- LOGIN
- MemberShip
- 2026-03-09 20:47:39
- Product
- Mounjaro arrives at pharmacies..₩310,000–350,000
- by Kang, Hye-Kyung Aug 21, 2025 06:07am
- Mounjaro (tirzepatide), which had been attracting considerable attention even before its arrival in Korea, has now been made available to pharmacies. With prescriptions also beginning on the 20th, pharmacies are bustling with activity. According to local pharmacies, two dosage forms, 2.5mg and 5mg, have begun arriving at pharmacies through wholesalers. Mounjaro Prefilled Pen 2.5mg, 5mg stocked at pharmacies A local pharmacist stated, “The 2.5mg and 5mg doses arrived this morning. Although no prescriptions have been made yet, we are receiving calls asking if the product has arrived. It seems that consumer interest is quite high.” The two main concerns for pharmacies are pricing and medication guidance. Mounjaro is priced in the ₩300,000 range, with the price lowest in Jongno at ₩290,000 ◆Prices vary between pharmacies, with the lowest price at KRW 290,000 = Since Mounjaro is a non-reimbursed drug, the price varies even between pharmacies. For this reason, pharmacies are struggling to set the initial price. According to telemedicine platforms, prices for 2.5mg in-hospital prescriptions at clinics range from KRW 320,000 to KRW 350,000 Pharmacies are pricing the medication between KRW 310,000 to KRW 350,000. While the number of pharmacies stocking Mounjaro is still limited, prices seem to be forming in the early to mid three hundred thousand won range. However, in Jongno, the price is set at KRW 290,000, which is the lowest price. ◆Medication guidance for Mounjaro?= Mounjaro comes in a package containing four single-use pre-filled pens. The outer case states, “Mounjaro is used once a week. Read the instructions carefully before using the medication and keep the instructions with the medication.” Mounjaro was approved as ▲an adjunct to diet and exercise therapy for improving blood glucose control in adult patients with type 2 diabetes; ▲an adjunct to low-calorie diet and exercise therapy for chronic weight management in adult patients; and ▲as an adjunct to a low-calorie diet and exercise therapy for the treatment of moderate-to-severe obstructive sleep apnea (OSA) in obese adult patients with an initial body mass index (BMI) of 30 kg/m2 or higher. When administered for weight management ▲Obese patients with an initial body mass index (BMI) of 30 kg/m2 or higher ▲Overweight patients with an initial body mass index (BMI) of 27 kg/m² or higher but less than 30 kg/m² and one or more weight-related comorbidities such as hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease, may use the treatment. The recommended starting dose is 2.5 mg subcutaneous injection once weekly, increased to 5 mg subcutaneous injection once weekly after 4 weeks, and maintained at that dose. The maximum dose is 15 mg subcutaneous injection once weekly. If a dose is missed, administer the missed dose as soon as possible within 4 days (96 hours) of the missed dose. If more than 4 days have passed, skip the missed dose and administer the next dose on the scheduled day. Mounjaro can be administered at any time of the day, regardless of meals, and should be administered as a subcutaneous injection in the abdomen, thigh, or upper arm. It is recommended to rotate (alternate) the injection site with each dose. The recommended that the injections are refrigerated at 2–8°C. If necessary, each single-use pen can be stored at a temperature not exceeding 30°C for up to 21 days without refrigeration. Do not freeze. If frozen, the medication should not be used and should be stored in its original container to protect from light. The most commonly reported adverse reactions include gastrointestinal disorders such as nausea (very common), diarrhea (very common), and vomiting (common). Generally, the severity of these reactions was mild or moderate, and occurred more frequently during dose escalation, and decreased over time. Meanwhile, Lilly Korea announced that it plans to first release 2.5 mg and 5 mg doses in Korea, but will also supply higher doses of 7.5 mg, 10 mg, 12.5 mg, and 15 mg in accordance with patient demand. Meanwhile, the Ministry of Food and Drug Safety sent a notice to medical organizations and other relevant parties to prevent misuse of the drug following the release of Mounjaro. The MFDS emphasized, “Please prescribe and dispense the drug appropriately in accordance with the approved efficacy, dosage, and precautions for use. To prevent side effects and misuse, please provide accurate information about the drug (efficacy, dosage, and precautions for use) to the patients and instruct them on how to take the drug.”
- Product
- Mounjaro set to hit shelves next week... pricing a dilemma
- by Jung, Heung-Jun Aug 13, 2025 06:07am
- With the imminent arrival of the diabetes and obesity treatment Mounjaro (tirzepatide) in Korea, pharmacies are struggling to set an appropriate price for the item. Due to the nature of high-priced non-reimbursable drugs, there are regional price differences, and price competition is expected in the future, so pharmacies are showing a cautious stance on their initial sales price. Recently, some distributors notified pharmacies of Mounjaro ‘s supply schedule for the 20th and surveyed the pharmacies’ willingness to handle the product to anticipate demand. Additionally, telemedicine platforms are guiding affiliated clinics and pharmacies to input their planned selling prices. Like Wegovy, Mounjaro’s supply price was disclosed before its domestic launch. However, unlike Wegovy, the supply price varies by dosage. While there may be minor differences depending on the distributor, the prices are currently set at KRW 278,000 for 2.5mg and KRW 369,300 for 5mg. The prices for the upcoming 7.5mg and 10mg doses are reported to be KRW 521,300. Pharmacists are expected to monitor the prices set by nearby clinics and pharmacies before determining their own sales prices. Pharmacist A from Seoul stated, “Based on the 2.5mg dosage, the price is expected to range from the mid-300,000 KRW to the 400,000 KRW bracket. Although distribution is scheduled for next week, we have not yet decided whether to carry the product, as we are unsure whether hospitals will issue outpatient prescriptions.” Pharmacist A added, “Although both products are used for obesity treatment, their mechanisms of action are different, so it is not appropriate to simply compare their dosage and price.” In the case of Wegovy, the selling price, which was in the range of KRW 500,000 to KRW 600,000 when it was first launched in Korea, gradually decreased, and the price was adjusted. In the case of obesity treatments, price information is quickly shared through telemedicine platforms and communities, so even among non-reimbursable drugs, the sales price can fluctuate greatly. Some pharmacists are hesitant to handle the drug because the supply price is known and there is a risk that it could become dead stock. Pharmacist B from Seoul stated, “It has a competitor, so it's difficult to predict how much demand there will be for Mounjaro, so we're waiting to see how demand develops. We also have remaining stock of Saxenda and Wegovy. Price competition is intense, so expanding our inventory can be a burden.”
- Product
- Wegovy supply price cut by 40%... to counter Mounjaro
- by Kang, Hye-Kyung Aug 13, 2025 06:06am
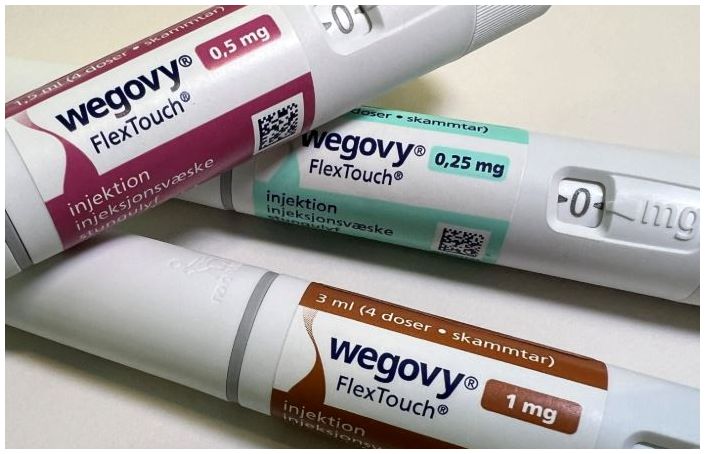
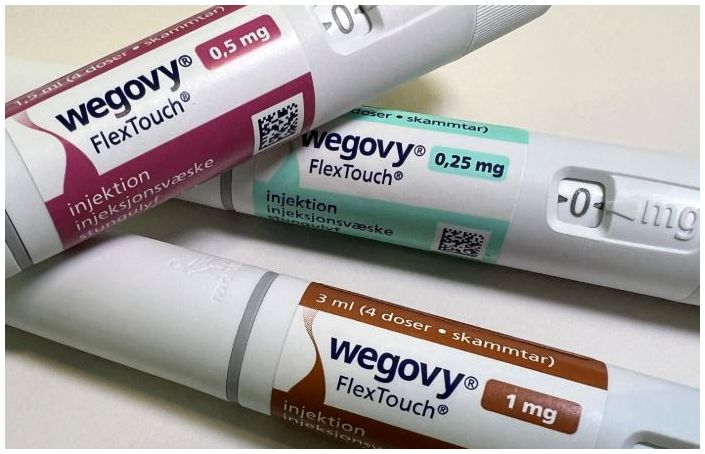
- The supply price for Wegovy will be reduced by approximately 40% starting on the 14th. Refund measures are also expected to be implemented for pharmacies with inventory. Novo Nordisk notified pharmacies and other relevant parties of the changes through Julic and other channels. According to Dailypharm's coverage, the prices for four dosage forms—0.25mg, 0.5mg, 1.0mg, and 1.7mg—will be reduced, excluding the highest dosage of 2.4mg. The previous single price system will be replaced with a tiered pricing system based on dosage, with the 0.25mg starting dosage set to receive a price cut of around 40%. Once the price reductions are implemented, the purchase prices for pharmacies and clinics will be around KRW 220,000. However, to prevent confusion in medical and pharmacy settings, the specific price reduction rates will not be disclosed. Refunds for existing inventory held by pharmacies will also be processed according to established procedures. Details regarding refund procedures and amounts per pharmacy will be communicated sequentially through Zuellig Pharma sales representatives and the suppliers. Some have speculated that the price reduction policy for Wegovy may be in response to the upcoming launch of Mounjaro this month. Mounjaro, which is expected to be launched around mid-month, will apply tiered pricing across six dosage forms, with the starting dosage being approximately KRW 100,000 cheaper than Wegovy. However, Novo Nordisk stated that it had been reviewing a tiered pricing policy by dosage for a long time after the launch and had decided to switch from a uniform single price system to a tiered pricing system by dosage. A Novo Nordisk representative said, “We decided to lower the shipping price and notify customers to minimize confusion among distributors, pharmacies, and patients who have been prescribed the drug.” They added, “In supplying innovative treatments like Wegovy, we prioritize treatment continuity and accessibility for Korean obesity patients as our top principle, and this principle also applies when determining the shipping price of the treatment. We will continue to strive to improve the obesity treatment environment in Korea through a patient-focused approach.”
- Product
- Platforms promote Wegovy's lowest price is ₩390,000
- by Kang, Hye-Kyung Jul 30, 2025 06:10am
- Non-face-to-face treatment platforms are facing backlash from pharmacies after launching activities promoting the lowest prices for non-reimbursable drugs. Doctornow, a leading non-face-to-face medical consultation platform, is being criticized for encouraging price competition for non-reimbursable drugs and causing confusion among consumers. A local pharmacist stated, “Recently, Doctornw has posted promotional content on its social media accounts, such as ‘How to buy diet injections for KRW 200,000 less,’ 'The real way to buy semaglutide diet injections for KRW 390,000,‘ and 'Hair loss medication consultation fee + medication cost: KRW 9,060.’ I believe this is likely to cause distrust and confusion among consumers toward pharmacies.” For example, in a video titled ‘How to buy diet injections for KRW 200,000 less,’ it says, 'Wow! I used to buy weight-loss injections for KRW 600,000, but I was paying KRW 200,000 more. If you don't know the latest way to buy diet injections cheaply, you're losing out 100%. If you know this method, you can get a prescription for KRW 200,000 less, at an unbelievable price!“ The video states that all you need is to access Doctornow. The video explains that you can find the lowest price at a pharmacy near you by clicking ”Find a Pharmacy,“ then clicking ”Get a Prescription" to select a nearby and affordable doctor, going to the hospital for a consultation, and then getting the injection at the lowest-priced pharmacy. However, the video has been criticized for causing consumer distrust and confusion about pharmacies. This pharmacist said, "Doctornow claims that the real price for semaglutide diet injections is KRW 390,000. It suggests that pharmacies are taking a middleman margin. However, the purchase price of the semaglutide diet injection, Wegovy, far exceeds the 390,000 won suggested by Doctornow.” When credit card transaction fees are added to the purchase price, it is practically impossible to meet the criteria for ”buying cheaply and well" suggested by Doctornow. When Dailpharm searched for the lowest price of Wegovy on Doctornow, the price varied depending on the region, but was usually over KRW 430,000. It was found that there are pharmacies in the Gyeonggi area that sell the drug for KRW 394,000 and KRW 395,000, but in the Gyeongsangbuk-do, Chungcheongbuk-do, and Gyeongsangnam-do areas, the price of the non-reimbursable injectable was set around KRW 430,000 to KRW 450,000. Doctornow also presented KRW 9,060 as the lowest hair loss medication consultation fees available. Another pharmacist said, “There are differences between pharmacies when it comes to the price of non-reimbursable drugs. However, I think it is problematic under the Pharmaceutical Affairs Act for non-face-to-face consultation platforms to arbitrarily set the lowest price and misrepresent it as the ‘standard’. Shouldn't sanctions be imposed on the platform for such excessive promotional activities?” Pharmacists also voiced concerns about the institutionalization of telemedicine, as the majority of non-face-to-face prescriptions made are for non-reimbursable drugs for cosmetic purposes. According to a survey conducted by the Guro District Pharmaceutical Association and the Jungnang, Gwangjin, and Gangdong District Pharmaceutical Associations among their members, 46% of pharmacists stated that they had accepted non-face-to-face prescriptions in the past three months. When asked about the proportion of non-face-to-face prescriptions for cosmetic purposes, such as hair loss, weight loss, and eye drops, among the prescriptions received, over 80% of respondents answered that they accounted for the majority of non-face-to-face prescriptions. The associations stated that the majority of pharmacists who accepted non-face-to-face prescriptions in the last three months responded that they were for cosmetic purposes. We are concerned about the reality that non-face-to-face medical consultations are being used as a means to purchase non-reimbursable drugs for cosmetic purposes, such as hair loss, diet, and eye drops, which deviates from the original purpose. We believe that non-face-to-face prescriptions for cosmetic purposes should also be excluded or restricted by the system, similar to psychotropic drugs.” Meanwhile, during her confirmation hearing as nominee for Minister of Health and Welfare, Eun-kyung Jeong stated, “The institutionalization of telemedicine should be aimed at ensuring the stability of medical care and improving primary care, rather than expanding platform profits. During discussions with the National Assembly, appropriate regulatory measures should be discussed to address concerns that platforms will effectively take over clinics and pharmacies or promote profit expansion.”
- Product
- KPDS ‘Gov’t action needed to counter price hike pressure’
- by Jul 08, 2025 06:35am
- The Korean Pharmacists for Democratic Society (President: Kyung-Lim Jeon, KPDS) urged the government to take decisive action against Trump and multinational pharmaceutical companies' pressure to raise drug prices. In a statement released on the 4th, the KPDS stated: “Trump signed an executive order on May 12 demanding a reduction in drug prices within the United States, claiming that ‘the United States accounts for less than 5% of the world's population but generates three-quarters of the global pharmaceutical industry's profits.” This is intended to support U.S. pharmaceutical companies by expanding their access to foreign markets, enabling them to lower drug prices within the United States while increasing profits in other countries.” However, ironically, the U.S. pharmaceutical industry has expressed opposition to President Trump's “most-favored-nation (MFN)” policy for the U.S., citing concerns that its double standard could lead to reduced investment and lower new drug development. The KPDS also pointed out that the U.S. pharmaceutical industry has long opposed policies that view health as a basic social security for citizens and seek to establish a foundation for the health of individuals and society as a whole through a national health insurance system and drug price control policies. Rather, the companies demanded a society where pharmaceutical companies can sell drugs at monopolistic prices that guarantee maximum profits, packaging the anti-market system of patent monopolies as “fair trade.” They have also sought to extend patent periods or operate various monopolistic systems, such as through the new drug product exclusivity system, and have exploited loopholes in the patent system. The KPDS stated, “US President Trump's executive order to lower drug prices acted as a catalyst, turning into a storm of drug price hikes for South Korea and many other countries. The Korean government must recognize this change in the trend and respond proactively.” The KPDS proposed 3 countermeasures. The first is to improve the drug pricing system being promoted by the government to reflect the innovative value of treatments. The currently promoted system is not a system for patients, but rather a capitulation to the demands of multinational pharmaceutical companies to raise drug prices. Efforts to increase bargaining power to counter the rapidly rising prices of new drugs should be prioritized. They also urged the government to seek international cooperation to counter the pharmaceutical companies' unreasonable demands for price hikes. KPDS stated, "The Korean pharmaceutical market accounts for only about 1% of the global market. The Korean government's bargaining power is so weak that multinational pharmaceutical companies can easily abandon the Korean market if they so desire. Recently, Europe has been countering the high prices of new drugs by forming alliances with neighboring countries to negotiate drug prices with companies. Korea must also build alliances with neighboring countries in various ways to strengthen its bargaining power with multinational pharmaceutical companies in drug price negotiations." In addition, they proposed the need to establish policies that would substantially reduce drug costs. The fundamental reason for the high price of drugs in the United States is that the government has excessively overestimated the value of drugs under the pretext of fostering domestic pharmaceutical companies and has made no effort to control drug prices. KPDS added, “The reason why rebates by Korean pharmaceutical companies continue is that the drug pricing policies that are lenient to domestic companies provide incentives for companies to sell drugs even if they have to pay extra to doctors. Amid rapid aging, South Korea's drug pricing policy needs to be readjusted, and it is urgent to establish new policies to resolve drug pricing issues, such as the 2006 ‘Drug Price Rationalization Plan.’” "From the perspective of reasonable corporate profits, drug prices in South Korea are not low. There is a growing trend of high-priced new drugs receiving economic evaluation waivers during review, and generic drug prices are among the highest in the world. From the U.S. perspective, demanding that all countries pay high drug prices on the grounds that South Korea's drug prices are low is not justifiable. Instead, the U.S. should review and reform its overly protective pharmaceutical patent monopoly system.
- Product
- 'Govn’t must take strong action against pharma rebates'
- by Kang, Hye-Kyung Jun 26, 2025 06:08am
- The Korean Pharmacists for Democratic Society (President Gyeong-rim Jeon, KPDS) has urged the government to take strong action against pharmaceutical company rebates. KPDS issued a statement regarding rebates made by a major domestic pharmaceutical company that JTBC reported. In the statement, KPDS said, “This is not an individual incident, but clear evidence of widespread illegal rebates rampant throughout the pharmaceutical industry. “Medicines prescribed by doctors are not ordinary commodities, but special goods that directly affect patients' lives and health. The Pharmaceutical Affairs Act and Medical Service Act fundamentally prohibit pharmaceutical companies from providing economic benefits to medical professionals.” However, according to the JTBC report, the pharmaceutical company in question blatantly violated legal restrictions. The KPDS criticized, “What is even more outrageous is the government authorities' incompetent response to these clear illegal acts. Using a shortage of personnel as an excuse to avoid addressing the issue raises suspicions that there may be an intent to cover up the incident.” They went on to say, “The explanation that this was a legitimate new drug promotion activity is a clear lie, and the government must immediately implement strong measures to eradicate illegal rebates.” In other words, the KPDS believes the MOHW and the police must reinvestigate the case and severely punish those involved. The KPDS proposed the following measures: ▲reinvestigation through the formation of a special investigation team; ▲a comprehensive investigation by the MOHW into the provision of financial benefits such as support for academic conferences, lecture fees, and consulting fees; and ▲strong administrative measures such as drug price reductions when illegal rebates are detected. They emphasized, “We can no longer tolerate the illegal rebate cartel that threatens the health insurance budget and public health. The government must use this incident as an opportunity to demonstrate its firm commitment to eradicating illegal rebates in the pharmaceutical industry.”
- Product
- Patient organization proposes 6 policies as election pledges
- by Kang, Hye-Kyung May 14, 2025 06:10am
- A patient group has proposed six policies, including a “rapid reimbursement for new drugs and post-marketing adjustment system.” The Korea Alliance of Patients Organization submitted a 'Statement regarding Six Major Patient Policies' to political parties on the first day of the official campaign for the 21st presidential election on the 12th, urging them to reflect the proposals in their election pledges. The six proposed policies are: ▲Enactment of a Framework Act for patients to promote their treatment journey and protect their rights ▲Establishment of a Patient Policy Division within the Ministry of Health and Welfare ▲Establishment of an integrated support platform for patients fighting illness ▲Introduction of a system for rapid reimbursement and post-marketing adjustments for new drugs directly related to life ▲Institutionalization of a nursing care system ▲Promotion of a national responsibility system for essential organ transplant costs. KAPO stated, “The medical crisis that has lasted over a year and three months, triggered by the government's policy to increase medical school quotas. The resulting conflict with the medical community has caused immense suffering and harm to us patients. The conclusion of this medical-political conflict has left only a visual lesson: the government cannot defeat doctors by using patients' lives as a tool. Now, we patients and patient groups demand a government that will protect patients' lives and rights no matter what medical crisis arises.” They called for a government that will not hesitate to push forward policies and legislation for patients' treatment and rights even in the face of opposition from the medical community, and for a presidential candidate who will create a “patient-centered healthcare environment” where patients are no longer objects or targets but as “subjects” that actively participate for their treatment and rights. They stated, “We have conveyed patients' voices to presidential candidates as 6 major patient policies and hope they will be adopted as campaign pledges.” KAPO was established on February 4, 2010, under the slogan “Listen to Patients,” and currently includes the Korean Leukemia Patients Organization, the Korean GIST Patients Association, the Korea Kidney Cancer Association, All. Can Korea, the Korea Congential Heart Disease Patient Group, the Korea Psoriasis Association, the Korean Society of Type 1 Diabetes, the Korea Neuroendocrine Tumor Society, the Korean PROS Patient Organization, and the Korean Parkinson's Hope Association, among others, with over 92,000 patient participants.
- Product
- Indiscriminate in-hospital prescriptions of Wegovy
- by Jung, Heung-Jun May 13, 2025 06:06am
- Criticism arose regarding the indiscriminate in-hospital prescriptions of Wegovy, which is currently allowed with an exception. According to the Korean Pharmaceutical Affairs Act, in-hospital prescription is allowed only when an 'injectable is injected.' However, it has been pointed out that Wegovy is being sold without a single act of injection. According to a local pharmacy, more hospitals have begun in-hospital prescriptions after Wegovy's non-face-to-face prescription was restricted as of December 2024. Pharmacists complain that "out-of-hospital prescriptions are being made only when hospitals run out of stock." In fact, it has been pointed out that proper monitoring has not been done despite frequent in-hospital prescriptions without meeting the requirement of 'when injected.' A Pharmacist 'A' in Seoul said, "Prescriptions are being made only when hospitals run out of stock and until restocked. We heard from patients coming to pharmacies that hospitals are selling the medication without giving injections," and added, "We are aware that in-hospital prescriptions are being restricted and doing so raises problem." A Pharmacist 'A' added, "Based on the guideline's recommendation, dosage must be initiated from Level 1, but there are cases where Level 2-3 are prescribed." Proper monitoring is deemed necessary because indiscriminate and misuse cases of in-hospital prescriptions were also found in hospitals and clinics that give injections. At a Wegovy online community with over ten thousand members, information on 'shared injection' cases in hospitals where a needle part of a single Wegovy is swapped and injected into multiple patients. Furthermore, supply price may differ, so when hospitals offer in-hospital prescriptions at a lower price, nearby pharmacies' selling prices are heavily affected. A Pharmacist 'B' in Seoul said, "Although we are seeing lower prescriptions compared to the early period of the launch, a few patients still visit. Due to the non-face-to-face platform, checking the Wegovy price got easier, and patients are sharing information online, so we have lowered the price at the pharmacy." Since Zuellig Pharma Korea does not directly transact with pharmacies, pharmacists purchase Wegovy through collaborative wholesale merchants, such as Kyungdongsa and Geo-young. Zuellig Pharma Korea reported that they have not changed the distribution price since the first domestic distribution in October 2024. It has been reported that there is a difference of about KRW 20,000 between the distributing price and that purchased through the collaborative wholesale merchants. Consequently, if pharmacies were to lower the selling price, adjusting to several hospitals that offer extremely low-priced in-hospital prescriptions, they must sell Wegovy at the buying cost.
- Product
- Paxlovid transition to general health system effective June
- by Apr 11, 2025 06:01am
- Product photo of Paxlovid Paxlovid, a treatment for COVID-19, will be transitioned to the general healthcare system starting in June. The free supply previously provided to centers designated for COVID-19 treatment (prescribing facilities) will be terminated entirely due to stock depletion. According to local pharmacists, on the 9th, various local public health centers recently announced that they would discontinue new supplies. The public health centers stated, '"Although the government has been supplying Paxlovid through both market distribution and government-supplied stocks, new supplies from the government are scheduled to discontinue starting in June due to exhaustion of stock.' The announcement is interpreted as a plan to transition Paxlovid entirely to the general healthcare system after the government-held stocks expire at the end of May. Pharmacy's applications and distribution for remaining stock are still ongoing. Pharmacist A stated, "I ordered four units through the public health center,” and added, “We placed the order as a precaution since there have still been occasional COVID-19 cases," and added, "I am now concerned about how to place Paxlovid orders once it transitions to the general healthcare system." Concerns over placing Paxlovid orders during the transition to the general healthcare system continue. Pharmacist B, who operates a Paxlovid-dedicated pharmacy, commented, "We received notice from the public health center that free supply will be discontinued at the end of May," and added, "This means that the distribution channels will be switched to a single market channel, but the specifics on orders are still undecided." Since October 25 of last year, when health insurance coverage for Paxlovid was first applied, a portion has been transitioned to the general healthcare system so that all clinics and pharmacies can handle it. However, the high price of KRW 950,000 per package (30 tablets) remains a significant burden, as discussed among pharmacists. Pharmacist B explained, "We have inquired with several wholesalers, but they all responded that there is currently no stock available," and added, "Since the acquisition cost is KRW 941,940, nearly KRW 950,000, I am concerned that pharmacies might end up bearing the stock, which would be problematic." Pharmacists point out that wholesalers have not yet released clear policies on the return of Paxlovid, raising concerns that without such policies, they could incur losses due to non-returnable stock. Pharmacist C also remarked, "Once the government's free supply of Paxlovid is depleted, the number of pharmacies ordering it will inevitably decrease," adding, "While it would be ideal if COVID-19 does not resurge, in the event of another outbreak, the burden of inventory and the inconvenience of dispensing for patients are expected to be unavoidable." However, the co-payment fee remains at approximately KRW 47,090 per package, maintaining the current level of around KRW 50,000. Earlier, Lee Jung Kyu, Director of the General Bureau of Health Insurance Policy at the Ministry of Health and Welfare (MOHW), stated, "In order to minimize gaps in practices due to the system transition and to efficiently utilize the purchased stock, the Korea Disease Control and Prevention Agency (KDCA) plans to maintain both market distribution and government supply for awhile," and added, "With health insurance coverage for COVID-19 treatments, we expect that patients will be able to use these treatments stably in response to COVID-19 resurges, and we will continue working to expand health insurance coverage for essential treatments needed in society."
- Product
- Distributors of Neurontin, Lyrica, and Celebrex to change
- by Kim JiEun Feb 14, 2025 05:58am
- With the transfer of sales rights between domestic pharmaceutical companies for major original products expected, the change is expected to affect not only the pharmaceutical and distribution industries but also front-line pharmacies. According to the pharmaceutical wholesale industry sources on the 13th, the transfer of rights to domestic pharmaceutical companies for some of Viatris and Takeda Pharmaceutical’s products is being confirmed or under discussion in the first quarter of this year. The products for which the recent transfer of rights has been confirmed and related notices are being issued to the pharmaceutical wholesale industry one after another are: Viatris' Neurontin Tab, Neurontin Cap, Lyrica Cap, Lyrica CR Tab, and Celebrex Cap. Their supplier will change from Jeil Pharmaceutical, the current supplier of these products, to a domestic pharmaceutical company on the 4th of next month. With less than a month left for the change of suppliers, some of these products are already in short supply. In the case of Neurontin, no new stock has been supplied to the distribution industry since last month, and the industry believes that the pharmaceutical company has adjusted its supply in anticipation of the change in sales channels. In the pharmaceutical and distribution industries, the change in the supplier of some of Takeda Pharmaceutical’s products is also rising as an issue. According to industry sources, information about the transfer of sales rights of Dexilant DR Cap and Lanston LFDT Tab, which have been supplied by Jeil Pharmaceutical, has spread throughout the industry, with the timing of the change expected around April 1. It is also reported that Dexilant's stock has been rapidly depleted recently. Jeil Pharmaceutical, which is at the center of the issue of changing suppliers, responded, “We are aware that relevant information is circulating in the industry, but it is difficult to confirm the details as it is a sensitive part of our contract.” The pharmaceutical and distribution industries believe that the market will be affected to some extent, as the products for which the change of suppliers is expected are major items. There may be some instability in the supply and demand of related items in the process of transferring sales rights, therefore, pharmacies that handle related items may need to manage their inventory. “Pharmaceutical companies and the distribution industry are keeping a close eye on the situation because the items under discussion are large,” said an official from the wholesale industry. “As the quantity of goods shipped may be adjusted during the process of changing suppliers, there may be temporary instability in the supply and demand of related items.”