- LOGIN
- MemberShip
- 2026-03-10 00:45:05
- Product
- Illegal errand companies deliver Wegovy for ₩10,000
- by Jung, Heung-Jun Oct 22, 2024 05:52am
- Amid the craze for obesity treatment drug Wegovy, errand companies are also thriving by offering pharmacy proxy pick-up and delivery services. These are illegal businesses that take advantage of the fact that consumers are looking for a low-priced source of the non-reimbursed drug, as the price varies greatly depending on the pharmacy. There are posts on diet communities that claim to have purchased Wegovy at a low price in the KRW 400,000 range. Some people said that they called the pharmacy to check the price, while others said that they found a lower price through non-face-to-face treatment venues. Some people also wrote that they received Wegovy packaged in a cooler from another region. When the reporter checked with the pharmacy that offered Wegovy delivery, they were using an errand delivery service. The pharmacy representative said, ‘We don't offer delivery services for Wegovy,” but gave me the contact details of a courier service. They explained that the pharmacy has been contacted repeatedly due to the courier service’s promotion. Although the pharmacy does not deliver directly, they are tacitly allowing the act. “They charge KRW 10,000 for 1-2 pens and KRW 12,000 for 3-5 pens,” said A, who introduced himself as an errand-running company. If you use non-face-to-face treatment services, you can send the prescription to the pharmacy and then contact them for us to receive and deliver the medicine,” A explained. After submitting the prescription to the pharmacy, the customer can exchange the detailed address and account number for the Wegovy delivery through A. A also added a condition that the customer should leave a review on online cafes and communities. “I don’t make much with the deliveries. To make a profit, we need multiple delivery orders,’ said A, adding, ’Please leave your reviews online.’ Meanwhile, pharmacies are also focusing on stocking up and selling Wegovy in line with shifting attention from Saxenda to Wegovy. Low doses are selling out quickly. Lawmakers are also posting reviews of Wegovy use on social media to promote in-patient prescriptions. The selling price of the drug, which can be checked through non-face-to-face treatment websites, varied greatly on the first day of its release, but as of today (21st), the drug’s price is set in the KRW 500,000 range.
- Product
- Consumers use tricks to receive Wegovy prescriptions
- by Kang, Hye-Kyung Oct 22, 2024 05:51am
- With heating interest in the obesity drug Wegovy, the way-around measures the consumers are taking to receive prescriptions are causing controversy. From how to receive Wegovy through non-face-to-face treatment venues without the legwork to how to split the high-dose formulations being shared online, concerns are growing over the drug’s misuse. The first issue is non-qualified prescriptions. Prescriptions that ignore the prescribing criteria, such as obese patients with a body mass index (BMI) of 30 kg/m2 or more, are becoming widespread, and one of the outlets for such is the non-face-to-face treatment platforms. As influencers with tens of thousands to hundreds of thousands of followers are revealing the fact that they have been prescribed Wegovy, non-face-to-face treatment platforms such as ‘Dr Now’ and ‘My Doctor’ are being mentioned as venues for such prescriptions. You can receive a prescription for up to 5 pens of Wegovy through non-face-to-face treatment, and can also compare drug prices at pharmacies. The reason for choosing non-face-to-face treatment is that it is easier to receive prescriptions and dispense compared to face-to-face treatment. Although some prescribing medical institutions have been promoting Wegovy prescription through press releases, blogs, and social media including Instagram, hospitals and pharmacies have not yet been able to secure enough supplies, which is why patients are turning their attention to non-face-to-face treatment, which is relatively easier to receive prescriptions and dispensing. These platforms allow users to check the information, contact details, and prices of Wegovy in pharmacies with stock at a glance, making it easy to receive Wegovy without having to make many phone calls. Looking at the non-face-to-face treatment platforms, the price of Wegovy prescriptions by pen ranges from KRW 5,000 for 1 pen, KRW 7500 for 2 pens, KRW 10,000 for 3 pens, and KRW 10,000 to KRW 15,000 for 4 pens. For dispensing, a pharmacy in Gwangju had the lowest price in the country at KRW 419,000. In addition, some blogs and other websites have been sharing advice on how to get prescriptions easily, with tips such as ‘raising your weight.’ Another problem is the indiscriminate spread of information on its off-label use, such as ‘how to use Wegovy at half the price’. The correct way to start from the 0.25mg dose, then increase to 0.5mg, 1.0mg, 1.7mg, and 2.4mg over a four-week titration period, but many people have been prescribed 2.4mg and have been sharing tips such as splitting the dose into multiple doses. In fact, one doctor described it as ‘using the same drug at half the price’ and said, ‘I just use a 2.4mg syringe from the start. Then you can use a single syringe for four 0.25mg doses = 1/4 syringe, and four 0.5mg doses = 1/2 syringe, and so on.’ The argument is that since the price per dose is the same, it's more cost-effective to take one pen of 0.25mg over 4 doses than one pen of 0.5mg over 8 doses. It's also easier to stock the higher doses than the starter doses of 0.25mg and 0.5mg. Pharmacists are wary that their fears are being realized. Pharmacist A pointed out that “non-face-to-face treatment is promoting medical shopping in conjunction with the Wegovy craze. Shouldn't there be sanctions against random prescriptions and off-label use that are being conducted with non-face-to-face treatment?” Pharmacist B also said, “I am concerned that the Wegovy craze is spreading like a fad on social media without any warnings or side effects.” In particular, regarding dividing the high-dose formulation, B added, “Wegovy can be stored for up to 6 weeks after opening. If you split the dose, you'll be forced to take it beyond that period. I don't think I can give such advice from a professional perspective.” ‘The role of the government, pharmaceutical companies, and healthcare professionals will be crucial in tackling this Wegovy craze,’ he added. The Korea Pharmaceutical Association also said it plans to respond strongly to concerns such as the abuse of non-face-to-face Wegovy prescriptions and the delivery by documenting cases. Meanwhile, Novo Nordisk has warned patients in its patient instructions not to use Wegovy Prefilled Pen if they are hypersensitive (allergic) to semaglutide or any of the drug's excipients, or if they are pregnant, nursing mothers, etc., and emphasized patients to refrain from using the drug unless indicated.
- Product
- Wegovy's price varies by pharmacy in Korea
- by Jung, Heung-Jun Oct 17, 2024 05:51am
- The price of Wegovy, which has been attracting much attention since its launch in Korea, has been found to vary greatly, ranging from KRW 420,000 to KRW 800,000. Some pharmacists who have not yet decided on the selling price are referring to the prices of in-house prescriptions at hospitals and local pharmacies. The prices of Wegovy at pharmacies listed on some non-face-to-face treatment platforms this morning (16th) varied widely. The platforms disclose the prices of non-reimbursed drugs set by pharmacies, such as those for diet and hair loss, so viewers can check the prices of not only Wegovy but also Saxenda. Even pharmacies in the same district had large price differences, as identified through non-face-to-face treatment platforms While the price of Saxenda is somewhat established, varying by 5-10% among pharmacies, the price range of Wegovy, which is in its early stages of release, varies by over twofold among pharmacies. The selling price set by pharmacies varies from 420,000 won to 800,000 won. Most pharmacies in the Seoul metropolitan area set the selling price at over KRW 500,000. However, there are cases where the price varies by KRW 200,000 even in the same district, so the price is expected to be adjusted gradually after distribution begins in earnest. Saxenda, which was launched in Korea in 2018, was also initially priced differently by clinics and pharmacies but gradually stabilized to form a price range. A pharmacist in Seoul said, “There is a lot of talk in the pharmacist community about how much to charge. Hospitals are also sharing their selling price. We had to set the price, but it became difficult when the supply price was released in advance in the media. Large pharmacies or hard-to-reach areas may sell Wegovy at that level.” As hospitals showed much interest in the drug, receiving preorders for Wegovy before the launch, they are expected to market the drug in earnest after its supply. While there are expectations that the Saxenda craze may shift to Wegovy, its relatively high cost is also expected to raise some price resistance. It is expected that many people will call hospitals and pharmacies to check the selling price. Pharmacist B in Seoul said, “The price difference is inevitable because it is non-reimbursed and everyone is watching each other closely. I think it will take some time for the price to stabilize. Also, since consumers are aware of the supply price, many people will call hospitals and pharmacies to check their price.”
- Product
- ‘Disclose the pricing rationale used for COVID-19 drugs'
- by Kang, Hye-Kyung Oct 02, 2024 05:49am
- The Korean Pharmacists for Democratic Society (CEO Kyungrim Jeon, KPDS) has called for the disclosure of the cost-effectiveness evaluation results of the COVID-19 treatments Paxlovid and Veklury Inj. “The Ministry of Health and Welfare is engaging in the atrocity of setting excessive drug prices for COVID-19 treatments,” said the KPDS on the 30th, adding, “Much question remains on the clinical utility of current COVID-19 treatments among vaccinated elderly patients, and the KPDS criticizes the Ministry of Health and Welfare for setting an unreasonable price and the arbitrary co-payment rates, which only filling the bellies of pharmaceutical companies.” “In addition to filling the medical gap with national health insurance finances, the MOHW is now lining the back pockets of pharmaceutical companies,” criticized KPDS. “One course of Paxlovid and Veklury is priced at KRW 941,940 and KRW 312,000, respectively. In principle, the price of drugs is set based on clinical utility, taking into account the cost of existing treatments, but the cost of COVID-19 drugs is tens of times more expensive than drugs for similar diseases.’ Oseltamivir (brand name: Tamiflu), a treatment for influenza, a type of respiratory system infection, costs about KRW 17,000 per course, and zanamivir (brand name: Relenza Rotadisk) costs KRW 23,000. In addition, the influenza treatment PeramiFlu, which was not reimbursed in 2012 despite its high clinical utility but high price, is currently purchased by patients at KRW 100,000 to KRW 150,000. “According to the statutory infectious diseases classifications set by the Korea Disease Control and Prevention Agency, novel influenza is a Class 1 reportable disease. On the other hand, COVID-19 is not highly contagious or fatal - which is why it falls under Class 4. Why is the price of a Class 4 treatment drug set several times more expensive than the price applied for the common respiratory infectious disease treatments?,” questioned KPDS. He also demanded an explanation as to why cancer patients and those with severe, rare, and incurable diseases have to pay higher coinsurance rates than those infected with COVID-19. The claim follows the MOHW’s decision to limit the out-of-pocket costs for Paxlovid and remdesivir to KRW 50,000. “Considering the prices of the drugs - being KRW 940,000 and KRW 312,000 - the co-insurance rates of the drugs are 5% and 1.6%, respectively, which is the same or lower than the 5% co-insurance rate paid by patients with severe, rare and incurable diseases or cancer to purchase the drugs,’ the KPDS pointed out. “The amendment to the enforcement decree was made in response to the provision which stipulates the Minister of Health and Welfare can lower the co-insurance rate for infectious disease treatments to reduce the burden of infectious disease treatment and increase access, but the logic of the bill does not disallow lowering access to treatment for patients with severe, rare and difficult diseases and cancer,” he said. The general consensus is that the co-insurance rate needs to be lowered for all essential medical treatments to increase access. The KPDS pointed out, “The Ministry of Health and Welfare should not our requests for clarification while charging suspicious prices for treatments that are not yet clear in terms of reimbursement standards or proven effectiveness, and applying suspicious co-insurance rates to increase the burden on health insurance finances. The national health insurance finances are not the MOHW’s private finances.” ‘The MOHW should stop its recent rush of determining drug benefits at questionable prices in the name of rewarding innovative value. It must increase transparency in the drug price determination process and consider the public interest first in drug production and supply.”
- Product
- Zomig distributor changes from AZ to SK Chemicals
- by Kim JiEun Sep 25, 2024 05:49am
- Product photo of Zomig Tab.The distributor of Zomig Tab, a triptan used in the treatment of migraine, will change from AstraZeneca Korea to SK Chemical. The distribution industry suggests that the change in a distributor could impact the supply and demand chain. AstraZeneca Korea recently sent an official letter to the pharmaceutical wholesaler community of 'termination of a distribution agreement and changes to distributor' of Zomig Tab. The company stated in the official letter that starting on October 1st, due to the sales agreement, Zomig Tab 2.5 mg distributor will change from AstraZeneca Korea to SK Chemicals. Zomig Tab has been in shortage several times. The Ministry of Food and Drug Safety (MFDS) predicted drug shortages three times between last year and early this year. AstraZeneca reported MFDS that such supply shortages were due to laying in supplies and the company requested more supply volume to the manufacturer. The industry suggests that the current supply shortage may be partially due to AstraZeneca Korea's lack of domestic promotion of the drug after selling the global sales rights of Zomig Tab to another company. After officially learning about the Zomig Tab distributor change, the industry confirmed that an anticipated factor may have contributed to the supply shortages. The wholesaler community predicted that the distribution of the drug might be unstable for a while since the order requests have not been processed properly following the announcement related to the Zomig Tab distributor change. A wholesaler said, "Order request for Zomig Tab did not go through today, and we found that there has been a change to the distributor,' adding, 'Even if SK Chemicals take over the sales rights, distribution will be affected for a while. We think that the drug supply will be impacted for at least 15 days."
- Product
- Concerns arise about securing COVID-19 treatment shortages
- by Kang, Hye-Kyung Aug 14, 2024 05:51am
- COVID-19 is spreading due to the circulating KP.3 variant. We have not learned lessons from the previous spread of the Omicron variant. As demand for test kits surges, there are no remaining stocks at online pharmacies. Even test kits with an expiration date within the end of October are no longer available. Due to shortages of oral medicines, patients prescribed Paxlovid and Lagevrio visit one pharmacy after another or do not receive medicines. Now, the nation appears to be in chaos. Clinical practices are not aware of stock issues at the pharmacy, and pharmacies have no drugs. Local governments and the government are bombarded with inquiries. ◆"COVID-19 patients surged in a short period…prescriptions are made despite no drug availability"= The current situation is due to the surge in COVID-19 patients in a short period. After the announcement of the endemic, the number of patients showed a decreasing trend, but it has rapidly increased since July. Based on pharmacy data, an increased demand for COVID-19 self-test kits began on June 30th. According to the pharmacy data analytics service Care Insight (www.careinsight.co.kr), sales increased from ▲429 in June 30th-July 6th, ▲625 in July 7-13th, ▲1249 in July 14-20th, ▲2223 in July 21-27th, and ▲5850 in July 28th-August 3rd, doubling every week. A pharmacist 'A' in the metropolitan area said, "We have experienced an increase in COVID-19 patients since the end of July. We suspected it after seeing increasing demand for the test kits. The price for the test kits increased on demand from the end of July and the beginning of August, and now we are out of stock." The pharmacist added, "Even the remaining COVID-19 medicines sold out quickly." This pharmacy started receiving the distribution of COVID-19 medicines at the end of July. The pharmacist explained, "A public health center requested us to manage COVID-19 medicines tightly. Because we previously did not have that many number of patients, most pharmacies may not have a large quantity of stocks." The number of patients had been decreasing. When the medicines became charge-based, there were only two prescription cases within a month. The problem is that the number of patients is increasing but, there are not enough medicines. The Korea Disease Control and Prevention Agency (KDCA) expanded the distribution of medicines from once per week to twice per week, and the KDCA promised to distribute as much as pharmacies requested supplies. However, it is uncertain whether they can deliver this. This situation has arisen because we do not know how much COVID-19 medicines the KDCA has in stock. As the nation's demand for medcines keeps increasing, the supply requested from individual pharmacies is in short. Pharmacies that requested supplies from July 30th to August 5th have been rejected, and less than half of the stock is being delivered. A pharmacist 'B' in Seoul said, "We requested 96 drugs, but the quantity we received was 12, which we sold out of in 2 hours." The pharmacist added, "Other pharmacies within the same area are experiencing the same. There are cases where they requested but received none." As a result, the KDCA requested that prescriptions be made only for individuals over 60 years old with underlying diseases. The KDCA emphasized, "Please be advised to check with and prescribe COVID-19 medicines to high-risk patients with symptoms who are likely to progress to severe cases, thereby requiring oral medications, by the COVID-19 medicines guidelines." The KDCA requests clinical practices to prescribe only to people with ▲Tumors or hematological cancers ▲Congenital immunodeficiency disorders ▲'immuno-compromised individuals' like post-lung transplant patients ▲Diabetes ▲Hypertension ▲Cardiovascular diseases ▲Chronic kidney diseases ▲Chronic lung diseases ▲Body mass index (BMI) of 30kg/m2 or higher ▲Underlying diseases, such as neurodevelopmental disorder or mental illnesses. ◆Local authorities notify pharmacists to "come to local healthcare centers for drugs"'= Confusion occurs when the local government attempts to distribute medications during shortages. The KDCA has distributed additional quantities of medications through local public health centers. However, these quantities are insufficient to cover all designated pharmacies, leading to issues of fairness and equity between pharmacies. As of August 8th, it is understood that the distributed medication amounts to a supply of 15,000 people. In region 'D,' the government has announced that they will distribute and allocate medication in the same proportion, considering the orders placed this week and last week and the current usage levels at designated pharmacies. A local pharmacist said, "We received a message apologizing for a limited quantity due to evenly distributing additional medicines to all pharmacies." The pharmacist said, "However, we were told to come to local health centers to receive medicines since they cannot make deliveries to all pharmacies." In region 'E,' the distribution was confirmed to be handled on a first-come, first-served basis. A pharmacist from area 'E' stated, "On the afternoon of August 8th, the public health office official notified us via a social media group chat that only a tiny quantity would be available. They were accepting orders on a first-come, first-served basis. When I checked the message later, the stock had already been depleted." ◆KDCA says, "Temporary shortage, concerns are limited to particular areas"= The KDCA, the control tower for oral COVID-19 medications, shows an entirely different response. In response to criticism for rapid COVID-19 medicine usage and emptying Paxlovid inventory, the KDCA explained that "Particular regions may experience temporary shortages, but it is not true that stocks are running out." The KDCA said, "We thoroughly monitor real-time usage and inventory levels to prevent drug shortages. Collaborating with cities and provinces, we provide additional supply quantities to cities and provinces to respond to real-time demand in each region." They added, "If pharmacies are concerned about a shortage of medicines before the regular supply arrives, they can receive the supply management quantities from the local health centers." This response is entirely different from the situation at local pharmacies. The KDCA said, "However, the supply amount for individual pharmacies is determined based on actual usage, inventory levels, and the amount that can be distributed within the region. Therefore, it may not always match the requested quantities." The KDCA added, "We are working on additional purchases to protect high-risk individuals until stable supply within the general healthcare system is achieved."
- Product
- 92 patient groups implore doctors to stop the strike
- by Kang Hye-Kyung Jun 14, 2024 05:46am
- “We strongly demand the doctors withdraw their resolutions to take a collective leave of absence and indefinite leave of absence as they threaten the patients' lives and health." Ninety-two patient advocacy organizations have called for the withdrawal of the medical community's collective strike that is set to start on the 18th. Ninety-two patient advocacy organizations, including the Severe Atopic Dermatitis Association, the Union of Korea Breast Cancer Patients, the Korean Alliance of Patients Organization, and the Korean Organization for Rare Diseases, held a press conference in front of the main gate of the National Assembly on the morning of the 13th to criticize the medical community's plans for a collective leave of absence. The PAGs said, "We are deeply concerned about the Korean Medical Association's collective leave of absence and the indefinite leave resolution of the Seoul National University College of Medicine and Seoul National University Hospital Faculty Council. We strongly demand that they withdraw the collective leave of absence and indefinite leave resolutions that threaten the lives and health of patients.” "Over the past four months, patients have suffered great anxiety and harm due to the prolonged medical gap caused by the collective action of doctors. We are devastated to see another collective strike launched at a time when we are just beginning to see hope for solving the situation." The PAGs called on the government and the National Assembly to enact relevant systems and laws to prevent the recurrence of collective actions by doctors.
- Product
- Selection dilemma rises in IBD mkt due to increased options
- by Moon, sung-ho Jun 11, 2024 05:48am
- With the recent surge in treatment options for inflammatory bowel disease (IBD), which is represented by ulcerative colitis and Crohn's disease, developing an appropriate treatment strategy for IBD is emerging as a rising topic in clinical practice. This is due to the recent health insurance reimbursement extensions granted for treatments by multinational pharmaceutical companies, which have lowered the burden on site. #Amid intensifying sales and marketing competition within the industry, the medical community is expected to revise its guidelines on selecting appropriate treatments. Until now, tumor necrosis factor (TNF) blockers Humira (adalimumab) and Remicade (infliximab) have dominated the field of IBD treatment in Korea. According to the pharmaceutical industry and medical community on the 8th, the IBD treatment market has been rapidly reshaping with the competitive entry of global pharmaceutical companies’ treatments this year. First, in the first half of this year, Lilly Korea received approval for its interleukin-23 (IL-23) inhibitor ‘Omvo (mirikizumab) from the Ministry of Food and Drug Safety. This added another IL inhibitor option to ‘Stelara (ustekinumab),’ the only anti-interleukin drug that had been available in the market until then. As a result, the anti-integrin agent ‘Kynteles (vedolizumab, Takeda),’ and anti-interleukin agent ‘Stelara,’ ‘Omvo,’ and the Janus kinase (JAK) inhibitors ‘Xeljanz (tofacitinib, Pfizer),’ ‘Rinvoq (upadacitinib, AbbVie),’ ‘Jyseleca (filgotinib, Eisai) can now be prescribed for IBD in Korea. Also, in addition to the JAK inhibitors, which were the only oral treatment options for severe IBD until the first half of this year, the launch and reimbursement approval of ‘Zeposia (ozanimod, BMS),’ a sphingosine-1-phosphate (S1P) receptor modulator broadened the options available on site. This means that the doctors have more options to use on patients who have failed initial treatment. In other words, the next treatment they choose can change the direction of each patient’s care. As such, the choice of IBD therapies, typified by ulcerative colitis (UC), has risen as a hot topic in clinical practice. While clinical research has made it possible to customize treatment for each patient, it has also increased competition between pharmaceutical companies. A professor of gastroenterology at A University Hospital in Busan said, "Previously, there were no options for IBD other than anti-TNF inhibitors. But many more options have become available in recent years, making the situation more complicated for prescribing clinicians.” The pharmaceutical industry is also scrambling to prioritize the lines of their treatment amid the various options becoming available. One representative example is BMS's Zeposia, which entered into a co-marketing agreement with Yuhan Corp, which owns strong domestic sales and marketing capabilities. An industry official said, "In fact, Yuhan Corp’s co-marketing agreement with BMS for Zeposia was considered quite unusual in the field. The collaboration seems to fall in line with the current situation, as the IBD market has recently become more competitive with an increasing number of treatment options and Yuhan had a need to increase its drug lineup." Also, with the increasing number of therapies available from global pharmaceutical companies, "sequencing" between existing and new drugs has become a hot topic in clinical practice. In other words, the increase in treatment options to use after initial treatment failure has created a "dilemma" as patients are allowed to switch between drugs, but not immediately. Another professor of gastroenterology at a university hospital said, "Recently, JAK inhibitors and small molecule drugs have been approved for ulcerative colitis and are being used in practice. However, the issue of side effects needs to be considered as well as benefits. Because complications such as herpes zoster and blood clots can occur when using these drugs, the new drugs are more commonly used on patients that have received shingles vaccinations and relatively young patients." He added, “In the JAK inhibitor class, Jyseleca is the only JAK inhibitor that can be prescribed directly after azathioprine. In clinical studies, RInvoq has shown promise in ulcerative colitis and Crohn's disease. However, the use of other JAK inhibitors requires azathioprine to be first removed, which is problematic for physicians because they have to remove azathioprine, which they consider effective for the patients.” In other words, while prioritizing the use of the right treatment for each patient, the sequencing of the treatments is currently being determined at the doctors’ discretion based on clinical research. As a result, therapies that are deemed to be the most effective based on the clinical studies that support their approval and reimbursement are being placed in the "last line of defense" and used as a last resort in practice. However, there is an opinion that this approach is not optimal and that prescriptions should be based on a comprehensive analysis of the patient's disease severity and socioeconomic status. There are also those who believe that they should wait and refer to the revisions made to the guidelines published by the Korean Association for the Study of Intestinal Diseases, which specializes in the disease. Professor Byong Duk Ye, Professor at the University of Ulsan College and Treasurer of KASID, said, "This is always a problem because there is no right answer. Especially, in the case of Rinvoq, there are opinions that its use should be delayed as much as possible because it is superior in clinical or endoscopic aspects compared to other agents. The drug should be used in combination with the patient's personal disease status and socioeconomic status." Ye added, "It is not necessarily a good treatment strategy to postpone the use of Rinvoq because it becomes less effective when used in later stages of the disease. It may be better to use it earlier to control inflammation. The final decision should be made in consultation with the patient, and we plan to publish revised KASID guidelines regarding the increased number of IBD treatment options available.”
- Product
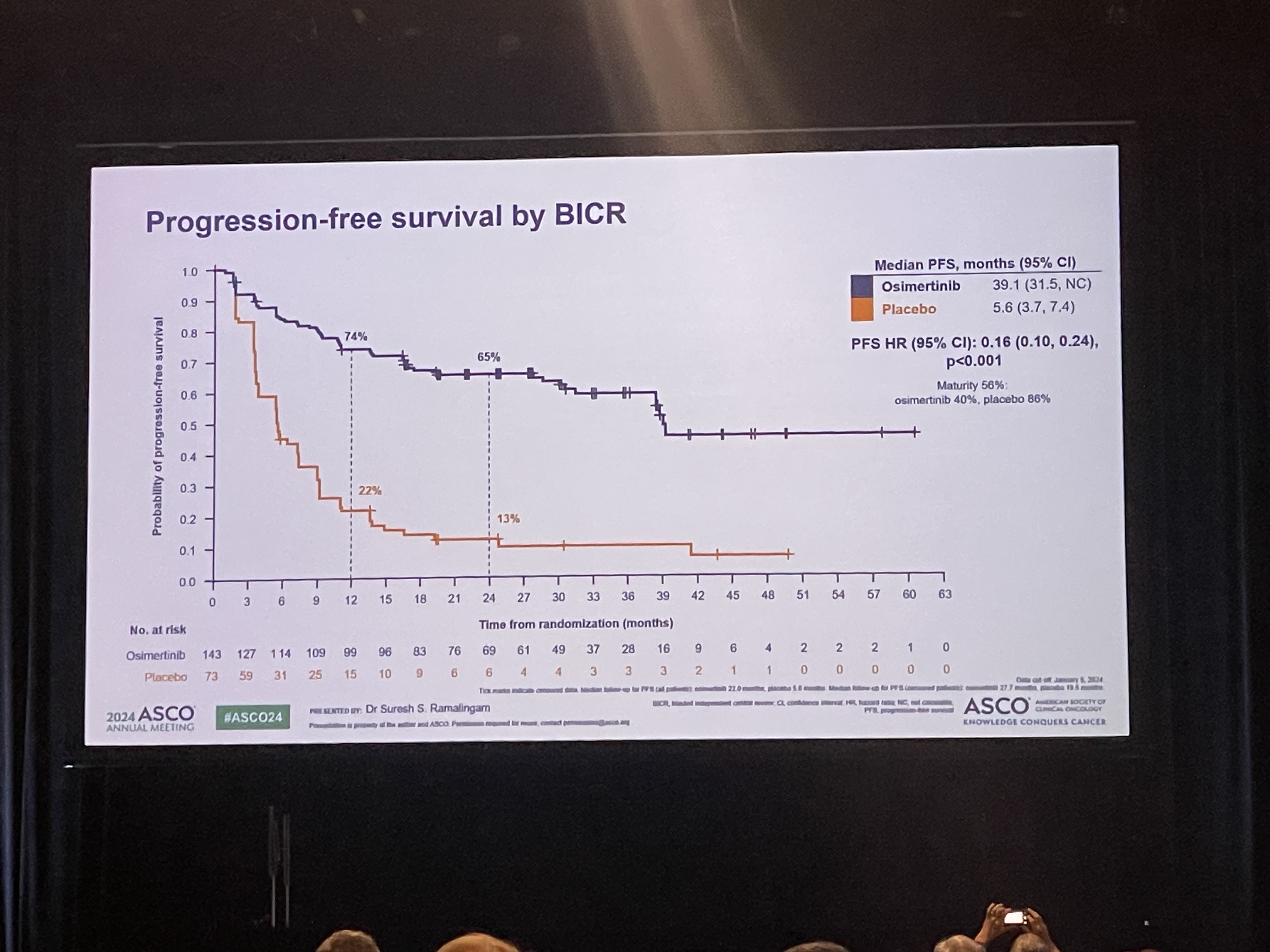
- Will Osimertinib emerge as the standard of care
- by Park, sang-jun Jun 10, 2024 05:41am
- The LAURA trial, which evaluated osimertinib’s effect in patients with unresectable Stage III EGFR-mutant non-small cell lung cancer who received chemoradiotherapy (CRT), was presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting. The results were also concurrently published in NEJM. # The LAURA trial evaluated progression-free survival (PFS) in 216 patients with unresectable stage III EGFR-mutant NSCLC who received chemoradiotherapy (CRT). The patients were randomized to receive osimertinib or placebo. The results showed a median PFS of 39.1 months and 5.6 months in the osimertinib and placebo arms, respectively, with an 84% reduction in the risk of disease progression and death in the osimertinib arm. The overwhelming numbers were met with spontaneous resounding ovation. Although the overall survival rates were not clear yet, researchers also added a positive interpretation based on the fact that overall survival did show a clear trend toward improved survival in the osimertinib arm, even though 80% of the placebo arm switched to osimertinib. Professor Suresh S. Ramalingam from the Winship Cancer Institute at Emory University School of Medicine, who presented results of the phase III LAURA study during the Plenary Session at the 2024 ASCO Annual Meeting, said, “The current standard of care for unresectable stage III EGFR-mutant NSCLC patients following CRT is durvalumab, but the benefit of the immunotherapy agent, specifically among patients with EGFR mutations, is uncertain. Based on the clear benefits, osimertinib after CRT will most likely emerge as the new standard of care for EGFR-mutant disease in this setting.” The next big question will be in setting the eligible subjects and timing of administration. Patients with unresectable stage III EGFR-mutant NSCLC who have received chemoradiotherapy (CRT) are regarded as an incurable group of patients, who have a high likelihood of relapse in the future. This is why drug use in this patient group needs to be reviewed from various aspects. Professor Lecia V. Sequist from the Massachusetts General Hospital and Harvard Medical School, who attended the presentation as a discussant for the abstract, regarded the results as a half glass of water, explaining that osimertinib may and may not be a viable treatment option depending on the perspective.” He emphasized that the positive benefits of osimertinib in terms of preventing brain metastases are a clear advantage, but the cost of the drug and increased side effects are a disadvantage. Professor Beung-Chul Ahn of the National Cancer Center, said, "The positive outcome of osimertinib in this group of patients is very welcome evidence, but if we evaluate it soberly, the cost of the drug cannot be ignored in clinical practice, and there are groups of patients who do not necessarily need it, so we need a treatment strategy that reviews its use according to the situation.”
- Product
- The Court rejects petitions on drug pricing negotiations
- by Kang, Shin-Kook May 03, 2024 05:53am
- The Constitutional Court of Korea. The Constitutional Court of Korea (hereafter referred to as the Constitutional Court) made a decision to reject pharmaceutical company’s petition, which alleged a constitutional violation related to the drug pricing negotiations order. The Constitutional Court recently announced that the clause of a claim, including the violation of the constitution, related to drug pricing negotiation does not fall under the category of exercising governmental authority subject to a constitutional petition. As a result, the request for petition has been declared invalid. The clause in dispute was 'Rules on Criteria for the Health Care Benefits of the National Health Insurance Service,' which allows the Minister of the Ministry of Health and Welfare (MOHW) to order the President of the National Health Insurance Service (NHIS) to negotiate with a manufacturer related to a pharmaceutical that already is reimbursable for health care benefits, acts related to the Minister of MOHW ordering President of the NHIS to negotiation with the petitioners, and acts related to the President of the NHIS disclosing negotiations schedule to the petitioners and notifying of submitting required documents. The Constitutional Court stated, "The clause in question is an organizational regulation that merely specifies the Minister of the MOHW to instruct the President of the NHIS to negotiate. Therefore, the rule does not infringe upon the petitioners' fundamental rights." The Constitutional Court ruled that "The Minister of the MOHW issued an internal order directing the President of the NHIS as a supervisory agency. It does not fall under the criteria of exercising governmental authority subject to constitutional appeals." And added, "Moreover, the Court highlighted that the notice does not directly affect the petitioners' rights and obligations. Therefore, historically, it does not qualify as a subject of a constitutional appeal."