- LOGIN
- MemberShip
- 2025-12-22 13:55:52
- Company
- 'Trodelvy' approved for prescription at general hospitals
- by Eo, Yun-Ho Aug 20, 2024 06:34am
- Product photo of Gilead Science Korea 'Trodelvy,' still not covered by reimbursement, has now been quickly approved for prescription in general hospitals. Sources said that Gilead Science Korea's Trodelvy (sacituzumab govitecan), a treatment for triple-negative breast cancer (TNBC), has cleared drug committees (DC) of 'Big 5' tertiary general hospitals, including Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary's Hospital, Seoul Asan Medical Center, and Severance Hospital in Sinchon, and 34 medical centers nationwide. Although not covered by reimbursement, it has been approved for prescription at all medical centers with a prescription code issued. More prescriptions are anticipated if it succeeds in listing for insurance reimbursement. However, the reimbursement process for Trodelvy has not progressed since clearing the Cancer Disease Review Committee of the Health Insurance Review and Assessment Service (HIRA) in November last year. It was expected that Trodelvy would be considered for the Drug Reimbursement Evaluation Committee (DREC) review this month, but it was not. The upcoming DREC review is scheduled to be on September 5th. It is to be watched whether the first Trop-2 targeting Antibody-Drug Conjugate (ADC), Trodelvy, is to be considered for reimbursement listing. Triple-negative breast cancer is an aggressive form among breast cancer types that is more likely to recur and metastasize. Patients with triple-negative breast cancer who have progressed metastasis despite undergoing chemotherapy have a life expectancy of several months. However, chemotherapy has been used as the standard therapy for a long time because an effective method to target cancer cells has not been discovered. The current guidelines in the United States and Europe recommend Trodelvy as a priority treatment for patients with metastatic triple-negative breast cancer who have a treatment history. According to the Phase 3 study, patients treated with Trodelvy had an overall survival of 11.8 months, close to a year, whereas patients undergoing chemotherapy had 6.9 months. Furthermore, Trodelvy has been shown to be effective in regulating symptoms and pains associated with cancer and improving overall well-being, thereby improving patients' quality of life. Trodelvy received the highest score of 5 on the ESMO-MCBS, a value-evaluation tool for anticancer medicines rated by the European Society for Medical Oncology (ESMO). Drugs with Score of 5 are indicated to not only extend patient survival but also be effective in improving quality of life. Trodelvy is the only drug to receive a Score of 5 among triple-negative breast cancer treatments. In South Korea, several petitions requesting reimbursement listing of Trodelvy were posted on the website, and over 100,000 people agreed to the petition. However, despite these efforts by patients and caretakers, the petition was ended due to the session ending of the 21st National Assembly. Accordingly, the Korea Alliance of Patients Organization has submitted a statement to the Ministry of Health and Welfare (MOHW) requesting reimbursement review to be made quickly for drugs with high patient demand, such as Trodelvy, in May. Meanwhile, in the United Kingdom, Trodelvy was granted a preferential status on a cost-effectiveness analysis based on extending survival of the patient group, a population less than rare disease, who have a life expectancy of less than two years. As a result, it was approved for reimbursement after receiving a twofold higher incremental cost-effectiveness ratio (ICER) than other drugs.
- Company
- Reimb remains an issue despite broadened options for UC
- by Moon, sung-ho Aug 20, 2024 06:34am
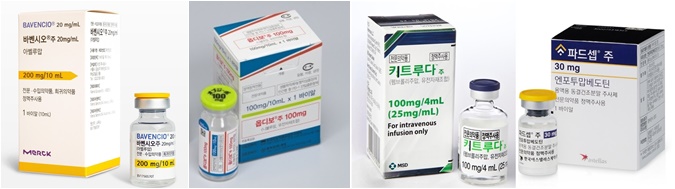
- Urothelial carcinoma, which is a type of bladder cancer, accounts for 90% of all bladder cancers and is considered a typical cancer type in the field. However, unlike other cancers like lung and breast cancer, where the standard of care changes quickly with the introduction of new drugs, UC has remained a barren area for decades, leaving a large unmet need for first-line treatment options. For the past 30 years, platinum-based chemotherapy has remained the first-line standard of care for UC. # Recently, new drugs, including immuno-oncology drugs and antibody-drug conjugates (ADCs), have been introduced for UC in Korea, shifting the treatment paradigm. However, until the health insurance reimbursement issue is resolved, their active use on-site seems unlikely. According to the medical and pharmaceutical industries on the 16th, platinum-based chemotherapy using gemcitabine, cisplatin, carboplatin, etc. had been used as a first-line treatment for UC. However, the median overall survival (OS) was only 12-15 months with their use due to toxicity, with patients often having to take a drug holiday from chemotherapy after 3-4 months of treatment, and disease progressing around 6-9 months after initiation. However, in the past year or two, the treatment paradigm for UC has been rapidly shifted with global pharmaceutical companies introducing treatments based on clinical research to Korea. One of the first treatments to receive attention is Merck's immuno-oncology drug Bavencio (avelumab). Bavencio is a fully human monoclonal antibody specific for PD-L1 and has demonstrated prolonged survival as a first-line maintenance therapy for UC. Since August last year, its use as first-line monotherapy has been reimbursed by Korea’s health insurance and is being used in practice. Bavencio’s efficacy was confirmed through the long-term follow-up Phase III JAVELIN Bladder 100 trial, which involved 700 patients with locally advanced or metastatic urothelial cancer in 29 countries, including Korea, for over 38 months. The trial results showed a median OS of 29.7 months with Bavencio+maintenance therapy. This is over 9 months longer than the 20.5 months found in the maintenance monotherapy arm. In July this year, the combination of the ADC cancer drug Padcev (enfortumab vedotin, Astellas) and the immuno-oncology drug Keytruda (pembrolizumab, MSD) was approved in Korea, marking another shift in the first-line UC treatment paradigm. The Phase III EV-302/KEYNOTE-A39 trial, which became the basis of its approval, showed a median OS of 31.5 months in the Padcev+Keytruda arm compared to 16.1 months in the control arm, which was a significant difference. Median progression-free survival (PFS) was 12.5 months in the Padcev+Keytruda arm versus 6.3 months in the control arm Around the same time the Padcev+Keytruda combination was approved, BMS and Ono Pharmaceutical’s immuno-oncology drug Opdivo (nivolumab) received approval in Korea as the first-line treatment for UC as well. The approval was based on results from the CheckMate 901 trial, which evaluated Opdivo versus the standard of care gemcitabine+cisplatin (GemCis) in 608 patients with metastatic UC. Results showed that at a median follow-up of 36 months, the primary endpoint, median OS, was 21.7 months with the addition of Opdivo versus 18.9 months in the control arm, resulting in improved treatment outcomes. The treatment paradigm for UC, which had remained unchanged for 30 years, has changed dramatically in the past 1-2 years after 30 years of unchanged treatment. Based on the results of the study, Padcev+Keytruda is expected to rise to the forefront among first-line treatment options on-site. “Based on the efficacy seen in the studies, the Padcev+Keytruda combination would be my first choice,” said In-Keun Park, Professor of Medical Oncology at Asan Medical Center in Seoul. ”The Opdivo+GemCis combination that has been approved at a similar period, has proven to be less effective. Also, we need to consider the availability of the cisplatin option and utilize drugs with caution, so I would consider using the Opdivo+GemCis option for patients with uncontrolled diabetes.” “The currently reimbursed Bavencio has fewer side effects and proven efficacy, but is indicated for patients who have not progressed after receiving platinum-based chemotherapy as a first-line treatment,” explained Professor Park. ”If Padcev+Keytruda or Opdivo+GemCis becomes a common first-line treatment option, Bavencio may lose more ground.” Now that the first-line treatment options for UC have been approved in Korea, attention is now focused on how quickly they will be available on-site. As Bavencio is covered as a maintenance therapy for first-line urothelial cancer, the industry is eyeing whether the Padcev+Keytruda combination will also be granted reimbursement. However, the Padcev+Keytruda combination presents a dilemma for pharmaceutical companies. This is because 2 different companies - Astellas and MSD - own the drugs that are included in the combination. In order for a combination therapy to be reimbursed, both companies must apply for reimbursement and participate in the process for the agenda to be discussed with the government. However, it is currently difficult for such discussions to take place due to the headquarters policies of global pharmaceutical companies and the possibility that discussions with other pharmaceutical companies may be judged as 'collusion' under domestic and international fair trade laws. In other words, both pharmaceutical companies must separately apply for reimbursement. If one of the companies is not interested in applying for reimbursement, reimbursement of the combination therapy is effectively off the table. This is the case for Padcev+Keytruda, and Astellas and MSD are known to be separately reviewing the need for reimbursement. However, the 2 companies are doing so at their discretion, as discussions between them could be regarded as collusion. As a result, the pharmaceutical industry has recently called for the need to establish regulations in the health insurance reimbursement process to address the combination therapy research being conducted between pharmaceutical companies. In other words, the industry has pointed out that a system needs to be established so that if one of the combination therapy developers applies for reimbursement, government agencies such as HIRA can notify the other companies so that the other companies can decide whether to participate in the reimbursement application process. A pharmaceutical industry official said, “If the developers of drugs used as combination therapies are different companies, the Fair Trade Act forbids the companies from discussing insurance reimbursement and insurance prices on their own. It is difficult for the government to proceed with the reimbursement process if one of the drug developers applies for health insurance benefits and the other does not show any willingness for its drug’s reimbursement.” This is why the industry sees it difficult for combination therapies to be applied reimbursement benefits. “Even though the number of first-line treatment options for UC has increased, it is difficult to actively utilize them on-site unless the cost issue is resolved,” said a professor of oncology at a tertiary hospital. ”It takes longer for combination therapies to be reimbursed. It may even be impossible if two separate pharmaceutical companies need to receive reimbursement together for one combination therapy regimen.”
- Company
- K-Pharma focuses on developing CAR-NK cell therapies
- by Son, Hyung-Min Aug 20, 2024 06:34am
- Korean biopharmaceutical industry speeds up the development of chimeric antigen receptor (CAR)-NK cell therapy. GC Cell has recently entered the Phase 1 clinical trial in South Korea. Companies are collaborating on developing CAR-NK therapies through joint-research agreements: GI Cell and Y-Biologics and TS Bio and Maru Therapeutics. NKMAX has confirmed the potential of its candidate in a preclinical study. According to industry sources on August 19th, GC Cell received approval of Investigational New Drug (IND) application from the Ministry of Food and Drug Safety (MFDS) for a Phase 1 clinical trial of its CAR-NK therapy 'GCC2005' on August 14th. GCC2005, an allogeneic cell therapy utilizing NK cells derived from umbilical cord blood, targets CD5, which is highly expressed in T-cell lymphoma. CD5 is a biomarker highly expressed in many T-cell lymphoma subtypes. GCC2005 is a CAR-NK cell therapy designed to co-express CAR and interleukin (IL)-15, improving the short persistence of existing NK cells and enhancing efficacy. GC cell developed a technology to mass culture high-purity and highly active NK cells from a small quantity of umbilical cord. CAR-NK, an allogeneic therapy, has the advantage of improving side effects compared to existing CAR-T therapies. CAR-T induces cytokines, such as interleukins associated with neurotoxicity, whereas activated NK cells generally produce interferon-gamma (IFN-γ) and granulocyte-macrophage colony stimulating factor (GM-CSF). Accordingly, CAR-NK is less likely to cause cytokine release syndrome (CRS) or neurotoxicity. GC2005 demonstrated anticancer effects in various in vivo CD5+ T-ALL models (RPMI-8402, CCRF-CEM). GCC2005 showed a higher survival rate and tumor-suppressive efficacy than a vehicle. Additionally, GC Cell is developing the CAR-NK cell therapy 'AB-201' with the U.S. affiliate company Artiva. AB-201 targets solid cancers, including HER2-overexpressed breast cancer and gastric cancer. In 2022, Artiva received IND approval from the U.S. Food and Drug Administration (FDA) for Phase 1/2 trials of its AB-201. Then, in December last year, GC Cell received IND approval from the MFDS and Australia's Human Research Ethics Committees (HREC) for a Phase 1 trial of AB-201. GC Cell and its U.S. affiliate company Artiva is developing CAR-NK therapies (photo source=Artiva). NKMAX is developing a CAR-NK candidate product, HER2 CAR-SNK02. NKMAX's HER2 CAR-SNK02 comprises HER2 gene targeting CAR and a cytokine that prolongs survival and activation of NK cells in the body, which is then stored by cryo freezing. Based on a preclinical study recently disclosed by NKMAX, HER2 CAR-SNK02 was confirmed to survive in vivo and exert anticancer effects in a mouse model engrafted with human gastric cancer cells. Collaborative research to co-develop CAR-NK therapies Korean biopharmaceutical companies are conducting collaborative research to develop CAR-NK therapies. GI Cell, an affiliate of GI Innovation, and Y-Biologics have signed a collaborate research agreement last year, commencing the new CAR-NK anticancer drug development. These companies plan to develop new anticancer drugs utilizing GI Cell's CAR-NK cell development and mass cell culture technology and Y-Biologics' nanobody-based antibody discovery platform technology. GI Cell has previously completed the domestic Phase 1 trial of the NK cell therapy 'T.O.P. NK' in patients with solid cancer and blood cancer. Since GI Cell proved T.O.P. NK's tolerability and safety, it began conducting a Phase 2a trial last month. Additionally, GI Cell is conducting collaborative research with HK inno.N for CAR-NK. HK inno.N and GI Cell are jointly conducting a basic research on CAR-NK targeting seven targets. In addition to this joint research, HK inno.N is also conducting two basic research cases on its own. TiCRos has entered into a license-based collaborative research agreement with Catherics, an Australian company focusing on developing iPSC-derived CAR-NK cell therapy. Based on the agreement, the companies are developing CAR-NK therapy with improved tumor-killing effects by incorporating TiCRos' 'CLIP-CAR' on Catherics' CAR-NK therapy. TiCRos will manufacture a CLIP-CAR targeting an antigen designated by Catherics. Catherics will incorporate this into iPSC-derived NK cells and evaluate the function in vitro and in vivo. TS Bio has been conducting joint research with Maru Therapeutics for CAR-NK cell therapy since last year. Both companies plan to actively develop CAR-NK cell therapy targeting blood cancers and solid cancers through the agreement.
- Company
- Will Handok’s Empaveli be reimbursed in 1H in KOR?
- by Eo, Yun-Ho Aug 19, 2024 05:47am
- Empaveli, a treatment for paroxysmal nocturnal hemoglobinuria imported by Handok Pharmaceuticals, has entered the final gateway to insurance reimbursement in Korea. According to industry sources, Handok will enter into drug price negotiations with the National Health Insurance Service for its new drug Empaveli (pegcetacoplan). Empaveli is a treatment for paroxysmal nocturnal hemoglobinuria (PNH) developed by the U.S. company Sobi. It is the first C3 protein-targeted therapy for the treatment of adult patients with PNH. Gaining attention as the first PNH drug that can prevent intravascular and extravascular hemolysis, Empaveli is approved for use in many countries, including the United States, Europe, Australia, and Japan. The drug was approved by the U.S. Food and Drug Administration (FDA) in May 2021 and the European Medicines Agency (EMA) in December 2021, and has completed two Phase III trials. According to the results of the Phase III PEGASUS trial that was published in the NEJM, Empaveli demonstrated superiority to eculizumab in changing hemoglobin concentration over 16 weeks. In an extension study, LDH levels, a marker of intravascular hemolysis, remained below 1.5 times the upper normal limit for 48 weeks in the Empaveli treatment arm. The percentage of patients who remained transfusion-free for 16 weeks was also higher in the Empaveli arm, at 85%, compared to 15% in the eculizumab arm. Empaveli also showed a 9.2-point improvement in the FACIT-fatigue score, a quality-of-life measure. A2-point or over change from the baseline of the FACIT-fatigue score is considered clinically significant. The efficacy of Empaveli was also confirmed in the PRINCE trial in complement inhibitor-naïve PNH patients. After 26 weeks of follow-up, 85.7% of patients in the Empaveli arm showed stabilized hemoglobin levels, and LDH levels were well controlled to below normal levels in the Empaveli arm. Meanwhile, Handok has entered into a strategic partnership with Sobi to introduce the rare disease drugs Empaveli and Doptelet (avatrombopag) to Korea. In April, the company officially launched its joint venture, Sobi-Handok, to strengthen its rare disease pipeline.
- Company
- Will Astellas’ gastric cancer drug Vyloy be approved in 2H?
- by Eo, Yun-Ho Aug 19, 2024 05:47am
- Zolbetuximab, a new targeted cancer therapy with a new mechanism of action for gastric cancer, may be commercialized in Korea in the second half of the year. According to industry sources, the Ministry of Food and Drug Safety (MFDS) is currently reviewing Vyloy (zolbetuximab), a treatment for patients with CLDN18.2 positive, unresectable, advanced, or recurrent gastric cancer. The drug was approved in Japan in March. Vyloy is an immunoglobulin (IgG)1 monoclonal antibody that targets Claudin 18.2, a cell surface protein expressed by gastric cancer cells. It binds to the claudin 18.2 protein expressed on the surface of cancer cells in gastric epithelial cells. However, unfavorable conditions to its approval also do exist. In January, Vyloy was denied approval by the U.S. FDA. The rejection was not based on any efficacy issues, including the drug's clinical data, but rather because of questions raised during the manufacturer's review. Astellas resubmitted its application to the FDA in June and the application is on track for Vyloy’s approval. Meanwhile, Vyloy has demonstrated efficacy in the Phase III SPOTLIGHT and GLOW studies. The POTLIGHT study evaluated the efficacy and safety of zolbetuximab+FOLFOX (fluorouracil, leucovorin, oxaliplatin) versus placebo+FOLFOX in 557 adult patients with CLDN18.2-positive, HER2-negative, locally advanced unresectable or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma. In the trial, the zolbetuximab arm achieved a primary endpoint, progression-free survival (PFS), of 10.6 months, compared with the 8.7 months in the control arm. Additional study results showed that the zolbetuximab combination arm achieved an overall survival (OS) of 18.2 months, a 2.7-month improvement over the control arm. The zolbetuximab combination arm also showed an improvement in PFS and OS in the GLOW study. The study compared zolbetuximab+CAPOX therapy (capecitabine, oxaliplatin) with placebo+CAPOX therapy. Study results showed that the zolbetuximab arm had a PFS of 8.2 months, 1.4 months longer than that of the control arm. OS was 14.4 months in the zolbetuximab combination arm and 12.2 months in the control arm. In terms of safety, the most commonly reported adverse events were nausea and vomiting.
- Company
- Entresto faces competition in the heart failure market
- by Moon, sung-ho Aug 19, 2024 05:47am
- As the volume of treatment for patients with heart failure increases due to the aging population, the competition is accelerating in the market for the treatment. This is because there are more treatment options in clinical practices and higher chances of generics entry into the market. Growing competition in the market for heart failure, previously dominated by Entresto.According to the medical and pharmaceutical industry on August 13th, the medicine market saw significant growth following major academic associations' revision to the guidelines for treating heart failure and the release of major new drugs. In the current clinical practices, there are '4 pillars' strategy of standard therapy for treating heart failure consisting of 'ARNI/Angiotensin-Converting Enzyme Inhibitor (ACEI),' 'Beta-Blocker (BB),' 'Mineralocorticoid Receptor Antagonist (MRA),' and SGLT-2 inhibitors, which is now expanded its use in addition to diabetes. Novartis' chronic heart failure treatment 'Entresto (sacubitril/valsartan)' has shown significant growth among these treatments. Entresto is the first-in-class dual blocker ARNI combining valsartan, an angiotensin II receptor blocker (ARB) inhibitor, and sacubitril, a neprilysin inhibitor. The turning point for this drug was being approved as a first-line treatment for patients who had not been administered ACEI or ARB since March 2022. In the same year, it became available for prescription to hospitalized patients and outpatients. When the Korean Society of Heart Failure (KSHF) revised 'A Complete Revision of the Heart Failure Guidelines,' it advanced Entresto as the first-line standard therapy for treating heart failure with reduced ejection fraction (HFrEF). According to the pharmaceutical market research firm UBIST, Entresto generated KRW 57.5 billion in the clinical field last year and recorded KRW 32.9 billion in this year's first half. It has become true that the sales have surpassed those of the previous year. Another medicine that is receiving attention is 'Verquvo (Vericiguat, Bayer).' Verquvo, available with reimbursement coverage, can be administered to patients with chronic heart failure (NYHA class Ⅱ-Ⅳ) with an impaired left ventricular contractility and left ventricular ejection fraction (LVEF) of less than 45%. It is a second-line treatment that can be used for patients who have worsened disease despite undergoing standard therapy for over four weeks. However, it has not yet demonstrated sales growth in clinical practices. A year after obtaining reimbursement in the second half of the previous year, Verquvo only generated sales of approximately KRW 400 million in the first half this year, based on UBIST. One reason for this might be the wide variety of treatment options due to the expanded reimbursement range of the original SGLT-2 inhibitor diabetes drugs, including Jardiance (empagliflozin), for chronic heart failures. Furthermore, while Forxiga (dapagliflozin, AstraZeneca) withdrew from the domestic market, AstraZeneca Korea granted clinical documents to HK inno.N's generic Dapa N, thus Dapa N succeeding Forxiga's heart failure and kidney disease indications. Consequently, Dapa N is the only generic product with the original indication. The analysis shows that Verquvo is considered a last resort treatment option for treating heart failure in clinical practices. Currently, clinicians have more treatment options they can use. A cardiology professor from a university hospital in Seoul, who requested to remain anonymous, stated, "Verquvo is used as a second-line treatment following four types of first-line treatment options, so it is not in competition with the first-line treatments." He explained, "SGLT-2 inhibitors were already being prescribed in private practices for diabetes, which reduced the psychological burden and made it easier to prescribe for heart failure. Verquvo is initially prescribed in tertiary hospitals and used as patients move to secondary and primary care hospitals." "It would have been a different scenario for SGLT-2 inhibitors if it had been initially released as heart failure treatment," he added. "After being used primarily as a diabetes drug, it has gained attention for its effectiveness in treating heart failure. The situation might be different if it were the other way around."
- Company
- Twice-yearly Uplizna enters last stage to reimb in KOR
- by Eo, Yun-Ho Aug 16, 2024 05:56am
- Uplizna, a new drug for neuromyelitis optica spectrum disorder (NMOSD) that is administered twice a year, has entered the final gateway for insurance reimbursement in Korea. According to industry sources, Mitsubishi Tanabe Pharma Korea entered pricing negotiations with the National Health Insurance Service (NHIS) for Uplizna (inebilizumab), a treatment used to treat adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are positive for anti-Aquaporin-4 (AQP4) antibodies. The company accepted the ‘below the evaluated amount’ condition set by the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee for the reimbursement of Uplizna (inebilizumab) last month. The drug is administered at an initial 300 mg dose, followed by an additional 300 mg dose 2 weeks later, and then every 6 months thereafter from the date of the initial dose. NMOSD occurs when AQP4 autoantibodies, a disease-specific biomarker produced by B cells, bind to AQP4, a target antigen present on glial cells in the central nervous system, and activate the immune responses, causing nerve damage. Uplizna is an anti-CD19 human monoclonal antibody that selectively binds to CD19, a B cell-specific surface antigen, depleting B cells that produce AQP4 antibodies, thereby preventing disease relapse. The safety and efficacy of Uplizna were demonstrated in the N-MOmentum study, which evaluated the use of Uplizna monotherapy in 230 patients without the use of concomitant immunosuppressive agents. Study results showed that 89% of patients treated with Uplizna did not experience a relapse during 197 days of follow-up, resulting in a 77.3% reduction in the risk of relapse compared to placebo. Safety evaluations Uplizna also showed comparable rates of adverse events to the placebo group. Also, in an extension study, Uplizna continued to reduce the risk of relapse for at least 4 years, with an 87.7% relapse-free rate. In terms of long-term safety profile, Uplizna was generally well tolerated, with no increase in infection rates due to B-cell depletion. NMOSD is a serious autoimmune disease in which most patients experience persistent relapses and incomplete recovery, resulting in accumulated nerve damage that can cause vision loss, gait disturbances, and even death from respiratory failure.
- Company
- OraTicx signs a distribution deal with U.S.-based Stratum
- by Son, Hyung-Min Aug 16, 2024 05:56am
- OraTicx, a company specializing in developing oral probiotics, announced that it has signed an exclusive distribution agreement for the North American region with Stratum Nutrion (hereafter referred to as Stratum), a U.S.-based dietary supplement ingredient supplier. Through this agreement, OraTicx will exclusively distribute its proprietary oral probiotics 'oraCMU,' 'oraCMS1,' and 'oraCMU' in the North American market. Stratum, a company under ESM Technologies in the United States, provides natural ingredients based on scientific research. Through this partnership, OraTicx is expected to utilize Stratum's broad distribution network to accelerate the entry of its products into the North American market. OraTicx has 10 patents in South Korea and the United States. It has expertise in researching oral probiotics, completing 10 cases of human studies, and publishing 36 research articles. Notably, its oral probiotics strain, oraCMU, was developed in an OraTicx-affiliated research center. Through joint research with a research team at Seoul National University School of Dentistry, oraCMU's human study results demonstrating an improvement in halitosis were published in the SCIE international journal, 'Frontiers in Microbiology.' oraCMU demonstrated the quality and safety of the ingredient through FDA GRAS (Generally Recognized As Safe) registration. oraCMS1 has been confirmed to prevent plaque formation and exhibit antibacterial efficacy against respiratory pathogens. Recently, OraTicx has obtained a patent for its composition aimed at preventing and treating respiratory diseases. OraTicx has launched oral probiotics products using proprietary research. It is expanding its product line-up focused on oral probiotics, including Green Breath, Dentee, kids' dental probiotics, Implantics with twice the units, and Pet Biome for pets. OraTicX President Yoon Eun-seop said, "The current exclusive distribution agreement with Stratum is an important opportunity to introduce our innovative oral probiotics to North American customers." He added, "We aim to establish a new value in the North American dietary supplement market and become a leading global company contributing to people living heathy lives until the age of 100." Stratum President Micah Osborne said, "OraTicx's advanced oral probiotics will strengthen our portfolio even more." He added, "Through this partnership, we are delighted to provide various and effective healthcare solutions to North American customers."
- Company
- K-Pharma speeds up marketing new drugs for bile duct cancer
- by Son, Hyung-Min Aug 16, 2024 05:55am
- Korean pharmaceutical companies are speeding up the development of new drugs for cholangiocarcinoma (also known as bile duct cancer). Handok's partnering company in the United States, Compass Therapeutics, has recently completed registering patients for the Phase 2/3 trials. Once the efficacy of the novel drug is confirmed, Handok plans to apply for approval in South Korea. According to pharmaceutical industry sources on August 16th, Compass Therapeutics has recently completed registering patients for the Phase 2/3 trials in the United States. The trials will evaluate the efficacy of HDB001A(CTX-009) for metastatic or recurrent cholangiocarcinoma and compare the efficacy of HDB001A in combination with paclitaxel to the paclitaxel monotherapy. HDB001A is a novel drug candidate developed by the Korean company ABL Bio. Handok has the domestic distribution rights, and Compass Therapeutics has the global distribution rights. This novel drug candidate is a bispecific antibody targeting Delta-like ligand 4 (Dll4) and vascular endothelial growth factor (VEGF), and it is known to form new blood vessels in the tumor microenvironment. Handok has confirmed the efficacy and safety of HDB001A in the Phase 2 trial conducted in South Korea. The clinical trials evaluated and compared HDB001A in combination with paclitaxel to paclitaxel monotherapy in 24 adult patients with advanced, metastatic, or recurrent cholangiocarcinoma who have received prior treatments. After a median follow-up of 12 months, patients treated with the combination therapy had an objective response rate (ORR) 37.5%, measuring the reduction in tumor sizes. The median overall survival (OS) was 12.5 months. The median duration of response (DOR) was 9.4 months, and the median progress-free period (PFS), duration without progression, was 9.4 months. However, the adverse events of HDB001A plus paclitaxel occurred at the highest rate. Treatment-related adverse events (TRAE) of all Grades, from 1 to 5, were reported in HDB001A plus paclitaxel combination therapy. 75% of patients had adverse reactions of over Grade 3. The most common adverse reactions over Grade 3 were neutropenia (50%), hypertension (16.7%), anemia (12.5%), and thrombocytopenia (8.3%). One case of Grade 5 pneumonia was reported. 25% of patients had confusion state, pulmonary embolism, and an increase in blood creatinine level. Once Handok confirms the efficacy and stable safety data from the U.S. clinical trial, it will apply for approval in South Korea. Confirming the potential of Keytruda combination therapy…enters the clinical trial Besides Handok, Genome & Company, GI Innovation, and SMT bio are developing novel drugs for cholangiocarcinoma. These companies are conducting clinical trials for Keytruda combination therapies. Korean pharmaceutical companies are conducting clinical trials for their candidate drugs in combination with various immune checkpoint inhibitors, including Keytruda.Keytruda was approved in April as a first-line treatment of cholangiocarcinoma in South Korea. Genome & Company, GI Innovation, and SMT bio plan to increase the possibility of commercializing their candidate products in combination with an efficacy-confirmed drug. Genome & Company is developing a new candidate product, microbiome-based immune checkpoint GEN-001, in combination with Keytruda for cholangiocarcinoma. Genome & Company aims to improve the efficacy of existing immune checkpoint inhibitors through combination therapy of 'GEN-001' and Keytruda. The company also plans to develop 'GENA-104,' an immune checkpoint inhibitor 'GENA-104' that suppresses novel target CNTN4, for patients who had not benefited from Keytruda targeting PD-L1/PD-1. The Phase 2 trial of GEN-001 for cholangiocarcinoma was approved for changes to the Investigational New Drug (IND) application in June. Genome & Company plans to evaluate the efficacy and safety of three-drug combination therapy, adding FOLFOX (fluorouracil, leucovorin, and oxaliplatin) to the existing GEN001 plus Keytruda combination therapy. Genome & Company confirmed the tolerability and safety of GEN-001 in the Phase 1 trial and determined the recommended dosage for the Phase 2 trial. The trial recently finalized patient treatments. SMT bio is conducting Phase 2b trials for allogenic Natural Killer cells (SMT-NK cells) in combination with Keytruda. The company is evaluating the efficacy of the combination therapy compared to the Keytruda monotherapy. Based on clinical results until now, the combination therapy had a PFS of 4.1 months, which is higher than 1.5 months of the Keytruda monotherapy. It showed improved ORR compared to monotherapy. Last year, the Ministry of Food and Drug Safety (MFDS) acknowledged the effectiveness of SMT-NK cells and approved the therapeutic use of under-trial pharmaceutical for treating patients with cholangiocarcinoma. GI Innovation is conducting a Phase 1 trial in the United States to evaluate the effectiveness of GI-101 in combination with Keytruda. The company aims to secure an indication for treating solid cancers, including cholangiocarcinoma. GI Innovation is also evaluating the marketing possibility of its candidate product in combination with a wide variety of immune checkpoint inhibitors, such as Imfinzi, in addition to Keytruda.
- Company
- LG Chem begins Phase 2 trials for infant hexavalent vaccine
- by Hwang, Byung-woo Aug 14, 2024 05:51am
- LG Chem LG Chem prepares to commence the Phase 2 clinical trial for its hexavalent vaccine LR20062. The company aims to domestically produce infant combination vaccines in South Korea. Since South Korea has so far relied entirely on imported combination vaccines, the company plans to provide a stable supply network to meet mid- to-long term demand for immunization. On August 13th, LG Chem announced it will conduct the Phase 2 trials for the hexavalent 'acellular Pertussis (aP)' vaccine, 'LR20062' overseas and prepare to enroll study participants. The successful completion of the Phase 1 trial and entry into the Phase 2 stage indicate progress towards commercializing the first domestically produced combination vaccine. 'LR20062' is a 6-in-1 vaccine used to prevent Diphtheria, Tetanus, Pertussis, Polio, Haemophilus influenzae type b (Hib), and Hepatitis B. Compared to vaccine conjugates in commonly used 5-in-1 vaccines and Hepatitis B vaccines, it reduced the number of doses by two doses (6 doses to 4 doses). The Phase 2 trial involving healthy adults demonstrated vaccine reactions from all study participants. Regarding immunogenicity endpoints, the serum protection and conversion rates were above 90%, similar to the control group, which was the existing hexavalent combination vaccine. In terms of safety, the new vaccine also demonstrated similarly favorable endpoints compared to the control group. The Phase 2 trial will enroll 300 infants aged two months and older, the target immunization age group in the real-world, as study participants and evaluate the safety and immunogenicity of 'LR20062' compared to existing hexavalent combination vaccines. LG Chem aims to develop domestically produced combination vaccines and contribute to meeting mid- to long-term demand through developing hexavalent combination vaccines. To this end, the company plans to invest about KRW 200 billion in R&D and facilities. Jeewong Son, Head of LG Chem Life Sciences Company businesses, said, "Establishing the production technology for all six antigens is a significant milestone and crucial for establishing domestically produced vaccines." He added, "We will expedite the commercialization of domestic combination vaccine to ensure that parents can safely and reliably vaccinate their children." Additionally, LG Chem is conducting the Phase 2 trial for a while cell Pertussis (wP) hexavalent vaccine, ‘LR19114,’ intending to enter the procurement market of international organizations like UNICEF.