRA will make a final decision. The 11th DREC review is scheduled for July 2024. Attention is drawn to whether drug switching for severe atopic dermatitis will be considered for the agenda. When it passes the DREC review, the final reimbursement expansion will be decided through negotiation with the National Health Insurance Service (NHIS). Meanwhile, treatments for severe atopic dermatitis have been listed for reimbursement, starting with the biological agent, 'Dupixent Inj,' approved in January 2020. It was followed by the JAK inhibitors, Olumniant Tab, Linvoq ER Tab, and Cibinqo Tab. In May, another biological agent, Adtralza PFS, was listed for reimbursement. As a result, patients have more drug options. However, reimbursement and special cases do not cover drug switching between biological agents and JAK inhibitors, so it remains an issue. When clinical practices initially use high-cost drugs, and then the effects seen are not apparent, they may continue administering first-line treatments. Therefore, the medical community has been demanding the allowance of drug switching.
- Policy
- Novartis cuts price of Cosentyx after PVA negotiations
- by Lee, Tak-Sun Nov 07, 2024 05:46am
- Cosentyx UnoReady Pen The price of Novartis’s Cosentyx(secukinumab) is set to be reduced due to its increased use. This is because the National Health Insurance Service recently reached an agreement during Price-Volume Agreement negotiations with the company for Cosentyx. According to industry sources on the 6th, the insurance price ceiling of Cosentyx was adjusted for the second time under the PVA system after 2021. The affected products are the Cosentyx SensoReady Pen and Cosentyx UnoReady Pen. Cosentyx was listed for reimbursement in Korea in August 2017. Currently reimbursed indications include ▲chronic severe plaque psoriasis, ▲active and progressive psoriatic arthritis, and ▲severe active ankylosing spondylitis. According to the market research institution IQVIA, Cosentyx SensorReady Pen has seen an increase in sales every year since its reimbursement. In 2019, sales reached KRW 12.3 billion, then KRW 19.9 billion in 2020, KRW 25.1 billion in 2021, KRW 30.3 billion in 2022, and KRW 31.7 billion in 2023. In addition, Cosentyx UnoReady Pen, a high-dose product that was launched last year, generated sales of KRW 4.5 billion. Due to such high demand, the insurance price ceiling of Cosentyx had been adjusted in 2021 under the PVA system. At the time, the price of Cosentyx Sensoready Pen dropped by 7.3% from KRW 682,938 to KRW 630,084. At the time, 'Type A’ of the PVA negotiations was applied. Type A negotiations are applied to listed new drugs whose claims in the same product category have increased by more than 30% from the expected claims amount. In 2020, the drug had already well exceeded the expected claims amount. Three years have passed, and this year, ‘Type B’ PVA negotiations were applied. Type B negotiations are applied when the claims for the same product group, whose insurance price ceiling had been adjusted by Type A negotiations, or four years passed after the date of initial listing or the date the price ceiling was adjusted through negotiations, have increased by 60% or more than the previous year's claims, or by 10% or more but the increase is more than KRW 5 billion. Since Cosentyx had its price ceiling adjusted through Type A negotiations, it is possible that the additional negotiation for price adjustments was ordered because its claims increased by more than 60% or more than KRW 5 billion over the previous year's claims. Given that Cosentyx Sensoready’s sales grew 5% YoY in 2023 by KRW 1.4 billion (IQVIA), the introduction of Cosentyx UnoReady Pen, which falls in the same product group, may have triggered the PVA negotiation. Combined, the increase in sales amounted to more than KRW 5 billion compared to the previous year. Upon the agreement, the final price of Cosentyx is expected to be announced after deliberation by the Ministry of Health and Welfare’s Health Insurance Policy Review Committee.
- Policy
- 'Eutropin·Biktarvy' price drop amid sales hike
- by Lee, Tak-Sun Nov 06, 2024 05:52am
- Product photos of Eutropin and Biktarvy. Due to the increased volume of usage, drug pricing for 'Eutropin Inj (somatropin, LG Chem)' and 'Biktarvy (Bictegravir/Emtricitabine/Tenofovir Alafenamide, Gilead Science),' which recently showed a sales hike, is expected to be reduced. Sources said on November 5th that the Health Insurance Review and Assessment Service (HIRA) recently agreed on drug pricing adjustment of these products through the 'Type-Na' negotiation. As a result, the final adjusted prices will be listed on the reimbursement list following a review from the Health Insurance Policy Review Committee. Eutropin has been a top-selling growth hormone drug in the Korean market. 'Eutropin S Pen,' introduced in April 2022, is skyrocketing in sales. Eutropin S Pen's expiration date has been extended from 18 months to 24 months, and, based on UBIST, it generated sales of KRW 81.5 billion last year. Eutropin recorded KRW 21.3 billion in sales in the same period. The combined sales growth rate for the two products is 24% compared to last year. Biktarvy also successfully achieved two-digit sales figures. Based on IQVIA last year, it generated sales of KRW 54.5 billion, up 11% from the previous year (KRW 49.1 billion). Biktarvy received the MFDS approval in January 2019, and it has been in sales following reimbursement listing in July. It is a single-capsule combination drug for the treatment of HIV containing three active ingredients: Bictegravir, Emtricitabine, and Tenofovir alafenamide. With Yuhan and Gilead Sciences in a joint-sales agreement, Biktarvy is a top-selling drug in the HIV market. "Eutropin and Biktarvy show rapid growth in the market," an expert in the pharmaceutical industry said. "With the increased usage volume, reducing its ceiling price according to the Price-Volume Agreement (PVA) is inevitable." In 2022, the ceiling prices of Eutropin and Biktarvy were lowered due to the PVA agreement. Eutropin was reduced by 2.9%, and Biktarvy was reduced by 3.0%. Meanwhile, the negotiation of the PVA 'Type-Na' is conducted when the ceiling price is adjusted by the type-Ga price or when the bill amount of the same product has increased by more than 60% or 10% from the previous year's bill, and the increase is more than KRW 5 billion.
- Policy
- How will the new drug review process change with fee hike?
- by Lee, Hye-Kyung Nov 06, 2024 05:52am
- The Ministry of Food and Drug Safety (MFDS) announced plans to raise the fee for new drug approvals to KRW 410 million from January 1 next year. The agency has prepared the approval and review process to implement the plan and has begun collecting industry opinion. According to MFDS’s press corp coverage, the MFDS recently delivered a revised version of the “Operating Procedures for Approval and Examination of New Drug Products (Guideline for public officials)” to the pharmaceutical and biotech industries and relevant associations. The guidelines contain specific measures to implement the “Innovative Measures for New Drug Approval” that was announced by the MFDS on Sept. 9, including the operation of a dedicated review team for each product and prioritizing GMP-GCP on-site inspections. “Upon the announcement of the rise in the approval fee of new drugs under the Innovative Plan for New Drug Approval, the pharmaceutical industry raised the question of whether the actual approval period will be shortened,” said an MFDS official. ”By recruiting reviewers, GMP inspections will be prioritized to be completed within 90 days from the date of the application receipt, and pre-registration of supplementary materials and explanatory meetings will reduce unnecessary delays in the approval process, dramatically shortening the approval period for new drugs from 420 days to 295 days.” The enactment of the Guideline on Operating Procedures for New Drug Approval and Review was promoted in line with the redetermination of new drug approval fees, which will take effect on January 1, 2025, and includes various changes to differentiate it from the existing approval process, such as the formation of a dedicated team, the establishment of explanatory meetings, and the introduction of a procedure for pre-registration of supplementary materials. From next year, when a new drug approval application is filed, MFDS will conduct an independent preliminary review within 7 days. During the preliminary review period, 80% of the fee will be refundable, and the MFDS will check the submission requirements. If there are any missing materials during this process, the applicant will have 14 days to submit them before an “initiation meeting” is held. Given the overall review timeline, the preliminary review process is crucial as data not submitted by the initiation meeting will be difficult to submit later and the application will be categorized as an application that requires primary supplementary data. A key part of this process is the establishment of a dedicated team for each item before the initiation meeting. The head of the approval division will lead the team, and a representative from the approval division will take charge as each item’s manager, and the team will be composed of representatives from ▲safety, efficacy, ▲quality control, ▲GMP (good manufacturing practice), and ▲GCP (good clinical practice). In addition, an initiation meeting is held within 14 days of receipt to coordinate the entire approval and review process and to provide the applicant with the necessary materials and procedures. The initiation meeting may be held face-to-face, via video, or in parallel, which is expected to increase the efficiency of the new drug review process. According to the proposed Operating Procedures for Approval and Examination of New Drug Products, GMP inspections will be conducted within 90 days from the date of application receipt, and GCP inspections will be conducted within 60 days after the first supplement. By accelerating the inspection schedule compared to the existing procedure, the verification required for the manufacturing and clinical management procedure will be completed at an earlier stage. This is intended to speed up the approval process and ensure adequate quality control. A number of improvements have also been made to the supplement requests. Previously, there were only first and second supplementary notifications, but the reform introduces a first supplementary request briefing session and a second supplementary request briefing session to ensure that the applicant fully understands the supplementary request. In particular, the explanatory meetings provide detailed information on the scope and requirements of the required materials and provide time to answer the applicant’s questions. In particular, for the first round of supplemental data submissions, a pre-registration process and a request for an information session can be made by the applicant. Applicants can pre-register their supplementary data and request the KFDA to hold an explanatory meeting. This allows the adequacy of the supplementary data to be reviewed in advance, reducing additional supplementary work and increasing the efficiency of the approval process. In addition, a final meeting will be held 5 days before the final approval target date to discuss the results of the approval review. “In addition to the effect of shortening the approval period, the revised procedure will improve the transparency of the approval and review process and help pharmaceutical and biotech companies develop new drugs efficiently while protecting the public's health,” said an MFDS official. ”We will collect opinions from relevant associations and industries to prepare the final draft and continue to optimize and implement the approval review service for the development of the domestic pharmaceutical industry.”
- Policy
- Reimb standards that prevent abuse of HA Eye Drops imminent
- by Lee, Tak-Sun Nov 05, 2024 05:45am
- Health authorities are expected to establish reimbursement standards to prevent the misuse of hyaluronic acid eye drops after failing to reach a conclusion during last year's reimbursement adequacy evaluations. It is reported that the Ministry of Health and Welfare is preparing an administrative notice to announce the reimbursement standards set by HIRA. HIRA’s reimbursement standard plan is expected to focus on the issue of abuse and misuse of HA eye drops and includes some restrictions on the number of uses and combinations. According to industry sources on the 3rd, HIRA’s proposed salary standards for HA eye drops are expected to be administratively notified soon. HA eye drops received reimbursement reevaluations in 2023, but no conclusion was made at the time. In September last year, during the first deliberation by the Drug Reimbursement Evaluation Committee, the committee determined that its use for “exogenous diseases such as postoperative, pharmaceutical, trauma, and contact lens wear” is not adequate for reimbursement. On the other hand, endogenous diseases such as Sjögren's syndrome, skin mucosal eye syndrome, and dry eye syndrome were deemed reimbursable, and it was concluded that it was necessary to set reimbursement standards such as single prescription per patient visit and total number of prescriptions per patient per year to encourage its appropriate use. However, opposition arose due to reasons such as weakened access and increased costs for elderly patients who use HA eye drops, and the issue was raised during the NA audit. As a result, DREC was unable to make a conclusion during its second deliberation and decided to review it further. At the MOHW’s Health Insurance Policy Review Committee meeting, which was held in December last year, the committee also said, “In the case of HA eye drops, we will make a final decision at a later date based on the evaluation results as it is necessary to consider setting the reimbursement standard for disposable eye drops as a whole in consideration of the users’ switch to other disposable eye drops.” So in the light of the new year, the health authorities reportedly came up with a reimbursement plan focused on preventing misuse. “It is expected that there will be some restrictions on the number of doses, combined use, etc.,” said a pharmaceutical industry insider, adding, ”The new reimbursement standards will have more restrictions on its use than now, but I understand that they are more aimed at preventing abuse.” After the first round of deliberations last year, there was a proposal to limit the number of boxes containing 60 individual vials to 4 per year. The pharmaceutical industry opposed the proposal, saying it was too restrictive. The current proposal, while still limiting the number, is said to be more eased than the 4 boxes per year. HIRA reportedly prepared a reimbursement standard and reported it to MOHW. The industry believes that MOHW will issue an administrative notice soon after a long discussion. HA eye drop prescriptions amount to around KRW 230 billion a year. They are widely used for eye diseases. Therefore, it will be interesting to see how the proposed reimbursement standard will affect the drug market.
- Policy
- Anticipating reimb for DPP4·SGLT2 combination drugs
- by Lee, Jeong-Hwan Nov 01, 2024 05:51am
- The government has announced a plan to address the necessity of the National Health Insurance reimbursement·expansion for two-drug combination drugs containing DPP-4 inhibitors and SGLT-2 inhibitors for the treatment of type 2 diabetes. In South Korea, reimbursement for combination drugs containing DPP-4 inhibitors and SGLT-2 inhibitors is only provided for three-drug combination therapy that involves an additional prescription of metformin. Approval of reimbursement for two-drug combination therapy, excluding metformin, is expected to offer more treatment options and convenience for diabetes patients while preventing unnecessary national health expenditures. On October 31, the Ministry of Health and Welfare (MOHW) and Health Insurance Review and Assessment Service (HIRA) responded to the question regarding the National Health Insurance coverage of type 2 diabetes treatments asked by Rep. Seo Miwha, a member of the People Power Party, during the parliamentary audit. Rep. Seo pointed out that reimbursement of combination drugs containing SGLT-2 inhibitors and DPP-4 inhibitors for treating diabetes must be approved to broaden treatment choices for diabetes. Rep. Seo stated that the current reimbursement for combination drugs is complicated for physicians to prescribe and restricts treatment choices for patients. Since May 2023, the scope of reimbursement for combination drugs containing DPP-4 inhibtors and SGLT-2 inhibitors prescribed to patients with type 2 diabetes has been expanded·changed so that it can be reimbursed when metformin is additionally prescribed. The problem is that the use of SGLT-2 inhibitors and DPP-4 inhibitors in combination or a combination drug is not covered by the National Health Insurance reimbursement. The current reimbursement policy is illogical for diabetes treatment because patients who can benefit simply from two-drug combination therapy are unnecessarily prescribed three-drug combination therapy because three-drug combination therapy is reimbursed, but two-drug combination drug are not. The HIRA responded to such criticism that they would review the need to expand the National Health Insurance reimbursement for a two-drug combination containing SGLT-2 inhibitors and DPP-4 inhibitors or a combination drug. "We will assess the safety·clinical utility of oral combination drugs for treating diabetes and communicate with related associations so that improved policy for two-drug combination drugs can be generalized," the MOHW said. "However, reimbursement expansion of diabetes drugs requires an impact on the National Health finance. We will discuss this with related associations." The HIRA explained the reason for the exclusion of two-drug combination drugs from the current reimbursement list, and they announced a plan to pursue improvements to the scope of reimbursement. "In 2018, we have written reimbursement criteria for the concurrent use of SGLT-2 inhibitors and DPP-4 inhibitors by discussion with an advisory committee. However, we retracted our stance that reimbursement of concurrent use by drug classes is appropriate and decided to maintain the current policy," the HIRA said. "After the previous discussion, we have checked the updated approvals from the Ministry of Food and Drug Safety (MFDS) and assessed the revision plan by comprehensively reviewing the latest resources and expert opinions, such as clinical practice guidelines, clinical research documents." "In addition to restricting the overall general policy for diabetes drugs, we plan to review issues related to reimbursement expansion for the combination therapy containing SGLT-2 inhibitors+DPP-4 inhibitors, prescription ease, and patient choices by discussing with related associations and experts," they added.
- Policy
- Hyundai withdraws application for Mifegymiso approval again
- by Lee, Hye-Kyung Nov 01, 2024 05:50am
- Hyundai Pharm has reportedly voluntarily withdrawn the application it had filed for the marketing authorization of Mifegymiso (mifepristone and misoprostol), which the company had applied for approval as the first abortion pill in Korea. Hyundai Pharm has requested approval for the abortion drug twice in the past, first in 2021 and then in 2023, but the Ministry of Food and Drug Safety has repeatedly asked for supplemental data, due to which the company has not been able to receive proper review. According to the MFDS’s written response to a written inquiry made during the NA Audit by the Health and Welfare Committee on the 31st, the approval and review process for Mifegymiso was recently temporarily suspended due to the company’s withdrawal. “Currently, there are some conditions that only be reviewed and approved after the Criminal Act and the Mother and Child Health Act is amended to allow drug-induced pregnancy termination and the permissible period of pregnancy termination are established by law,” said MFDS. In other words, if the Criminal Act and the Mother and Child Health Act are not amended, it would be difficult to review Mifegymiso’s effect/efficacy, and risk management plan, among others. The MFDS said, “Currently, the approval review process has been temporarily suspended (withdrawn) by mutual recognition of the applicant and the MFDS,” adding, “If the relevant laws are amended in the future and the applicant submits data accordingly, we plan to speed up the review and promptly make a decision.” Hyundai Pharm signed an exclusive marketing and distribution agreement for Mifegymiso’s supply in Korea with the UK-based Linepharma International in 2020, but the company has not been able to launch the drug in Korea due to disruptions in the approval process. Mifegymiso comes in a combination pack that contains 1 200mg mifepristone tablet and 4 misoprostol 200ug tablets. The drug inhibits the action of progesterone, the progestational hormone that sustains pregnancy, while misoprostol works to contract the uterus. It is only indicated for use in the first trimester of pregnancy, up to 9 weeks, and is typically prescribed as one tablet of mifepristone followed by 4 tablets of misoprostol 36-48 hours later. In April 2019, the Constitutional Court ruled the criminal offense of abortion (termination of pregnancy) unconstitutional, citing respect for women's right to bodily self-determination. As of January 1, 2021, abortion was effectively legalized. Upon the ruling, the Constitutional Court ordered the National Assembly to come up with an alternative legislation to reflect the decision, but 4 years have passed without such legislation being enacted. In the 21st National Assembly, 7 bills to abolish the abortion law were introduced, including amendments to the Criminal Code and the Mother and Child Health Act, but were discarded with the closure of the 21st National Assembly.
- Policy
- Seretide withdraws from Korea due to poor performance
- by Lee, Tak-Sun Oct 31, 2024 05:55am
- GSK's asthma inhaler 'Seretide' is withdrawing from the domestic market. Once the market leader in the market, it seems that the company is reorganizing the product line as its performance has recently declined due to the entry of new products. According to industry sources on the 30th, GSK recently sent letters to long-term care organizations and distributors to notify them of the withdrawal of 6 seretide items and the removal of their reimbursement listing. The 6 currently licensed Seretide products are Seretide 100 Diskus, Seretide 250 Diskus, Seretide 500 Diskus, Seretide 50 Evohaler, Seretide 125 Evohaler, and Seretide 250 Evohaler. Among those, 3 of the Seretide Evohaler formulations have already withdrawn from the market as of April 29th. The remaining 3 Discus formulations have also applied for withdrawal and are expected to be removed in the near future. “We have carefully considered the fact that sufficient alternatives to the Seretide product line already exist in the Korean market and have decided to withdraw the Seretide product line in Korea to simplify and concentrate our respiratory portfolio,” said GSK. The company said the expected date of the items’ reimbursement withdrawal is Dec. 1, with prescribing and reimbursement available for approximately 6 months until the end of May 2025. Seretide was once the leading asthma inhaler in South Korea, but it has been on a downward spiral in recent years due to the entry of its generics and new drugs. Sales of Seretide Diskus have fallen to KRW 12.2 billion in 2019, KRW 11.7 billion in 2020, KRW 9.1 billion in 2021, KRW 7.6 billion in 2022, then to KRW 1.7 billion last year, according to IQVIA. Seretide Evohaler also generated only KRW 800 million in sales last year. In addition to Seretide, GSK has other asthma-COPD inhaler products such as Arnuity, Anoro, Flixotide, Relvar, and Trelegy. In particular, sales of Relvar Elipta was KRW 36.2 billion and Trelegy Elipta was KRW 8.1 billion last year.
- Policy
- Gvnt agrees on the need for dispensing substitute drugs
- by Lee, Jeong-Hwan Oct 30, 2024 05:54am
- In this year's NA Audit, the Minister of Health and Welfare Kyoo-Hong Cho said that he would prioritize the ‘dispensing of substitute drugs’ as a solution to the problem of unstable supply of medicines such as cold medicines that are often out of stock. Two bills related to alternative dispensing were submitted to the National Assembly on the 28th, including a bill to amend the Pharmaceutical Affairs Act, which was introduced as representative by Rep. Byung-Duk Kim and Rep. Soo-Jin Lee of the Democratic Party of Korea. The bills aim to enable pharmacy pharmacists to substitute prescribed drugs with other generic versions that have undergone bioequivalence tests certified by the head of the Ministry of Food and Drug Safety as having the same ingredients, formulation, and dosage as the drug listed in the doctor's prescription. By clarifying whether or not a pharmacist should notify the doctor of the substitution, the bill is intended to promote information sharing among pharmacists and improve convenience in prescribing and dispensing medicines. Specifically, Rep. Byung-Duk Kim's bill expands the post-notification subject for the pharmacists’ dispensing of substitute drugs to the Health Insurance Review and Assessment Service, while Rep. Soo-Jin’s bill expands the notification to HIRA and changes to the term from “dispensing of substitute drugs” to “international nonproprietary name prescriptions.” The 22nd National Assembly is regarded as being more proactive in addressing the issue of the unstable supply of drugs by changing the substitute dispensing method, as it has introduced 2 bills to activate the practice early in its term, compared to the 1 bill submitted during the 21st National Assembly. In addition, Rep. Young-Seok Seo and Rep. Yoon Kim of the Democratic Party of Korea are also considering legislation to streamline the dispensing of substitute drugs and international nonproprietary name prescriptions, so there is a possibility that more related legislation will be introduced in the future. In addition, Minister Cho is expected to actively participate in the parliamentary review of relevant legislation, as he had cited the promotion of dispensing substitute drugs as a solution to the unstable supply and demand of drugs after the COVID-19 pandemic. However, opposition from the medical community is one barrier that must be overcome. Doctors argue that increasing substitute dispensing could adversely affect patient health, as different products with the same ingredients, whether original or generic, can have different clinical effects when taken by patients. Nevertheless, lawmakers who agree with the need to promote dispensing substitute drugs say that the legislation is essential to overcome the 5-year-long problem of unstable drug supply. Some doctors find it difficult to understand why the government would consider promoting dispensing substitute drugs, which can hinder public health when it is already operating an incentive system that provides incentives to pharmacies for dispensing low-cost substitute drugs. “We shouldn't have another case of stalled legislation due to inter-functional differences among professionals about dispensing substitute drugs,’ said an official from the Health and Welfare Committee, adding, “The problem of unstable drug supply and demand has been infringing on the public's access to medicines for several years now, deteriorating public health.” “Many Health and Welfare committee members are in agreement with the need for legislation, and some are considering expanding the scope of INN prescriptions,” said the official, “As Minister Cho answered in the NA Audit that he will prioritize INN prescriptions, we plan to focus on passing the legislation together with the MOHW.”
- Policy
- Low reimb approval rate hinders Soliris’s use for aHUS
- by Lee, Tak-Sun Oct 29, 2024 05:49am
- The industry’s eyes are on whether the preliminary reimbursement review process for Soliris, a treatment for the rare disease aHUS (atypical hemolytic uremic syndrome), will be eased in Korea. Until now, patients wishing to use Soliris for aHUS with reimbursement had to pass a preliminary review process. However, the problem is its low approval rate. With an approval rate in the 30-40% range every year, it has been a regular topic of discussion in the medical field and at the NA audit. This year, the issue was again raised during the NA audit, and attention has been drawn to the fact that HIRA expressed its intention to conduct a prospective review of the process. According to industry reports on the 27th, Rep Yoon Kim, a member of the Democratic Party of Korea, said to HIRA, “Soliri Inj, a treatment for atypical hemolytic uremic syndrome, has a very low approval rate at the preliminary reimbursement review committee process. If it cannot be used because of too strict standards, the standards need to be improved.” “As it is an acute disease, its use should not be bound by the preliminary review system, but should be started first, and the decision on whether to continue the treatment should be made afterwards.” aHUS is a severe, rare, hereditary disease where 79% of patients die within 3 years of onset, require dialysis, and suffer permanent renal failure. Without Soliris, it can be fatal. However, there are many cases of pre-approval rejections, rendering it difficult to treat patients in the field. However, HIRA has been citing the lack of required application data from long-term care institutions as the reason for the low approval rate, and that there was no problem with the reimbursement standards and review process. However, whether Rep. Yoon Kim’s criticism will improve the system is gaining attention. In its response to the NA’s written inquiry, HIRA said, ‘We will collect opinions from relevant societies on whether first allow the initial treatment with “Soliris Inj,” a drug for atypical hemolytic uremic syndrome, and then conduct preliminary review for further administration of the drug.” However, it seems that the authorities will keep the preliminary review system will be maintained. NA Rep. Jong-Heon Baek pointed out, “It seems inappropriate to operate the pre-review system for aHUS, as it is an acute rare disease. In other countries, considering the characteristics of acute rare diseases, they do not place them in the pre-review system or process the review very quickly. Korea is the only country that does not consider such characteristics of the disease.” To this, HIRA only responded, ‘Considering the nature of the disease, the drug can be administered immediately after submission of the pre-review application if it is an emergency case, and if approval follows, It is retroactively applied.” This suggests that the authorities are not considering plans to convert the preliminary review process to regular review. The implication is that they will consider exempting the initial dose only. In November, the government decided to switch the preliminary review process for the reimbursement of Soliris and Ultomiris for PNH (Paroxysmal nocturnal hemoglobinuria) to regular review. This is because the existing approval rate has remained stable at over 90%. Upon the change in their reimbursement process, there have been growing calls in the medical community for the removal of prior authorization aHUS in addition to PNH.