- LOGIN
- MemberShip
- 2026-03-10 02:17:23
- Product
- “MA and marketer need experience, analytic mind, acumen”
- by Kim, Min-Gun Sep 18, 2020 06:29am
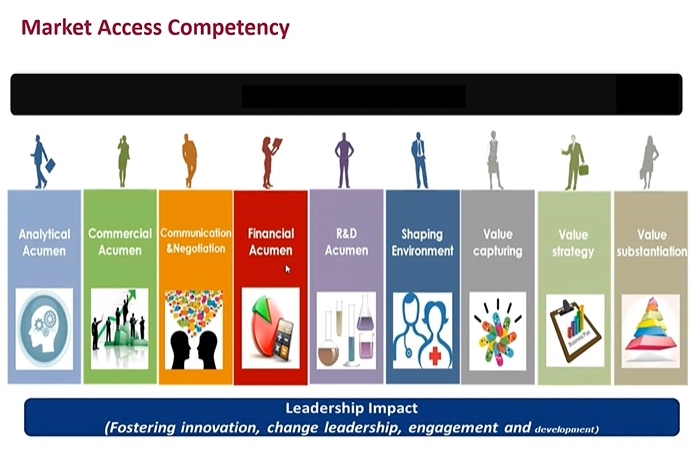
- “For a Market Access (MA) specialist, the most important skill set is to analyze the clinical trial data and to recreate them as a valuable message. They need to find any missing data required for pharmaceutical reimbursement review, and prepare the evidences prior to the review, if need be. If you are a pharmacy school student and want to become a MA specialist, you need to acquire rich experience and knowledge.” That was an advice from MA Director Lim Kyungwha at Jansen Korea to the students in college of pharmacy, who are looking into getting a job in the MA sector. The Pharmaceutical Marketing Professional Leaders (PPL) convened a sixth pharmaceutical industry seminar on Sept. 5 with the theme of ‘the Wave of Pharmaceuticals.’ At the seminar, Director Lim gave a lecture on the current industry status, and the importance of MA specialists. MA Director Lim Kyungwha at Jansen Korea Until few years back, MA specialists played a role of applying for reimbursement listing after a new drug approval. But things have changed—now the MA specialists participate from the clinical designing stage. In the initial clinical stage, new drug development and market access departments come together and talk about strategy as an ‘integrated MA’ to ponder on what the government would stress on when listing for reimbursement, areas to better reflect new drug’s value than other global alternative options, and which clinical outcomes would win the healthcare reimbursement. Director Lim said, “Nowadays, pharmaceutical companies do not consider MA as a single department, but rather treats it like a strategy. It’s because MA can intervene from the initial clinical stage to accelerate the reimbursement listing procedure and affect the entire drug approval and market access strategy.” In other words, MA is a key player in building a strategy for a new drug seeking for a proper acknowledgement of its value and to enter the market fast. Specialized and diversified MA specialist also has to serve two roles of communicating and negotiating with the government. So what kind of skill sets should a MA specialist have? Director Lim said, “MA specialist has to understand clinical data and capture the value in them. The reason why most of the companies hire master’s degree or doctorate degree graduates majoring in public health or social pharmacy is because we need people, who have wide experience and knowledge, skill to analyze finance and market, and understanding of R&D.” “Understanding the general pharmaceutical industry is crucial, as necessary strategy can be designed by understanding of the business. I also finished my MBA, but I went back to school to study social pharmacy,“ Director Lim added. Market access competency She encouraged the students and said, “Students who want to become a MA specialist need to acquire abundant knowledge, experience and strategic mind. As the industry is short of well-experienced MA specialists, the companies time to time hire entry level applicants, fresh out of university or graduate school, with potential seen during an interview.” She also reiterated, “Only because you choose your career path once, does not mean you would stay on it forever. Having richer experience is more important as all companies prefer people with more experience.” ’Acumen’ is critical asset for a marketer, while knowledge in pharmacology and toxicity is essential Executive Director Park Kwang-kyu at Gilead Science Liver Disease Business Department, emphasized ‘acumen’ is the most important factor in a student who dreams of being a marketer, who has to communicate with consumers in the sales department. They have to be highly empathetic to grasp when to be soft or firm during a conversation. Executive Director Park Kwang-kyu at Gilead Science Executive Director Park also mentioned insight, communication and leadership are important as well. He pointed out, “Even the marketers on the job struggle to distinguish ‘insight’ and ‘summary.’ Often the marketers have to deal with big data. And they need to digest the vast amount of data into a concise message,” so “they should be able to say easily in a single sentence to describe ‘what the company should do’ based on those data.” “We need people who are not afraid of challenges with confidence to work well in a multinational company. When it comes down to marketing, your own skill is more valuable than your degree,” he noted. However, Executive Director Park said the number of marketing jobs in multinational companies is declining. He said, “Two decades ago, a pharmacist-turned-marketer was treated as a specialist with expert knowledge. But now we have marketers from liberal arts, biology, pharmacy and nursing background, while [work] has gotten diversified and enhanced. But the company still thinks highly of pharmacy school graduates, so it would be worth it to knock on their doors.” Senior Director Kang Byung-chul at COSMAX BIO Senior Director Kang Byung-chul at COSMAX BIO leading both marketing and R&D departments recommended the participating students to make sure they study about pharmacology test and toxicity test before graduating to apply for a job in a pharmaceutical company. Senior Director Kang highlighted, “In any department, pharmacology and toxicity are the basics. And we all assume a pharmacy school graduates have full understanding of those.” The senior director did not recommend applying for the Business Development (BD) department for an entry level applicant. He said, “Only after working over a decade in R&D or marketing department, you can become a fully functioning member of the BD department. I would recommend transferring to BD department, after becoming a specialist in respective area for 10 years.”
- Product
- 10,000 pros & cons for generic substitution
- by Kim, Min-Gun Sep 10, 2020 06:23am
- There are a lot of unusual comments in the National Assembly legislation, where not even a single comment is usually posted. These are the 'Simplification Act for Generic Substitution Post Notification' and 'The Act on Restriction of 1+3 Joint Biological Equivalence'. All of them are laws related to the issue of generc prescription. Among the 304 bills posted on the National Assembly Legislative Advancement System on the 9th, a total of 13,000 comments were posted on two partial amendments to the Pharmaceutical Affairs Act, initiated by Seo Young-seok (Bucheon), Rep. of Democratic Party of Korea. List of legislative notices posted on the National Assembly Legislative Notice System on the 8th By around 11:30 a.m. on that day, 7681 comments were posted on the legislative notice of the Simplification Act for Generic Substitution Post Notification bill, and the number of views exceeded 69,934. Likewise, 5857 comments were posted on the legislative proposal for the The Act on Restriction of 1+3 Joint Biological Equivalence proposed by Seo, and the number of views was 48,395. This is three to four times more than the Wildlife Protection Act (2,454 comments) with the most opinions among the comments posted on 302 other legislative proposals. In particular, except for the top five legislative notices, most bills do not have less than 100 comments. There are only hundreds of views. As the two bills passing generic prescription spread to confrontation between doctors and pharmacists, it can be assumed that a systematic commenting campaign is underway. Lee * Eun, who appears to be a doctor, objected to the bill when he claimed that "even if the ingredient is the same and passed the bioequivalence test, the effects and side effects of the drug are not the same." Hwang * Deok also pointed out, "It is not the same drug because the ingredients are the same. The drugs used for each patient are different, which is contrary to the current trend of advocating precision medicine." However, Chansung, who appears to be a pharmacist, also confronted this. Kim *Ah argued, "We need to change the perception of the same ingredient preparation. Even now, it is awkward to say that doctors who prescribe the same drug to another drug company every few months to designate a company according to the patient." Mr. Kim *Han also said, "I agree with the legislation that changes the name of the generic substitution with the same ingredient name to reduce public confusion. It is very ridiculous for doctors to prescribe a company's medicine that is not clear to the manufacturer and rather changing it to an original. In many cases, not a single comment is posted on the legislative proposal of the National Assembly Legislative System The same comment was also occupied by the MFDS’ proposed The Act on Restriction of 1+3 Joint Biological Equivalence, which were unsuccessful contrary to the Regulatory Reform Committee. The main idea of this bill is to limit the number of generics per original drug to three. Rep. Seo cited the reason, "The outbreak of consignment generics due to unlimited sharing of biometric data weakens illegal distribution such as rebates and R&D of pharmaceutical companies." There is a reason for the pros and cons of the legislation to limit the number of generics produced by bioequivalence testing. This is because it also contains the content that 'because the type, content, and route of administration of the active ingredients are the same as for new drugs, bioequivalence test data must be submitted for drugs that are requested based on safety and efficacy data such as new drugs.' Oh *-hyun, who argued against this, commented, "It is said that it is the same, but if you take medicine, will you take the original, or will you take flour medicine from a third-rated pharmaceutical company that you have never heard of?" Regardless of the bill, Kim *young wrote, "If the pharmacy changes to a different drug with the same ingredient, patients can not take the medicine which doctor prescribe under specific manufacturer" He wrote a comment on a similar bill that would run on the bill. Mr. Kwon *-jin, who agreed to the bill, refuted, "People who disagree haven't read the text properly. The prescription for the brand name in force now leads to rebates between doctors and pharmaceutical companies, causing reckless competition for generic drugs." Kwon said, "The prescription of ingredient names will limit the number of generics. The case of using expensive original drugs without taking into account the patient's economic situation through rebates will disappear." He argued, "It can prevent the reckless competition for mass production of generics by pharmaceutical companies."
- Product
- Halted fight between government vs. doctors damages both
- by Kang, Shin-Kook Sep 09, 2020 05:46am
- The Korean Medical Association (KMA) has decided to temporarily halt but revisit their discussion with the government and the ruling party on increasing the quota for medical school and establishing public medical school, until COVID-19 is contained. The junior doctors are most likely to return for their duty after the last discussion on Sept. 7. Instead of revoking the policy, the government made up a pretext for re-discussing the medical reform policy, whereas the medical community paved their way to intervene in the future medical policy making process. With COVID-19 spreading relentlessly, the talks on medical reform plan saw no clear winner or loser. Ultimately the medical community also had to settle on a middle ground as they were to face the angry public, if the strike continued. ◆So did the ruling party ‘lose’ the battle?: Even within the ruling party, criticisms were made on the result of the ruling party and medical organization agreement. Democratic Party Lawmaker Representative Lee Su-jin posted a comment on her social media on Sept. 4, "We are now supposed to re-discuss expanding the medical school quota and establishing public medical school from square one, and obviously we are putting a pin on introducing the regional doctor system, indefinitely.” Lee continued and evaluated, “The doctors used medical strike to maneuver their say in the medical reform policies the public is highly interested in.” Lawmaker Yun Kun-young firmly defended the ruling party’s decision and responded to the criticism on the agreement between the ruling party and the Korean Medical Association (KMA), saying, "Even if the government couldn’t save its face, and the ruling party feels humiliated, there is nothing we can do,” because “saving the people’s lives come first amid COVID-19.“ Democratic Party Policy Committee Chair Han Jeongae, who led the mediation between the government and the medical community, also refuted the criticisms that the government and the ruling party had surrendered to the medical community. Lawmaker Han said, "The policy will be revisited again through persistent communication and discussion to resolve the unfair distribution of medical service in different regions, strengthen essential medical care and reform medical care system for the public. Also the ruling party would endeavor to deliver the agreement signed between KMA and the Democratic Party.” The medical community is also still torn from the result. KMA President Choi Dae-zip’s executives clashed with the interns and residents, who are already calling for President Choi’s impeachment. Resisting against the agreement, the medical interns, residents and fellows demanded the strike to continue, but they declared the strike would be over without a justification to continue as KMA signed the agreement. ◆Private clinic and hospitals were calmer, but junior doctors arose: The most unique part of the medical strike was medical interns, residents, fellows and students taking the action together. KMA President Choi Dae-zip signed an agreement with the ruling party and the government. The medical reform plan to increase the medical school quota and opening public medical seemed to have stirred the young doctors more than private hospital doctors. During the nationwide strike from Aug. 28 to 29, only about 6 percent of private hospitals and clinics went on a strike, but up to 84 percent of the medical interns and residents participated. The private hospital strike participation rate went down over time, but the junior doctors’ participation rate continued to rise. The medical community and the National Assembly analyze reaching the agreement would have been impossible without the participation and solidarity the young doctors showed. The government also took Korean Intern Resident Association (KIRA), instead of KMA, as a negotiation counter partner. This is the reason the ruling party agreement added a clause stating ‘fostering and supporting essential medical care, and practically improving medical interns and residents’ training environment.’ A National Assembly official commented, "It is true that the medical interns, residents and fellows play a significant role in the medical field," and “their absence in response against the pandemic would have pressured the government tremendously.” What the government feared the most was the actions taken by professors, interns and residents at tertiary hospitals. Amid the epidemic, dysfunctional tertiary hospitals would be detrimental. Specifically, the government was concerned with the inconvenience the patients requiring urgent care or fighting against severe diseases would experience. This proved the role of private hospitals and clinics is weaker than that of tertiary hospitals amid COVID-19. During the junior doctors’ fight against the government policy plan, KMA secured their seat in re-discussing the key agendas, such as the Health Insurance Policy Deliberation Committee structure, medical care delivery system reform, pilot program for Korean herbal medicine coverage, and non-contact medical service. The government and the ruling party would have realized by now that fighting with doctors is never easy. And the doctors now have the ‘strike’ as their open option whenever an unfavorable policy is on the table.
- Product
- Successful August KMA strike all depends on participation
- by Kang, Shin-Kook Aug 03, 2020 11:05am
- The doctors’ organization objecting against the South Korean government intending to expand medical school admission and provide coverage on Korean herbal medicine are drawing up a plan to call a private clinic doctors’ strike. The strike is most likely to be on Aug. 14. According to Korean Medical Association (KMA) on July 29, the issue of calling an organized strike on Aug. 14 would be addressed at the general assembly on July 31 and the organization would publicly announce the result on Aug. 1. A press conference convened in front of the NA Hall on July 23 to reprimand medical school admission expansion. President Choi Dae-zip declared the organization would plan an all-out single-day strike on either Aug. 14 or 18. The board members from 16 cities and provinces across the country gathered for an emergency meeting and shared their opinions on the strike date. Apparently, the meeting convened in Osong city on July 25 had the majority of the opinions leaning towards Aug. 14. But the administration of the organization is concerned of worsening the public opinion with the general strike amid COVID-19. Besides, the regional doctors’ communities are questioning how to convince private clinic doctors when the expected outcome of the strike is unclear, regardless of their justifiable objection against the government policy. However, the KMA administration claims its recent survey on the organization members found that the organization has enough willpower to fight against the government action. Over 95 percent of the doctor members answered they are opposing against the government policy, and over 85 percent said they are willing to participate in a fight against the government policy like an all-out strike to correct the wrongful policy. Moreover, the specialists’ all-out strike to be conducted on Aug. 7 could catalyze the private clinic doctors’ strike. On July 23, the ruling party and the government have decided to gradually expand the medical school admission size from 400 students to 4,000 students through ten years time. Leverage by the super ruling party’s power, the Ministry of Health and Welfare (MOHW) held a meeting with the Health Insurance Policy Deliberation Committee (HIPDC) to swiftly push the plan of conducting a pilot program of granting coverage on Korean herbal medicine that requires budget of 50 billion won. For now, the only option KMA has to reprimand and stop the so-called ‘Four Unjust Policies’ including stipulation of remote medicine, medical school admission expansion, coverage on Korean herbal medicine and establishment of public medical school is an all-out strike. In a letter sent out to specialist doctors on July 29, President Choi Dae-zip stated “The true role of the KMA president is not to hide behind the organization but to lead the fight against wrongful government action in the frontline,” and “I ensure I will serve the role. So please, join the fight against the unjust law. Come together, as we must seize the madness of the government and ruling party constantly pouring out bad healthcare policies.”
- Product
- Eventually, Fulcare’s YouTube Ad is deleted
- by Jung, Heung-Jun Jul 20, 2020 06:18am
- Deleted video capture When Mennarini Korea's athlete's foot drug Fulcare’s YouTube advertisement raised a problem saying that Ads excluded pharmacist skills, the pharmaceutical company deleted the video and began to rectify it. The content of the controversial advertisement is a scene where Another patient who was waiting for a patient who visited the pharmacy for athlete's foot symptom recommends Fulcare. Pharmacists protested that it was “an advertisement that ignored pharmacies and pharmacists,” and on the morning of the 15th, some local pharmacist societies called for an immediate cessation of advertising. When the controversy broke out, Menarini Korea apologized to the pharmacists for the inappropriate image, and immediately switched the controversial video to private. An official from Menarini Korea said, "The video in question was immediately removed. It will not be used in the future. This video has hurt the pharmacists who have worked hard and dedication in the front line of the public health." "We take the matter seriously and prepare a plan to prevent recurrence," he said. This is an explanation that the consultation and product recommendation were intended to be included in the video. However, it may be illegal for the pharmaceutical affairs law to be referred to as 'expert recommendation'. Also, it was intended to highlight the fact that it is OTC drug and can only be purchased at a pharmacy. Considering that it is an online video for consumers, he also conveyed the intention of making such as the point that the patient tried to express the part explaining his or her disease. The official said, "It is entirely wrong to fail to reflect carefully, and we are sincerely sorry for the confusion and inconvenience caused by this." also he added, "we will do our best to actively reflect the opinions of pharmacists in all future marketing activities and avoid recurrence." Then, the reason why this advertisement was controversial can be found by looking at the ‘Guidelines for Provision of Information on Drug Ads and Rx drugs’ by the MFDS. In the guidelines, doctors, dentists, and pharmacists are not allowed to recommend and guarantee medicines in advertisements. This is because, according to the guidelines, the characteristics of the social perception of the healthcare practitioner have a large impact on consumers perception of the medicine. In addition, it is judged that it is against the regulations for entertainer and the public to wear gowns to make them look like pharmaceutical experts. Accordingly, pharmaceutical companies produce advertisements in a manner recommended by the general public. "pharmacist should not recommend it. It can be seen as a violation for entertainer to wear a pharmacist's gown. Therefore, in the pharmaceutical industry, advertisements are presented in a way that the general public explains or recommends." he said. This Fulcare’s YouTube ad was shot especially in the pharmacy, so the pharmacists were against it. The official said, "There weren't many commercials in the background of the pharmacy. If this was done outside the pharmacy, the situation may have been different."
- Product
- Will electronic masks by LG be commercialized?
- by Kang, Shin-Kook Jul 15, 2020 06:33am
- Electronic masks made by famous domestic home appliance companies are attracting attention. LG Electronics announced on the 12th that it has donated 2000 electronic masks made of electronics and IT technology to Severance Hospital to express appreciation and support to medical staff who need to work while wearing masks to overcome COVID-19. The electronic mask contains the patented technology and know-how of LG Electronics' puricare air purifier. Two replaceable HEPA filters (grade H13) are attached to the front of the mask, and the user inhales the air that has passed through the HEPA filters. Electronic mask developed by LG Electronics The amount of air entering the mask is controlled by an ultra-small fan mounted under each HEPA filter. The mask applies a sensor that detects the pressure generated during breathing and a breath recognition algorithm to increase the fan speed when the user inhales, increasing the amount of air entering the mask and reducing the speed when exhaling. In order to design a mask that fits well with the shape of the face, LG Electronics analyzed the face type in collaboration with the Ergonomics Design Laboratory of the Department of Industrial Management Engineering, Korea University. The product also received certification mark for EMF, which certifies that electromagnetic waves generated from electrical products are released below a certain level from the KTC. However, the timing, method, and price of selling to the public have not yet been determined. LG Electronics is also considering donating electronic masks for workers in public institutions who need to always wear masks due to frequent contact with people following medical staff. Therefore, outpatient pharmacies predicted that if there is little maintenance cost and the product price is reasonable, there is a possibility that it will work in the market. "COVID-19 outbreak may be an opportunity for a company. It should be seen in product size, price, and convenience, but it is likely to be used in places where there is a high likelihood of exposure to COVID-19, such as pharmacies and medical institutions," said Pharmacist K, in gangnam, Seoul.
- Product
- Why Tylenol short in pharmacy, but still plenty in GS25?
- by Jung, Heung-Jun Jun 18, 2020 06:27am
- As of June 15, Tylenol is sold out in online pharmacy Pharmacies in Korea are experiencing a long-term shortage of Tylenol 500 mg and Tylenol ER 600 mg, but apparently convenient stores have no issue stocking up Tylenol 500 mg. As of June 15, Tylenol in 500 mg and ER 650 mg tablets were indicated to be sold out on an online pharmaceutical wholesale website used by pharmacists. Since the incident of the World Health Organization (WHO) initially recommending the use of acetaminophen in suspicious symptoms of the novel coronavirus in last March, pharmacies in Korea have been struggling to get their hands on Tylenols. However, major convenient store brands like GS25, Emart24 and CU had no problem with Tylenol 500 mg stock. Seeing the contrasting situations, pharmacists have started wondering if the pharmaceutical company is differentiating the stock control in convenient stores and pharmacies. Tylenol still sold at convenient stores like CU and GS25 A pharmacist from Gangwon who requested to be anonymous commented, “Following the 500 mg dose, our pharmacy is short on the ER 650 mg dose as well. The wholesale distributors also seem to be short on the stock according to their online websites,” and “Even if the bulk package in bottle for prescription dispensing is available, OTC stocks are sold out. Three regular vendors and an online shop are all having the same issue.” The pharmacist also noted, “Currently, the drug is strangely not available in pharmacy, but accessible in convenient store. Although pharmacists have requested the OTC first-aid kits to be sold in pharmacists as well, the request was rejected.” “The shortage should be resolved as soon as possible as many of customers specifically ask for Tylenol and they tend to be faithful to the brand,” added the pharmacist. Regarding the issue, Johnson & Johnson Korea stated the company is doing the best to streamline the supply to meet the rapidly changing demand, and the supplies to both pharmacies and convenient stores are delivered normally at the moment. And the company noted the supply amounts to both outlets are not differentiated. Johnson & Johnson official said, “The company has been normally supplying stocks of Tylenol to pharmacies and convenient stores. As Tylenol has been mentioned more usual and consumer demand has surged amid COVID-19, the company is committed to supply stocks smoothly.” On the shortage apparent in online shopping sites, the official explained, “When supplied to the distributor, some [of wholesale distributors] seem to be pacing the stock release.” Regardless, the distributors claim they are not the one controlling the supply, but actually they do not get access to the stock. A distributor insider said, “The drug stock has not been supplied to our company. Including Tylenol, some of items are having similar issues and we suspect it is the active pharmaceutical ingredient supply issue.” According to Daily Pharm’s analysis on top 100 drugs sold in 300 pharmacies around Korea with POS machine installed, Tylenol was highly demanded as it was on top third, fifth and ninth place in March, April and May, respectively.
- Product
- Metformin's alternative medicine sold out in an hour
- by Kim JiEun May 29, 2020 06:16am
- As the discontinuation of sales of 31 items of Metformin used as a treatment for type II diabetes patients is decided, the shortage of alternative products is intensifying. The MFDS announced that it will temporarily manufacture, sell, and discontinue prescriptions for 31 items containing Metformin, which are used as a primary treatment for type II diabetes patients today (26). Along with the announcement of the MFDS, a notice was sent to the pharmacists of Korean Pharmaceutical Association, and pharmacists had a busy time checking the related items and organizing their inventory immediately after work. Pharmacists need to order replacement items immediately if the product they normally prepare is included among the 31 items that have been supended from today. In fact, as of 9:00 am today, Metformin alternative medicines have been rapidly sold out at major drug online malls. One of the alternatives to the metformin formulation that has been discontinued this time In the case of Glucodown OR 750mg, which were relatively prescribed in hospitals and clinics, among the discontinued items, was replaced by a pharmacy actually Yuhan’s Metformin XR 750mg, and the product was found to be sold out at major online malls less than 10 am. In addition, Yuhan’s Metformin XR 500mg 300T & 30T are currently out of stock in some pharmaceutical online malls. Pharmacists say that Daewoong’s Diabex XR is not easy to secure inventory as orders are concentrated in the morning as well as in major online retailers. A pharmacist in Seoul said, “I immediately ordered Yuhan’s Metformin as soon as I came to work today.” “Yuhan’s Metformin XR 750mg doesn't have any alternatives, so I think it will be more ordered. I know that the product is currently out of stock." Some pharmacies contacted a nearby internal medicine clinic this morning to discuss discontinued Metformin and to discuss alternative prescription products. A pharmacist in Seoul said, “I thought that Yuhan’s Metformin XR 750mg is the only substitute in a nearby hospital, but I know it is currently out of stock.” "Instead, there was a question about how to prescribe." Another pharmacist said, “It seems that pharmacists ordered quickly from online malls, etc., as information related to them was announced last night. "There seems to be some hoarding.”
- Product
- KDA “Agree with government action on metformin with NDMA”
- by An, Kyung-Jin May 28, 2020 10:07am
- Medical academic societies expressed their support for the Korean government’s decision to suspend manufacturing and sales of metformin with excessive level of impurity found. However, the scholars noted diabetic patients should not stop taking metformin without consulting their doctors. On May 26, Korean Diabetes Association (KDA) and Korean Endocrine Society (KES) issued a joint statement on the government suspension on manufacturing and sales of metformin upon discovery of N-Nitrosodimethylamine (NDMA) exceeding an acceptable level. The statement first expressed gratitude for the Ministry of Food and Drug Safety’s (MFDS) prompt but proactive action on the metformin products and their safety issue, and also showed support for the government’s decision. The two academic societies urged, “For similar cases in the future, the government should continue to directly conduct investigation, disclose the result transparently and provide solution for the people and healthcare providers to feel free of concerns.” Prior to the statement, MFDS has disclosed 31 out of 288 metformin products available in the Korean market were discovered with NDMA exceeding the acceptable daily intake limit (96 nanograms), and halted manufacturing and sales of those 31 products. Metformin is the most widely used first-line treatment for type 2 diabetes with outstanding effect of lowering blood sugar level and many other advantages. But when Singapore’s Health Sciences Authority (HSA) announced last December that three out of 46 metformin products were found with NDMA surpassing the acceptable level, KDA has officially requested the Korean government to run a full investigation on the contamination of NDMA in metformin ingredients and finished products used in Korea. However, the medical experts stressed the government’s action should not cause a confusion for the diabetic patients using metformin. The statement advised, “31 products exceeding the acceptable limit of NDMA should not be prescribed anymore, but as MFDS has elaborated, the risk of developing cancer only from taking those products is extremely low,” and “it is not advisable for diabetic patients to stop consuming those metformin drugs without consulting their doctors, but they should rather seek for new prescription for other metformin product with safe level of NDMA.” According to the human body impact assessment by MFDS, 0.21 out of 10,000 patients, who have been taking the maximum dose of metformin product with unacceptable level of NDMA since the point of approval to the end of this year, would be risked to develop cancer. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline (ICH M7: Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk) mentions the risk of developing cancer could be dismissed, if it is lower than 1 out of 10,000 people. The academic societies highlighted, “Metformin is an important drug recommended by international practice guidelines as a first-line medicine to treat patients with type 2 diabetes. And as there are nine different classes of diabetic treatments available, the government should amend the healthcare reimbursement standard to disperse concentrated use of one specific pharmaceutical substance and give various options for first-line drug based on patients’ condition.” They also added, “KDA and KES would closely cooperate with the government for enhance the health and the rights of diabetic patients, and actively participate in improving the medical system.”
- Product
- Hospitals ready to prescribe another CDK inhibitor Kisqali
- by Eo, Yun-Ho May 21, 2020 06:01am
- Following after Ibrance and Verzenio, a third cyclin-dependent kinase 4 and 6 (CDK 4/6) inhibitor is landing its prescription code in Korean general hospitals. Pharmaceutical industry sources reported drug committees at eight major general hospitals like Seoul National University Hospital, Seoul Asan Medical Center, National Cancer Center, Korea University Anam Hospital and Konkuk University Hospital have recently cleared Novartis’ Kisqali (ribociclib). While awaiting Drug Reimbursement Evaluation Committee’s (DREC) nod after passing Health Insurance Review and Assessment Service (HIRA) Cancer Deliberation Committee in last January, Kisqali is preparing for prescription code-in process at major hospitals. Unlike Ibrance (palbociclib) and Verzenio (abemaciclib), Kisqali can be prescribed to premenopausal and postmenopausal patients who have not had an oophorectomy. Phase III MONALEESA-7 study evaluated Kisqali plus endocrine therapy (either an aromatase inhibitor or ovarian function suppression) against existing endocrine single therapy as first-line treatment for pre and perimenopausal women with HR+/HER2- advanced or metastatic breast cancer. The result found that the combination therapy significantly extended patient’s overall survival (OS). Specifically, the study confirmed the patient group receiving Kisqali combination therapy had median progression free survival (mPFS) of 23.8 months, whereas the group receiving endocrine therapy alone had mPFS of 13 months. In an Asian subgroup analysis, Kisqali combination therapy group reached mPFS of 24.7 months, about 14 months longer than endocrine therapy only group. Moreover, MONALEESA-3 study confirmed Kisqali extending the OS of pre and postmenopausal women. At 42 months, estimated rates of survival were 58 percent for Kisqali combination therapy group and 46 percent for fulvestrant alone. Professor Im Seock-ah of Hemato-oncology Department at Seoul National University Hospital elaborated, “MONALEESA-7 was actively proposed and led by Asian researchers. The fact that 30 percent of patients registered for the clinical study were Asians reflects the demand for new breast cancer treatment option for premenopausal patients is high in the Asian region.”