- LOGIN
- MemberShip
- 2026-03-10 02:17:19
- Product
- α-GPC can't be replaced with other drugs in the field
- by Kim, Jin-Gu Nov 25, 2021 05:55am
- Apart from the steady increase in the number of patients over the past 20 years, there has been no significant change in dementia treatments used in Clinic sites. However, two major changes have been predicted in the past two years. One is the controversy over the preparation of Choline alfoscerate in Korea, and the other is the controversy surrounding Aducanumab, a new dementia treatment drug that has been released for more than 20 years. The controversy is whether the drug is effective enough. The solution to the controversy is quite similar. Regulators in South Korea and the U.S. have instructed the controversy surrounding the two drugs to properly verify their validity through "clinical re-evaluation." What do the front-line prescription sites think about these controversies? Regarding the controversy over Choline alfoscerate, Ha Sang-wook, head of On Hospital, said, "It seems to help clinically improve cognitive function in early dementia," adding, "There will be positive results that re-verify the effectiveness of clinical re-evaluation." ◆ The prescription amount is similar to the previous one in reducing the benefit of Choline alfoscerate The core of the controversy is the effectiveness of Choline alfoscerate. This is because drugs were recognized as medicines in Italy, where drugs were developed, while in other countries they were sometimes used as health functional foods. In the end, the Ministry of Food and Drug Safety ordered a "clinical re-evaluation" last year to re-evaluate the safety and effectiveness of Choline alfoscerate on its own. 57 companies, including Daewoong Bio and Chong Kun Dang, have begun clinical re-evaluation. The Ministry of Health and Welfare has reduced the benefit of the Choline alfoscerate. Since August last year, when patients who have not been diagnosed with dementia use Choline alfoscerate, the drug price rate has risen from 30% to 80%. Pharmaceutical companies have actively taken legal action. Currently, a lawsuit for revocation of administrative disposition is underway. Regarding the controversy over the effectiveness of Choline alfoscerate, manager Ha Sang-wook explained, "There are many studies that improve cognitive function when dementia or dementia drugs are not activated in the early stages." He said, "Clinically, when replaced with Choline alfoscerate from other drugs, it is observed that it leads to cognitive improvement." For this reason, he explained that despite the reduced benefit, the prescription amount is almost the same as before. Manager Ha Sang-wook said, "First of all, it is a drug that patients are very satisfied with. Even if the prescription is changed to another drug, it is often prescribed again as Choline alfoscerate, he said. "I understand that it is prescribed steadily not only in our hospital but also in other places." He said there was no suitable drug to replace Choline alfoscerate. He said, "Except for Choline alfoscerate, there is virtually no drug to prescribe to patients." He said, "In particular, in the case of vascular dementia, Donepezil is not applied, so it cannot be used. Choline alfoscerate is the drug to be used, and if this drug is not allowed to be used, it is quite difficult for patients and doctors to use it, he said. He was also optimistic about the results of clinical re-evaluation. Manager Ha Sang-wook explained, "The key will be how many patients with hidden cognitive impairment are discovered." He said, "I think more patients with cognitive impairment will get good enough results if they participate in clinical trials. The Dementia Association is also formed in a positive direction". ◆"Patients' interest in Aducanumab is increasing rapidly Expectations were high for the new dementia treatment "Aducanumab" recently approved in the United States. In June this year, the U.S. Food and Drug Administration (FDA) approved Aducanumab, co-developed by Biogen and Eisai, as a treatment for Alzheimer's dementia. It has been about 20 years since Allergan's Namenda in 2003. However, controversy over the validity of this drug has continued since the approval process. Conflicting results were found in two clinical trials conducted by Biogen. For this reason, the FDA Advisory Committee issued a non-approval recommendation in November last year. The FDA did not accept the advisory committee's opinion. However, in consideration of the controversy over its validity, the condition of "reevaluation after clinical trials" was attached. Biogen should reevaluate Aducanumab through post-marketing clinical trials. If the efficacy of Aducanumab is not proven in the re-evaluation, the approval will be revoked. In terms of controversy alone, it is almost similar to the case of Choline alfoscerate in Korea. Many patients ask a lot about this drug, he said. "The success of Aducanumab will significantly change the direction of dementia treatment itself. If proven effective, dementia will turn into a conquerable disease. On the contrary, if clinical trials fail, follow-up drugs of the same mechanism are likely to fail, he said. Since the approval of Aducanumab, several pharmaceutical companies have been developing drugs with beta-amyloid blocking mechanisms. In June this year, Eli Lilly's "Donanemab" and "Lecanemab," co-developed by Eisai and Biogen, were designated by the FDA for breakthrough therapy designation one after another. Both drugs are undergoing phase 3 global clinical trials.
- Product
- AstraZeneca vaccination is expected to end as of this year
- by Nov 24, 2021 05:53am
- The first inoculation will end at the end of November, and for the second inoculation, all inoculations will end on the 31st of next month. Medical institutions that have AstraZeneca vaccines should provide primary vaccinations with vaccines they have, and inform the inoculated that secondary vaccinations should be cross-vaccinated with Pfizer vaccines. However, if the vaccinated person want to have AZ shot, the AstraZeneca vaccine can also be vaccinated until December 31 of this year. The KDCA recently guided medical institutions entrusted with COVID-19 vaccination on the end of the AstraZeneca vaccination. Those who have already been scheduled for the second vaccination with AstraZeneca vaccine will be vaccinated according to their reservation details, but if they wish, cross-vaccination is also possible through the health center. However, those who are scheduled for the second vaccination with AstraZeneca vaccine in 2022 will be changed to cross-vaccination. The KDCA urged consigned medical institutions to cooperate to ensure safe vaccination by familiarizing themselves with the expiration date of the vaccine they have and how to inoculate them. The COVID-19 vaccination response promotion team explained, "There are no plans to introduce additional AstraZeneca vaccines, and the validity period of the previously introduced inventory is imminent, so it is necessary to review the inoculation plan considering the amount available."
- Product
- Expansion of non-face-to-face tx little by little
- by Nov 18, 2021 05:54am
- Despite opposition from the KMA to expand non-face-to-face treatment using regulatory sandboxes, it is judged that the satisfaction of overseas Koreans is high and it will help resolve medical blind spots. However, concerns about allowing regulatory special cases are also expected to increase, taking advantage of regulatory sandboxes. Ministry of trade, industry and energy deliberated and resolved a total of 14 cases such as on-face-to-face overseas Koreans' care services including digital switching of tasks and tasks, such as carbon neutral through the 5th Industrial Convergence Regulation Special Deliberation Committee. Temporary permission for the non-face-to-face overseas Koreans' care and counseling is a major medical foundation Myongji. Myongji Hospital provides services such as medical counseling and medical treatment to overseas Koreans through telephone and video using an online platform, and medical staff judges and issues prescriptions when requested by patients. This is the same as the approval agenda for Life Semantics Corp. and Inha University Hospital from 20 to 2021. There are many people who have difficulty using medical services due to language and medical accessibility problems or are excluded from access to local hospitals due to their citizens' priority policy abroad. In particular, temporary permission was granted through sandboxes with the aim of "protecting the Korean people to the end," saying that SOS is lined up from overseas workers in the Middle East, where the number of COVID-19 confirmed cases is soaring. This year, Ministry of trade, industry and energy said, "Under the current medical law, telemedicine is only allowed for doctor-medical people, and medical practices such as doctor-patient diagnosis and prescription are prohibited in principle. The Special Regulatory Committee approved additional temporary permits." However, to prevent diplomatic and trade problems, services were provided to the extent that they did not violate local laws, and the same conditions as the existing approval agenda, such as medical mediation, were added. Ministry of trade, industry and energy "non-face-to-face overseas Koreans ' care services, currently on projects on the table is high user satisfaction." said. The Ministry expected that in the future, overseas Koreans will be able to use various non-face-to-face medical services at more domestic medical institutions, which will increase their medical options. Last year, the KMA pointed out in a statement that telemedicine for overseas Koreans is ineffective. At the time, the KMA argued, "Telemedicine is likely to trigger competition between companies and industries that provide platforms and increase unnecessary demand, abandoning the basis of face-to-face treatment and the people's right to health."
- Product
- Claiming a more expensive drug than a generic
- by Kim JiEun Nov 11, 2021 06:00am
- Pharmacist A, who did not give a follow-up notice of general submission and charged for a drug that was more expensive than an alternative drug, filed a lawsuit claiming that it was an unfair disposition, but the court refused to accept it. The Seoul Northern District Court recently dismissed a claim filed by pharmacist A against the NHIS for the return of unfair gains. Pharmacist A did not notify the doctor after generic substitution of the drug from the MOHW in 2016, and was suspended for 50 days, claiming that the NHIS was unfairly paid about 34 million won. Afterwards, the NHIS disposed of the collection of about 34 million won in unfair claims through notification of the results of the on-site investigation by the MOHW. Since then, it has been executed in a way that deducts medical care benefits to be paid to pharmacies operated by pharmacist A. After the disposition, pharmacist A filed a lawsuit with the Seoul Administrative Court, and in 2018, the pharmacist won the case. For this reason, the court explained, "Some of the periods under investigation lack proof of the reasons for disposition, but the illegal parts of the disposition cannot be clearly distinguished, and some of the facts that were the basis for discretionary judgment are not recognized, forcing cancellation of all relevant dispositions." Immediately after that, the MOHW appealed to the Seoul High Court, but the pharmacist's victory was confirmed. Based on the ruling, the pharmacist filed a lawsuit against the corporation to recover unfair profits. Since the related disposition of the MOHW was canceled through administrative litigation, the disposition of the corporation, which recovered 34 million won in the name of unfair claims based on the same reason, is also illegal, so the recovered amount must be returned. However, the court did not accept it. It noted the reason why the court, which ruled in the previous ruling to cancel the administrative disposition of the MOHW, revealed it. The court explained that just because the MOHW's disposition was canceled, there was no reason to cancel the separate disposition, the NHIS' redemption measure, saying it was unfair. The court said, "The reason for canceling the disposition in an administrative lawsuit against the cancellation of the disposition of the MOHW is that there is a lack of proof of facts regarding 'part' of the reasons for the disposition." As a lack of proof of some of the reasons for disposition, it falls under the case where the presence or absence of the defect is revealed only when the facts are accurately investigated. It is difficult to say that the defect in the disposition of this case has reached a significant and obvious degree, it said. He then said, "It is a separate and independent disposition based on a separate provision of the (former) National Health Insurance Act, even though the facts and the previous disposition of the MOHW are common." The court said, "Even if the related disposition is canceled, the NHIS cannot be ordered to return the disposition because the degree of defect in separate disposition has not naturally reached invalidity. The pharmacist's claim is dismissed for no reason, it said.
- Product
- PO treatment for COVID-19 "Molnupiravir" will be released
- by Whang, byung-woo Oct 29, 2021 05:53am
- The emergence of Molnupiravir of MSD, known as the first oral treatment for COVID-19, is drawing attention to how it will affect the war against COVID-19. While it is compared to Tamiflu and is evaluated as a game changer, there are mixed views on the other side that its role may be limited due to price limitations. According to the interim results of phase 3 clinical trials of MOVe-OUT, which evaluated the efficacy of the oral corona treatment Molnupiravir by MSD on the 1st, 775 patients with mild and moderate symptoms had reduced hospitalization and mortality by about 50%, satisfying the primary evaluation index. At this time, the dose of Molnupiravir was taken twice a day, 10 times for 5 days, and as a result, 7.3% of patients worsened to severe and there were no deaths. 14.1% of patients taking placebo worsened to severe and 8 died. Based on these clinical results, MSD terminated the clinical trial early without the registration of additional clinical patients originally planned and submitted an application for emergency use approval to the FDA. Considering the trend so far, Molnupiravir, which has been effective in clinical trials, is cheaper than conventional injections, so it will not be too much to win the title of the first oral treatment for COVID-19. The FDA will closely review safety and effectiveness data to determine whether to approve or not, and a final conclusion is expected within a few weeks. "The reason for getting a vaccine is to prevent it from going from mild to severe, that is, how severe it is even if it is a breakthrough infection," said Kang Jinhan, head of the Vaccine Bio Research Institute at Catholic University. "In the case of flu, the prevention rate is only about 50%, but the oral treatment of COVID-19 will be meaningful in that way." Director Kang said, "There is a need for oral treatments as a strategy to go to the so-called With Corona like the flu," adding, "I think it will be a concept that prevents medical confusion by administering it early so that mild patients do not get serious." Can Molnupiravir, which has been proven effective, be like Tamiflu? One question here is whether Molnupiravir can play the same role as Tamiflu or Xofluza, a flu treatment, at a time when many experts predict that COVID-19 will become endemic like the flu in the future. According to the most recently developed CAPSTONE-1 clinical study by Xofluza, the median time required for the Xofluza administration group to relieve symptoms after administration was about 2.3 days (Tamiflu 3.3 days). In addition, the median time it took to fever was about 1 day (Tamiflu 1.8), and the effect of reducing virus levels was reduced by half in about 1 day (Xofluza about 4 days). Choi Young-joon (pediatric infection), a professor at Korea University Anam Hospital, said, "I remember that Tamiflu was first introduced as an endpoint and gradually expanded to shorten the duration of symptoms," adding, "Molnupiravir aims to treat death and severe infections in public health and may expand the scope of treatment in the future." In fact, according to the MOVe-OUT clinical evaluation index that evaluated the efficacy of Molnupiravir, the primary evaluation index is the rate of hospitalization, death, and side effects, but the second evaluation index is the decomposition or improvement time of the coronavirus, so the possibility remains. Another variable is that the expected price of Molnupiravir is set at about 830,000 won ($700). As a result, the current market for oral treatments for COVID-19 is expected to form from about 7 trillion won (6 billion dollars) to about 8 trillion won (7 billion won).
- Product
- Smoking cessation tx, sold out due to impurities
- by Kim JiEun Oct 01, 2021 06:08am
- According to local pharmacies on the 1st, most Bupropions used for smoking cessation treatment are sold out or lack of inventory, making it difficult to order at online malls. This out of stock is related to the recent controversy over the detection of impurities in the Varenicline. As NNV, a carcinogen, was detected in Champix (Varenicline), the company decided to voluntarily recover it. The MFDS explained that N-nitroso-varenicline (NNV) is very low in the domestic Varenicline, but concerns about the ingredients remained in the market. Pharmacists say that most hospitals and clinics, which have consulted and prescribed patients under the government's anti-smoking treatment support project, often prescribe alternatively with Bupropion. A local pharmacist said, "I was contacted by the hospital to recommend a replacement drug," adding, "It was extremely rare for the hospital to ask the pharmacy to prescribe the drug first, but I was also embarrassed." Pharmacies that need to prepare related drugs are experiencing considerable confusion. In particular, the situation is more serious in pharmacies where nearby hospitals and clinics participate in the government's anti-smoking treatment support project and have a large number of related prescription preparations. These pharmacy pharmacists complain that it is not easy to secure inventory of medicines that are usually prescribed. Currently, Bupropion, where hospitals and clinics have replaced the existing Champix (Varenicline), is a total of 6 products, including Nicopion Sr (Hanmi), Healthpion ER(Myungin), Addpion SR 150mg(Whanin), Papion(Korea Pharma), Well SR(Unimed), and Wellviewderma ER(Hutecs). These items can be prescribed for the government's anti-smoking treatment support project, and most of them are currently out of stock at online drug malls or only a few are in stock. Another pharmacist in Seoul said, "In the past, Champix monopolized the prescription of smoking cessation treatment," adding, "We contacted a nearby hospital and asked them to prescribe even a small amount of products in stock."
- Product
- Take Ibuprofen after Pfizer COVID vaccine??
- by Kim JiEun Sep 06, 2021 05:58am
- Following Tylenol, the purchase of related drugs is increasing as Ibuprofen-containing painkillers have been raised to prevent side effects of COVID vaccines in certain companies. According to outpatient pharmacies on the 6th, patients who have recently been vaccinated (Pfizer vaccines) have frequently sought Ibuprofen-containing anti-inflammatory drugs. If manufacturers have purchased Tylenol before or after COVID vaccine, they have recently been looking for Ibuprofen, particularly IBU 600mg, among Pfizer vaccine or Moderna vaccine. The reason why people only look for Ibuprofen is that videos of some specialists have been affecting online or YouTube recently. This is because some media have suggested that myocarditis, and pericarditis caused by Moderna vaccine or Pfizer vaccine should be prevented by Ibuprofen's anti-inflammatory action. Some of the experts who actually run YouTube recommend taking Ibuprofen if they find chest pain after vaccination or prevention of myocarditis, one of the possible side effects of Pfizer vaccine. Pharmaceutical companies with Ibuprofen also posted advertisements in online malls exclusively for pharmacists to take medicine for abnormal reactions such as fever and pain after vaccination. A pharmacist in Seoul said, "There are quite a few patients who get Ibuprofen 600mg," explaining on YouTube that it is good to take Ibuprofen-containing anti-inflammatory pain medication for muscle pain after getting the shot. "I dion't know how to explain this," he said. As the number of patients wishing to purchase Ibuprofen-containing anti-inflammatory painkillers before vaccination increases, many pharmacists are also explaining the related information through blogs and YouTube. Outpatient pharmacists are struggling with medication guidance to patients who believe in in information that has not been immediately confirmed. Another pharmacist in Seoul said, "Acetaminophen was initially recommended after COVID vaccine because it was believed that taking anti-inflammatory anti-inflammatory drugs could interfere with antibody formation. If there are any side effects to Acetaminophen, it is okay to take other anti-inflammatory drugs. However, it is not true to say that Ibuprofen is the only drug after the Pfizer vaccination. Another pharmacist said, "Patients mistakenly believe that Pfizer vaccine can lead to cardiomyopathy, and that Ibuprofen can cure myocarditis" However, if the patient still wants to take Ibuprofen, we have no choice but to do so.
- Product
- Disturbance to secure inventory of Diovan and Exforge
- by Jung, Heung-Jun Sep 05, 2021 08:26pm
- Pharmacists suffered from inventory after six products, including Diovan and Exforge, by Novartis Korea, were announced to local pharmacies on the afternoon of the 1st. When there were concerns that impurities were detected, Novartis Korea explained that the quality was fine, but it was a lot release that was decided due to administrative delays. According to local pharmacies on the 1st, four products including Diovan, Co-Diovan, Exforge, and Entresto by Novartis Korea and Kotarec and Tarec by Sandoz Korea will be suspended from September. It said the lot release was suspended because it did not submit safety inspection data to the MFDS until the end of August. Upon hearing the news, pharmacists rushed to secure inventory, and all products were sold out at pharmacies-only online malls. Pharmaceutical companies immediately explained that the decision to suspend lot release is not a matter of quality. "Azido impurities were not detected in Valsartan raw materials used in that product," a Novartis Korea official said. "We have been conducting investigations for many years to confirm that there is no problem, and right after the incident, the headquarters submitted the manufacturing process verification data to major European and overseas countries and confirmed that there was no problem." Although the related documents were recently submitted to the MFDS, it took some time to confirm, so the lot release was inevitably decided from September 1. "Lot release is scheduled as soon as the reply comes," the company added. Later in the day, Novartis sent an official letter to hospital doctors and pharmacists, explaining that there was no problem with the quality of the products mentioned. A pharmacist in Seoul said, "We received an answer that we will try to release the product as soon as possible once we prepare the prescription with our inventory." "I'm glad to hear that." Another pharmacist in Gyeonggi do (in an online mall) seems to have hoarded some pharmacies because of anxiety. "I think it's going to work out faster than I thought."
- Product
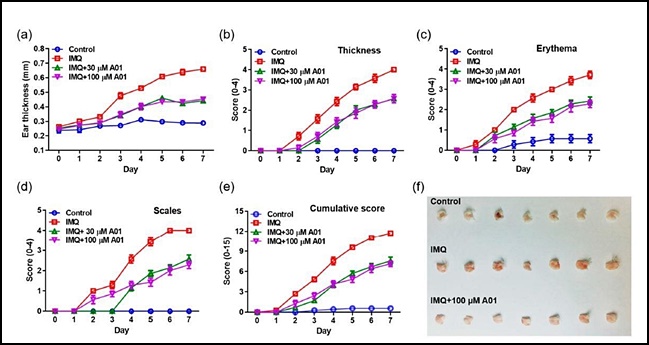
- "Inhibiting ANO1 activity has effect on treating psoriasis"
- by Lee, Jeong-Hwan Aug 05, 2021 06:02am
- A study result has shown that inhibiting the activity of the Anoctamin-1 (ANO1) protein may be effective in treating psoriasis, an intractable skin disorder. The results are expected to aid further research in developing ANO1 inhibitors for the treatment of intractable diseases such as psoriasis and cancer. On the 4th, the joint research team of Gyeonggi-do Regional Research Center and Professor Young Duk Yang’s team at the CHA University School of Medicine announced the results of the study that contained these findings. Psoriasis is an intractable skin condition that causes a build-up of extra dead cells and inflammation that occurs due to abnormal function of the immune system. The joint research team found that effectively blocking one of the ion channels that exist in the body – the ANO1 ion channel – reduced key symptoms of psoriasis such as rash, erythema, white scales, etc. while reducing inflammation-inducing substances that cause psoriasis Accordingly, the teams plan to expand their scope of research to develop new ANO1 inhibitors for the treatment of intractable diseases such as psoriasis and cancer. ANO1 is a membrane protein that acts as a channel for chloride ion transport. Professor Young Duk Yang said, “Psoriasis is an intractable skin disorder with no identified cause that is difficult to treat and recurs well. The study results may be used as a new breakthrough in the development of treating psoriasis.” The study results were published in the International Journal of Molecules Sciences in July 2021.
- Product
- Consult with MD about controversy over Champix?
- by Jul 07, 2021 05:53am
- Concerns over the detection of carcinogens in the anti-smoking supplementary drug Champix (Varenicline), pharmacies are also making a series of inquiries. However, there are no clear guidelines for existing patients, adding to the confusion. As part of preemptive measures related to the impurity crisis, the domestic supply has been completely suspended and the investigation is underway at the global headquarters level, but no guidelines have been prepared for refunds or suspension. Therefore, pharmacists explain that it is difficult to respond to questions about whether pharmacies continue to take them. Pharmacist A said, "There have been more than a few recent inquiries from existing users asking if they can continue taking the medicine after hearing media reports." "There is a risk of cancer, so we are asking the pharmacy whether the dose continues or not," he said. Pharmacist B also had patients taking Champix asking the same question through the pharmacy, and he was confused when he asked Pfizer whether to take it continuously. The pharmacist asked the patient how to guide him in terms of safety, and the pharmaceutical company replied, "We can't give you advice that you can't take it steadily, so I have to discuss it with the doctor in charge." "It is sensitive to give accurate and clear answers because it is currently under investigation. As there are people who have already been prescribed and taken, we expect many inquiries from pharmacies." The pharmacist said, "It is absurd why they ask us to consult a doctor about impurities," and added, "There should be guidance from pharmaceutical companies on how to respond." The pharmacist actually asked the lawmaker, but the doctors also did not give a clear answer. He stressed the need to prepare guidelines for refunds and inquiries. The MFDS has launched an investigation into the impurities of the Sartan hypertension drug and the anti-smoking drug Varenicline. Regarding the guidelines, Pfizer explained, "We are currently investigating the possibility of Nitrosamine impurities, so we are guiding you to consult with a doctor about whether to take them. It is said that it is difficult for pharmaceutical companies to express their position on the suspension or recommendation of medication because they have not received additional recommendations from regulators other than the suspension of supply. The official predicted that follow-up guidelines will be prepared based on the results of the inspection. "The risk of exposure to Nitrosamine from taking Varenicline is very low," a Pfizer headquarters spokesman said. "The benefits of taking drugs are better than the risks," he said.