- LOGIN
- MemberShip
- 2026-03-10 02:17:22
- Product
- AZ-Pfizer vaccine mix is "confirmed safe and effective"
- by Jul 05, 2021 05:54am
- With community pharmacists who have received their first vaccination of AstraZeneca’s COVID-19 vaccine awaiting their second jab with the Pfizer vaccine this month, the health authorities announced that there are no safety concerns regarding the ‘mix-and-match' of the vaccines. The authorities explained that though mild side effects were observed, no severe adverse events were reported after receiving the vaccine mix, therefore, there are no safety issues pertaining to mixing doses of the two vaccines. Guidelines with the said information was issued by the COVID-19 Vaccination Promotion Team in response to the safety concerns raised by some ahead of the ‘temporary mixed-dose inoculation’ that will be provided to the 76,000 people who are working in vulnerable facilities, flight attendants, healthcare workers, and essential workers that already received their 1st dose with the AstraZeneca vaccine from April to May. The move for this mix and match schedule passed deliberation of the Korea Advisory Committee on Immunization Practice (KAICP) with a review on the efficacy and safety of the mixed-dose vaccination to address the delay of the 835,000 vaccine doses that were initially scheduled to arrive by the end of June through the COVAX facility. In other words, the mixed two-dose schedule that uses the Pfizer vaccine as a second dose is a temporary measure decided upon in consideration of the current supply shortage. Community pharmacists under the age of 50 (born after January 1st, 1972) who already received their first vaccinations will have to receive their second shot with the Pfizer vaccine due to age restrictions that were set on using the AstraZeneca vaccine. However, pharmacists who are 50 years or older (born before December 31st, 1971) may receive the AstraZeneca vaccine if they desire. Reservations for the second dose are automatically made to the same institution at which the individual received their first dose, on the 7th day of the 11th week after his/her first vaccination. Individuals may change their vaccination date in the 11th-12th week period after their first vaccination. They may postpone the date by 1 week at most from the originally scheduled date, and it may not be pulled forwarded to an earlier date. “Other countries such as Germany, France, Canada, Norway, Sweden have been recommending mixing use of mRNA vaccines to those who were first vaccinated with the AstraZeneca vaccine. Also, the safety and efficacy of using the Pfizer vaccine as a second dose was confirmed in people who were first vaccinated with the AstraZeneca vaccine in Canada and Spain,” said the COVID-19 Vaccination Promotion Team. Studies from Spain and Germany have shown that vaccinating with the AstraZeneca-Pfizer mix showed a better immune effect with increased neutralizing activity against the COVID-19 variants than one or two shots of the AstraZeneca vaccine. The team added that a study on this mixed-dose vaccination is also currently underway in Korea and that the initial results will be shared in early July. Regarding the adverse events of the dose-mixing, the team added, “Dose-mixing studies abroad have shown that fever increased when the AstraZeneca vaccine was administered first and followed by Pfizer compared to when a same vaccine was administered both times. The results were also the same for the occurrence of mild adverse events such as fatigue, injection site pain, headaches, and muscle pain, but no serious abnormalities were confirmed." The inoculation schedule for the administration of the mixed second dose will follow the period set during the first vaccination. The mix currently planned is the 1st AstraZeneca-2nd Pfizer vaccine mix, and will be administered with an 11-12 week dosing interval according to the AstraZeneca vaccine’s dosing interval. People who must postpone receiving their second shot while following the AstraZeneca-AstraZeneca vaccine schedule are recommended to receive their shots within 16 weeks, which means the second dose should be administered from July 26th to July 31st. In cases where ‘an individual does not receive their second dose due to fear of mixing vaccines,’ the team said, “The second vaccination, as in the first vaccination, will be administered based on each individuals’ consent, so we cannot force an individual to vaccinate with a different vaccine if he/she is not comfortable receiving the AstraZeneca-Pfizer mix. Those who wish to receive the AstraZeneca vaccine rather than Pfizer’s may be able to receive their shots after August 1st, depending on the supply situation.”
- Product
- Sales prices have risen due to increased Tylenol demand?
- by Jung, Heung-Jun Jun 23, 2021 05:54am
- Pharmacists are upset when it is pointed out that pharmacies have raised the sales price of Tylenol due to increased demand for Tylenol. It is a misunderstanding that pharmacies are making great profits by raising prices unlike existing sales prices, which was actually a price change in some pharmacies due to differences in supply prices. Some media reported that the price of Tylenol, which was previously purchased at ₩2,000, rose due to the price adjustment due to the shortage. Recently, the price of Tylenol exceeded ₩2,000, forcing pharmacists to protest against the report. local pharmacist A also sent a protest mail to the media company, attaching a transaction statement with the unit price of supply. The unit price of pharmacies has already exceeded ₩2,000 (Pharmacist A said, "It is seriously distorted. Should consumers pay by credit card at ₩2,000, lower than the unit price supplied to pharmacies?" He said, "It further hurt pharmacists suffering from inappropriate comments on Tylenol." The supply price of 5 million tablets of Tylenol, which were recently divided into the first and second rounds, increased by about 10% compared to the supply price of individual pharmacies. Therefore, some pharmacies were adjusting their selling prices slightly. "As Tylenol is supplied equally across the country, the purchase price has risen slightly to pharmacies. Pharmacists are also unhappy about that," he said. "Some pharmacies have adjusted their sales prices and raised our pharmacies by ₩500 because they have already risen from the previous price." The first and second supplies to pharmacies nationwide will be distributed early this week. While 5 million tablets were supplied, Hangaram Pharmaceutical Co., Ltd., which is in charge of distribution, was not receiving any separate orders. With all the units supplied, Hangaram Pharmaceutical will resume its individual orders at pharmacies.
- Product
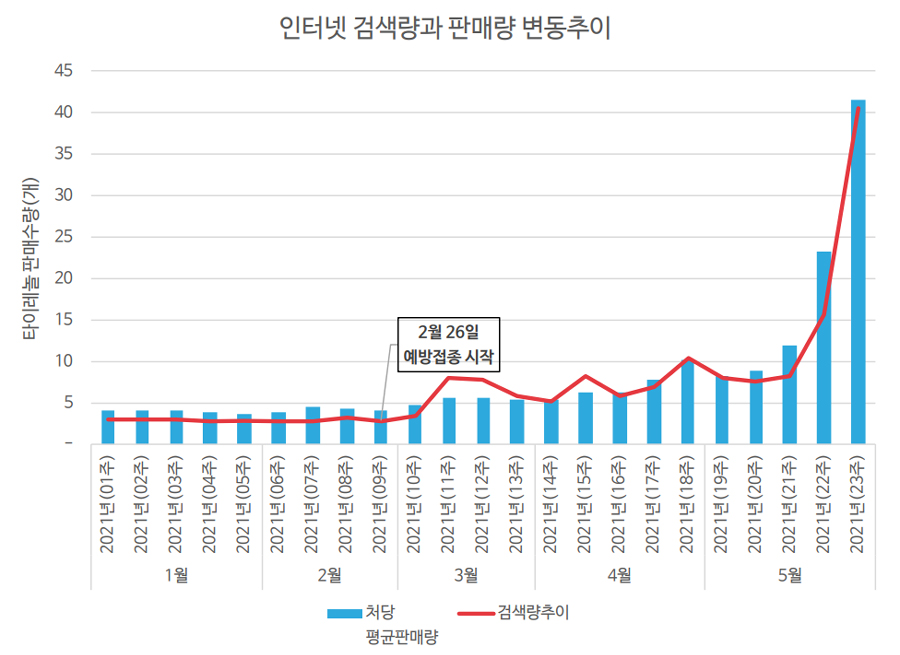
- Tylenol sales increased 10 times to peak in May
- by Jun 21, 2021 05:50am
- Pharmacies' difficulties have reached their peak due to the surging demand for Tylenol, which is said to be "more precious than COVID-19 vaccine." This is because Tylenol has become so precious that it is rarely found in pharmacies. Then how popular was Tylenol? Analysis of POS data from 185 pharmacies showed that sales of Tylenol 500 mg and Tylenol ER 8HR increased 10 times compared to the beginning of this year. According to a survey of sales at 185 pharmacies, Care Insights recorded a peak in May after steadily increasing in March and April since the COVID-19 vaccination began on February 26. In particular, demand more than quadrupled compared to the average year in May. According to this year's sales, 12,467 in January, 12,222 in February, 20,756 in March, 29,447 in April and 54,340 in May. From January to May, 129,232 units were sold. Compared to 126,590 in 2018, 138,327 in 2019, and 173,687 in 2020, the company sold a year's worth in five months. The market share of Tylenol in Pharmacies was also overwhelming with 92.4%. Other APAP formulations accounted for only 7.6%. Considering that the average Tylenol sales share was 82.6% last year, the market share itself has risen further. An official from Care Insights said, "The more Tylenol is mentioned through the media, the more Tylenol sales tend to increase." The official said, "Although 100 to 200 Tylenols are supplied per pharmacy, this is believed to be the amount that can only be sold for about 15 days," and added, "As the vaccination rate is increasing, it seems necessary to come up with measures."
- Product
- 5 mil Tylenols will be released to market…100 per pharmacy
- by Jun 14, 2021 05:56am
- ‘Public Tylenol will be supplied,’ ‘Why is the government unnecessarily supplying Tylenol to every pharmacy?’ On the 11th, the pharmacists’ community heat up over the Tylenol supply issue. The issue was sparked with some distributors spreading the news that ‘public rationed Tylenol will be released in the market from the 14th.' The social media chat rooms of pharmacists heated up with intense debate at the news that was spread before the government’s announcement. To clarify, the to-be-supplied Tylenol is not ‘public’ Tylenol. It is a measure made by the government to solve the current Tylenol shortage, under which the government plans to release the maximum amount of antipyretic analgesics to the market. From the released products, each pharmacy may order the amount needed, therefore the measure is not public rationing in any sense. Also, pharmacies that do not need Tylenol do not need to take up the supply, so there is no need for pharmacies to complain ‘Why the government is unnecessarily supplying Tylenol to every pharmacy?’ But it is true that the Ministry of Food and Drug Safety (MFDS) has called on relevant associations including the pharmacists', pharmaceutical, and distribution associations to address the issue. The MFDS had recently called on the Korean Pharmacists’ association, Korea Pharmaceutical and Bio-Pharma Manufacturers Association, and Korean Pharmaceuticals Distribution Association to resolve the shortage caused by the surge in demand. The government’s goal is to ‘allow the public to be able to purchase acetaminophen as needed after COVID-19 vaccinations without differences by region or pharmacy by resolving the supply-demand imbalance in pharmacies.’ As a result, 5 million 500mg Tylenol will be released on the market. This is the total amount that Johnson & Johnson had been stockpiling for the next year, and this total amount will be released in two phases, 2.4 million and 2.6 million, respectively. Each company may first secure 100 each, and the supply will be avaialbe as early as from the 15th, depending on the pharmacy. 40 wholesalers will oversee the distribution. The Korean Pharmaceuticals Distribution Association divided the pharmacies by region for 40 companies to cover. For example, a pharmacy located in Yeongdeungpo district in Seoul will receive 100 Tylenols from BaekGwang Pharm, which is in charge of the Yeongdeungpo area, even if the pharmacy uses multiple distributors like BaekGwang Pharm, Geo-young, and Yujin Pharm. The order method may also differ by the wholesaler. Some distributors may call the pharmacy or make visits to each pharmacy to distribute Tylenol. “To resolve the issue of pharmacies that works with various distributors, we divided the regions between 40 wholesalers,” explained an official from the Korean Pharmaceuticals Distribution Association. “The necessary amount may differ by the size and situation of each pharmacy, so after fairly distributing 100 to each pharmacy that wants Tylenol, the rest will be left to be ordered at an as-needed basis by each pharmacy.” The MFDS also called on each association to play its part in resolving the Tylenol shortage issue. First, the Korean Pharmacists’ association will inform consumers about the correct use of antipyretic analgesics after vaccination through medication counseling, and continue to conduct a campaign informing the public that all domestically approved acetaminophen products have the same efficacy and effect as Tylenol. The Korea Pharmaceutical and Bio-Pharma Manufacturers Association and acetaminophen producers will maximize the production volume of their drugs by releasing their inventory by mid-June as well as by mobilizing all capabilities available to adjust the production schedule to expedite supply. MFDS said, “The Ministry of Food and Drug Safety and relevant organizations will do the best to supply acetaminophen to the people in need at the right time according to the government's vaccination plan. Also, we will share the supply and demand status with relevant institutions through regular monitoring and immediately provide the necessary administrative support to suppliers to increase production of acetaminophen products.”
- Product
- Ask for Acetaminophen, instead of Tylenol
- by Kang, Shin-Kook Jun 08, 2021 09:12pm
- "If fever or muscle pain occurs after vaccination, ask for Acetaminophen to the pharmacy." As the Tylenol shortage continues, the movement to change public perception begins. Rep. Seo Young-seok of the Democratic Party of Korea visited the Korean Pharmaceutical Association Hall on the morning of the 4th and announced that he would start an SNS challenge with Chairman Kim Dae-up. Chairman Kim Dae-up & Seo Young-seok, a member of the Democratic Party of Korea, who started SNS challenge Representative Seo said, "The vaccination target is 13 million by the end of this month, and the quarantine authorities' plan is to start vaccinations by July." "The demand for painkillers is expected to surge due to the expansion of vaccinations, and if the stock is low, people will have to visit many pharmacies to buy Tylenol," he said. He said, "With this phenomenon of Tylenol shortage, we need to draw the right public perception of the same active ingredient. There are about 70 generics of the same active ingredient, and I hope it will serve as an opportunity to discuss why there was a phenomenon in which only certain brands, Tylenol, were favored and solutions," he explained the background of the SNS challenge. "The domestic pharmaceutical market is very strange," said Chairman Kim Dae-up. "There are many generics that cannot even be compared to foreign countries, and there are too many items of the same active ingredient as the consignment market." Chairman Kim said, "Tylenol was sold out as the KDCA promoted to take Tylenol after vaccination." "Some pharmacies have to say that Tylenol is out of stock 100 times," he said, criticizing the health authorities' initial response. Chairman Kim said, "The KDCA should be responsible for distributing unofficial note prescriptions and asking vaccination centers to take Tylenol." "I am happy and grateful that Rep. Seo Young-seok joined the movement to change public awareness under such circumstances," he said. He then pointed to Yoon Sun-hee, chairman of the Bucheon Pharmaceutical Association, and Nam In-soon, a member of the Democratic Party of Korea, after Suh, who started the first SNS challenge. Chairman Kim named Han Dong-joo, head of the Seoul Pharmaceutical Association, and Cho Sang-il, head of the Incheon Pharmaceutical Association, as SNS challenge runners.
- Product
- Tylenol's sales at convenience stores rose 125% year-on-year
- by Jun 04, 2021 06:06am
- Convenience stores are also benefiting from the shortage of outpatient pharmacies' Tylenol as vaccination begins in earnest.Tylenol sales at convenience stores designated as household medicine rose. Household medicine sold at convenience stores First of all, CU's Tylenol sales increased 125.5% year-on-year. According to sales from May 27 to May 31, when vaccinations for people aged 65 or older began in earnest, it increased 89.9% compared to the previous month (April 26 to 30), and it also increased 68.1% compared to the previous week (May 20 to 24). In the case of Seven-Eleven convenience stores, sales of Tylenol increased 23.3% compared to the previous month, especially sales from the 27th to 30th increased 99.6% from the same period last month. In the case of GS25 convenience stores, Tylenol sales rose 41.9% last month from a year ago and 38.8% compared to the previous month, the survey showed. In some convenience stores, Tylenol is in short supply like pharmacies. "The number of consumers visiting Tylenol has increased recently," said CU convenience store owner in Seoul. Both Tylenol 500mg and Tylenol160 mg are out of stock, he said. "We only have children's syrup in stock at the moment." Meanwhile, the Gyeonggi Pharmaceutical Association criticized the government and the vaccination center's inexperienced administration for causing Tylenol shortage on the 1st and insisted that Tylenol should not be sold at convenience stores for a while as it is time to manage side effects.
- Product
- Korean Tylenol is in stock
- by Jun 04, 2021 06:05am
- "It doesn't have to be original Tylenol." Pharmacists have launched a counterattack in connection with the Tylenol shortage. As Tylenol has been out of stock for months to respond to consumers due to recommendations from vaccination centers and health centers, pharmacists are trying to immediately inform them of fever and painkillers. They started to improve consumer awareness by spreading the message that It doesn't have to be original Tylenol through pharmacies and SNS. First of all, pharmacist Lee Jung-chul, the author of "Alssulshinyak" told by dispensing brothers, recently posted a message on blogs and YouTube saying, "You don't have to stick to Tylenol after the vaccine against COVID-19." The pharmacist recognizes that "the painkillers available after COVID-19 vaccine are Tylenol unconditionally, but there is no need to insist on Tylenol." Tylenol is just one of many drugs made of Acetaminophen, and said, "There are many drugs made of the same Acetaminophen even if they are not Tylenol." Since Tylenol has become an issue due to its high recognition of many drugs with Acetaminophen, there is no problem in taking Acetaminophen, the same active ingredient as Tylenol, in situations where Tylenol is not easy to obtain. "About 10 to 20 consumers a day visit and ask for Tylenol," said pharmacist Lee Jung-chul. "It doesn't matter if it's Acetaminophen, but if patients don't understand or if the media or hospitals nominate Tylenol, they often think they can't do other drugs," he said. "I'm filming a video out of frustration, and I hope the message will be shared widely." The pharmacist explained, "In fact, the number of cases where people accept and buy other products is increasing if they use the 'Ask a Pharmacist' card news produced by the Korean Pharmaceutical Association." Tylenol-related notice attached to the entrance of a pharmacy in Incheon A pharmacist in Incheon recently attached a sign that Tylenol is out of stock and there's a stock of same active ingredient to the entrance of a pharmacy and a consultation desk. Tylenol is Acetaminophen formulations made by Janssen, a foreign company, under the notice, "If you take fever and painkillers after the COVD-19 vaccine, take Acetaminophen-based medicine." There are 80 items such as Suspen, Tramol, Tacenol, Nasphen, and Setopen," a notice said. The pharmacist said, "When asked repeatedly by consumers if they had Tylenol in stock all day, the voices of working pharmacists were hoarse. "It was like the public mask crisis in the past," he said. "We changed our sales strategy because of the release of good domestic drugs." In addition, pharmacists who run SNS channels such as blogs, Instagram, and YouTube are also participating in "Knowing About Acetaminophen Right away," explaining the types of Acetaminophen, how to take it, and alternatives. The Korean Pharmaceutical Association and the MFDS urgently produced posters and distributed them to pharmacies about the ridiculous situation of trying to buy only Tylenol at pharmacies despite the presence of a number of Acetaminophenes. Posters distributed by the Korean Pharmaceutical Association & the MFDS The Korean Pharmaceutical Association and the MFDS posted a poster saying, 'Relax if some items are out of stock. There are about 70 Acetaminophen formulations approved by the MFDS. Talk to a pharmacist' in the message.
- Product
- Zuellig Pharma ends distribution at the end of this month
- by May 24, 2021 05:51am
- Zuellig Pharma Korea, a global drug distribution company, announced its order and return procedures to prevent confusion as of this month. According to pharmacies on the 24th, Zuellig Pharma Korea recently announced a re-announcement of the termination of the pharmacy distribution business to pharmacies and others. Although it has already provided guidance to pharmacies and others since early last month, it has been announced again about a week before the end. Zuellig Pharma Korea faced limited financial soundness due to worsening profits, and finally ended up abolishing its pharmacy retail division and conducting large-scale restructuring. About 80% of employees in the business department were reduced, leaving only a small number of employees. Announcement of Zuellig Pharma Korea Zuellig Pharma Korea said, "We decided to end our business of distributing medicines directly to pharmacies on May 31 and distribute them only through wholesale partners. We will service the distribution of Rx drugs directly through Kyungdongsa." The explanation is that drugs supplied through Zuellig Pharma Korea can be returned in accordance with normal procedures even after the business is closed. Pharmacies are confused. This is because pharmacies with relatively large transactions seem to continue through wholesale, but pharmacies with relatively small transactions may have returned them sequentially. "I've heard from the person in charge since April about the end of distribution due to accumulated deficits," a pharmacist at Pharmacy A said. "There was an advantage of direct transactions, but I don't know what will happen in the future." "It is expected that there will be no significant impact on continuous transactions, but I understand that some pharmacies with small transactions have already started organizing items," he said. Zuellig Pharma Korea plans to offer promotions for OTC through its affiliate ZP Therapeutics and its medical merchandiser. Sales of Zuellig Pharma Korea rose every year, but its operating loss in 2017 was ₩2.6 billion, in 2018 was ₩8.4 billion, and in 2019 was ₩6.8 billion. The debt ratio in 2016 exceeded the 200% level, which is a risk level of 1,720%, and it recorded 30,000% in 2019
- Product
- Will Kypris become dominant in multiple myeloma markets?
- by Whang, byung-woo May 10, 2021 05:56am
- "New treatments will also emerge in the treatment of myeloma. However, its importance is still expected to continue when Kypris compares combined therapy features with a single formulation and other formulations." As multiple myeloma is difficult to completely heal and many recurrences occur, recurrence and non-responsive polymyeloma is considered the key to treatment. In the domestic treatment situation, Kyprolis-focused KRd therapy (Carfilzomib, Lenalidomide, and Dexamethasone) became the main treatment strategy, as third-generation proteasome inhibitors (PI drugs) became the standard of treatment. However, since March, IRd therapy (Ixazomib+Lenalidomide+Dexamethasone), which is centered on the oral PI drug Ninlaro, has become convenient, and the market for prescribing recurrent and non-responsive multiple myeloma has begun to become active. Accordingly, let's listen to the strategies and perspectives of treating multiple myeloma through Joseph Mikhael, a professor of applied genomics at the Korean Society of Hematology International Conference. First of all, what he emphasized in his presentation at the Korean Society of Hematology conference is the importance of early treatment strategies for patients with recurrent and unresponsive multiple myeloma. As recurrence and non-responsive multiple myeloma have various options not only in the U.S. but also in Korea, optimal measures that take into account the characteristics of patients should be considered first. Professor Joseph Mikhael said, "It is very encouraging that there are more treatment options to choose from now on compared to the past. We expect more options for treating recurrent and unresponsive multiple myeloma in the future." Professor Joseph Mikhael pays attention to the fact that Kyprolis has achieved a 12-month extension of the PFS (Present Progressive Survival Period) compared to conventional treatments in treatment of recurrent and non-responsive multiple myeloma. "The improvements in the duration of the existing treatment are significant, compared to four to six months and eight months at the most," he said. "It's a great achievement in that about six months is a common improvement in other treatments." Professor Mikhael emphasized that Kypris clearly has an advantage in terms of efficacy compared to other PI agents, Velcade (Bortezomib) and Ninlaro (Ixazomib). "Kyprolis still has the greatest effectiveness of PI drugs used to treat multiple myeloma," he said. "A study directly compared to Velcade concluded that Kyprolis is the most effective treatment needed by medical staff and patients." Treatment options for increased myeloma. What's the situation in the U.S.? In Korea, as IRd therapy has recently been reimbursed, options for clinical doctors have also increased. In the United States, where both KRd and IRd therapy were available, how would the choice of prescription between the two treatments appear? Professor Mikhael said that KRd therapy is preferred, assuming that there are many options. "If a patient has an autologous hematopoietic stem cell transplant, there is a maintenance therapy based on Levlimide, and Kyprolis and Ninlaro are available options," He said. "KRd therapy is mainly chosen because Kyprolis is more effective." IRd therapy can be selected if patients want to avoid Kyprolis because they are concerned about abnormal reactions related to the heart, but IRd therapy is less used in the United States than KRd therapy. However, as Professor Mikhael mentioned earlier, Kyprolis' biggest concern is that patients with heart-related comorbidities require attention. Considering that multiple myeloma comes from older people aged 65 or older, concerns about heart failure and hypertension abnormalities of Kyprolis cannot be ruled out. Professor Mikhael acknowledged the abnormal response that could appear as Kypris, but evaluated it as manageable. "After more than 10 years of use of Kyprolis, experience in abnormal reactions can be accumulated and reduced to 2%," he said. "2% of patients should stop taking medication, but this situation is very small in the actual field."
- Product
- Pharmacists & employees in their 20s receive Pfizer vaccine
- by May 07, 2021 06:01am
- Pharmacists and employees under the age of 30 who were excluded from the AZ vaccination are expected to receive the Pfizer vaccine next month. The KCDA announced on the 4th that the Pfizer vaccine for social essential personnel and military soldiers under the age of 30 who have been suspended from vaccinating AZ vaccine due to "thrombotic side effects" will begin in June. According to statistics of the Korea Institute for Pharmaceutical Policy affairs, 1,784 pharmacists under the age of 30 have completed their personal reports to The Korean Pharmaceutical Association, and 2,000 including pharmacists who have not reported. "Pharmacists are also among the essential social workers," a pharmacist said. "We have not yet been informed of specific vaccination personnel by the KCDA, but pharmacists and employees under the age of 30 who were excluded from the vaccination will be vaccinated this time." According to COVID-19 vaccination status released byCOVID-19 vaccination task force, 197,092 out of 335,017 people who were vaccinated as of midnight on the 2nd completed the vaccination rate, recording 58.8%. If this includes personnel who will receive the Pfizer vaccine, the inoculation rate is expected to increase slightly.