- LOGIN
- MemberShip
- 2025-12-22 07:31:08
- Company
- Novo Nordisk tops KRW 14T in sales, thanks to Wegovy
- by Whang, byung-woo Nov 08, 2024 05:46am
- Novo Nordisk has recorded a strong sales performance in Q3, driven by the steady growth of its major products, including Wegovy and Ozempic. According to Novo Nordisk's business reports on November 6th (local time), they recorded US$10.267 billion (KRW 14.3378 trillion) in Q3 sales, up 21% from the previous year. Its operating profit amounted to US$4.877 billion (KRW 6.8112 trillion), up 26% from 2023, and it recorded US$3.9325 billion (KRW 5.4921 trillion) in net revenues, up 21% from 2023. By region, the sales rose by 22% to US$3.85297 billion (KRW 5.4861) in the North American market and by 21% to US$2.59707 billion (KRW 3.6275 trillion) in the global market, excluding the North American market. It recorded cumulative sales of US$29.483 billion (KRW 41.1818 trillion) up to Q3 2024. Sales growth of 23-27% and an operating profit increase of 21-27% for the fiscal year 2024 are forecasted. The company analyzed increased sales across the entire portfolio contributed positively to the sales growth in Q3. By products, entire GLP-1 agents recorded sales of US$446.59 million (KRW 623.5 billion), up 14%. Among these, GLP-1 drugs for obesity showed a growth rate of 55%, and GLP-1 drugs diabetes showed a growth rate of 15%. During the same period, rare disease treatments and insulin agents recorded sales growth of 17% (KRW 577.7 billion) and 10% (KRW 1.5805 trillion), respectively. Also, its key product Wegovy (semaglutide), an obesity drug, generated US$2.4997 billion (KRW 3.49 trillion), which rose 79% from US$873 million (KRW 1.2194 trillion) from year-over-year (YoY). The diabetes drug Ozempic generated sales of US$7.82581 billion (KRW 10.9287 trillion) in Q3, up 32% YoY. In contrast, Saxenda (liraglutide), an obesity drug, recorded sales of US$135.45 million (KRW 189.1 billion), down 43% YoY, due to Wegovy's success. Victoza, a diabetes drug, also recorded a negative growth of -41%. "We have achieved significant sales growth due to the rising demand for GLP-1 treatment targeting diabetes and obesity," Lars Fruergaard Jørgensen, CEO of Novo Nordisk, said. Meanwhile, Novo Nordisk announced they have confirmed Wegovy's effect in patients with metabolic dysfunction-associated steatohepatitis (MASH) through the latest ESSENCE study. Based on these clinical trial results, the company will file for approval from the United States and EU in the first half of 2025.
- Company
- Oral psoriasis drug 'Sotyktu' lands at Big 5 hospitals
- by Son, Hyung Min Nov 08, 2024 05:46am
- Product photo of Oral psoriasis drug 'Sotyktu' is now available for prescription at tertiary general hospitals. According to industry sources, BMS Korea’s TYK2 inhibitor, Sotyktu (deucravacitinib), has passed the drug committees of the 'Big 5' tertiary hospitals, including Samsung Medical Center, Seoul University Hospital, Seoul St. Mary's Hospital, Seoul Asan Medical Center, and Sinchon Severance Hospital. Prescription code is also available in major hospitals nationwide, including Gangnam Severance Hospital, Konyang University Hospital, Soonchunghyang University Bucheon Hospital, Seoul National University Bundang Hospital, Soonchunghyang University Hospital Seoul, Ewha Womans University Medical Center, Chosun University Hospital, and Soonchunghyang University Cheonan Hospital. Since being added to the reimbursement list in April, this drug is increasingly available in hospitals. Sotyktu is the first TYK2 inhibitor approved for adults with moderate-to-severe plaque psoriasis. It was approved in August 2023 in South Korea to treat adult patients with moderate-to-severe plaque psoriasis who are candidates for phototherapy or systemic therapy. The insurance coverage was made available eight months after receiving approval. It is covered by insurance reimbursement for the treatment of patients over 18 with chronic severe plaque psoriasis who have symptoms lasting over six months. Coverage requirements include ▲Plaque psoriasis with over 10% of the total skin areas ▲Patients who have Psoriasis Area and Severity Index (PASI) score of over 10 ▲Patients who have undergone methotrexate or cyclosporine for over 3 months but cannot continue the treatment due to no response or side effects ▲Patients who have undergone light therapy or UPV photo therapy for over 3 months but cannot continue the treatment due to no response or side effects. Clinical efficacy of Sotyktu was demonstrated in Phase 3 POETYK PSO-1 and POETYK PSO-2 clinical studies, which compared the drug with placebo or Otezla in 1,684 adult patients 18 years or above with plaque psoriasis. POETYK PSO-1 studies have shown that the Sotyktu treatment group had a PASI 75 response rate of 58.4% at week 16, which was significantly higher than the apremilast group's 35.1% and the placebo group's 12.7%. Furthermore, 53.6% of the Sotyktu treatment group achieved sPGA score of 0 or 1, higher than 32.1% in the apremilast group and 7.2% in the placebo group. In POETYK PSO-2 study, the Sotyktu treatment group had a PASI 75 response rate of 53.0% at week 16, which was significantly higher than the apremilast group's 39.8% and the placebo group's 9.4%. Additionally, 49.5% of the Sotyktu treatment group achieved sPGA score of 0 or 1, higher than the apremilast group's 33.9% and the placebo group's 8.6%. Sotyktu’s high response was maintained for up to 52 weeks. "Previously, patients who did not respond to or have had side effects when treated with conventional treatments, such as systemic therapy or light therapy, had biological agents as their only option. Sotyktu, which offers the convenience of once-daily oral administration, is expected to meet the unmet needs of psoriasis patients," Choe Yong-beom, President of the Korean Society for Psoriasis, said.
- Company
- Reimb discussions discontinued for NMOSD drug Uplizna
- by Eo, Yun-Ho Nov 07, 2024 05:47am
- Uplizna, a new drug for neuromyelitis optica spectrum disorder (NMOSD) that is administered twice a year, has failed to pass the final gateway to reimbursement in Korea. According to Dailypharm coverage, Mitsubishi Tanabe Pharma Korea’s pricing negotiations with the National Health Insurance Service (NHIS) for Uplizna (inebilizumab), a treatment used to treat adult patients with for neuromyelitis optica spectrum disorder (NMOSD) who are positive for anti-Aquaporin-4 (AQP4) antibodies, has been discontinued recently. The company had accepted the “below the evaluated amount” condition set by the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee for the reimbursement of Uplizna (inebilizumab) in August but was unable to reach an agreement. NMOSD occurs when AQP4 autoantibodies, a disease-specific biomarker produced by B cells, bind to AQP4, a target antigen present on glial cells in the central nervous system, and activate the immune responses, causing nerve damage. Uplizna is an anti-CD19 human monoclonal antibody that selectively binds to CD19, a B cell-specific surface antigen, depleting B cells that produce AQP4 antibodies, thereby preventing disease relapse. The safety and efficacy of Uplizna were demonstrated in the N-MOmentum study, which evaluated the use of Uplizna monotherapy in 230 patients without the use of concomitant immunosuppressive agents. Study results showed that 89% of patients treated with Uplizna did not experience a relapse during 197 days of follow-up, resulting in a 77.3% reduction in the risk of relapse compared to placebo. Safety evaluations Uplizna also showed comparable rates of adverse events to the placebo group. Also, in an extension study, Uplizna continued to reduce the risk of relapse for at least 4 years, with an 87.7% relapse-free rate. In terms of long-term safety profile, Uplizna was generally well tolerated, with no increase in infection rates due to B-cell depletion. NMOSD is a serious autoimmune disease in which most patients experience persistent relapses and incomplete recovery, resulting in accumulated nerve damage that can cause vision loss, gait disturbances, and even death from respiratory failure.
- Company
- 20-valent pneumococcal conjugate vaccine set to launch
- by Moon, sung-ho Nov 06, 2024 05:52am
- Attention has been drawn to another competitor set to launch in the market for the 'pneumococcal vaccine.' This year, the pneumococcal vaccine market has been fiercely competitive with newly released products. As the 20-valent vaccine is likely to come out right after the 15-valent conjugate vaccine has entered the market after 13 years, competition between global pharmaceutical companies to take the market share is becoming intense. As clinical practices draw attention to the next generation of vaccines, they expect that 'price' will be a determining factor in the non-reimbursed market. According to pharmaceutical industry sources, the Ministry of Food and Drug Safety (MFDS) granted approval of Pfizer Korea's 'Prevenar 20 Prefilled Syringe (hereafter, Prevenar 20).' Prevenar 20 is Pfizer Korea's next-generation product that follows 'Prevenar 13,' which currently dominates the Korean market for pneumococcal vaccines. In addition to serotype components in the 13-valent vaccine, Prevenar 20 contains seven additional serotypes, including 8, 10A, 11A, 12F, 15B, 22F, and 33F. The MFDS has approved the drug's indication to prevent invasive pneumococcal disease, pneumonia, and acute otitis media caused by pneumococcus in infants, children, and adolescents aged six weeks and below 18 years old. It is also used to prevent invasive pneumococcal disease and pneumonia caused by pneumococcus in adults 18 years of age and above. As Prevenar 20 has been officially approved, Pfizer Korea will prepare to launch the product. Pfizer Korea will likely aim to launch the product in the first half of 2025. Analysis suggests that an official launch is possible when Prevenar 20 is included in the vaccine guidelines of major medical sciences organizations and the National Immunization Program (NIP). The process is similar to that of MSD's 15-valent vaccine, 'Vaxneuvance,' which has been officially launched into the clinical practices. MSD Korea received the official approval of Vaxneuvance from the MFDS on October 31, 2023. Considering Pfizer Korea's Prevenar 20 was approved on October 31, 2024, Vaxneuvance was approved precisely a year before. After the approval, Vaxneuvance was included in the Korean Society of Infectious Diseases (KSID)'s '2024 Updated Guidelines for Adult Immunization' that "KSID recommends use of the 15-valent pneumococcal conjugate vaccine (PCV15, MSD's Vaxneuvance) over the 13-valent pneumococcal conjugate vaccine (PCV13, Pfizer's Prevenar 13) for individuals subjected to adult pneumococcal conjugate vaccine (PCV) immunization." MSD launched the vaccine in April this year soon after its inclusion in the NIP, and Pfizer Korea is likely to follow a similar launching schedule. "We have been trying to receive Prevenar 20's approval. The specific schedule for launch has not been decided," a representative from Pfizer Korea said. "We will prepare for the launch next year." "We have yet to confirm our Korean partner for Prevenar 20," a representative from Pfizer Korea said. "We are in a partnership with Chong Kung Dang for Prevenar 13 for adults, and we have an agreement with 'Koreavaccine' for Prevenar 13 for young children." The industry draws attention to the competition in the pneumococcal vaccine market for the clinical practice. The question is who will dominate the market, given the competition between two global pharmaceutical companies. Following the withdrawal of the 10-valent PCV vaccine (Synflorix, GSK) from the Korean market, the 13-valent vaccine Prevenar 13 and the 15-valent vaccine Vaxneuvance are currently competing in the clinical practice. In particular, MSD Korea chose Boryung Biopharma as its Korean partner. Upon launching Vaxneuvance, MSD Korea actively engaged in sales‧marketing by hiring Paik Jong Won, a businessman, TV celebrity, and CEO of TheBorn Korea, as its commercial model. According to a pharmaceutical market research firm UBIST, the number of NIP and non-reimbursed immunizations for adults in nationwide hospital‧private clinics is rising after Vaxneuvance launched in April. In detail, based on UBIST, the cumulative number of PCV vaccine immunizations from April to June this year is 994 counts. Among these, 809 immunizations were with Prevenar 13. 185 Vaxneuvance immunization was made. After MSD Korea launched Vaxneuvance in April this year, they chose Paik Jong Won, TheBorn Korea CEO, as their commercial model, putting efforts into raising awareness. To the right is the product photo of Prevenar 20, Pfizer Korea's pneumococcal vaccine, which is expected to launch in the first half of 2025. The number of immunizations with Prevenar 20 is below that of market dominating Prevenar 13, but the number is rising. Based on the assumption that Vaxneuvance has been well established in clinical practice as a new vaccine, the number of immunizations were likely increased. In fact, a director of the pediatrics and adolescent clinic said, "When we used Prevenar 13 for immunizations, we had several inquiries if it's possible to switch to Vaxneuvance. Since medication switching is possible during the immunization course, we answer patient inquiries." And added, "If patients have not completed the immunization schedule, medication switching is possible. We had several cases of switching after inquiries." Considering changes brought by the Vaxneuvance launch this year, Prevenar 20 will likely bring changes to clinical practices such as the department of internal medicine and the department of pediatrics and adolescents. Sang Hyuk Ma, a Director of Pediatrics and Adolescents at Changwon Fatima Hospital, said, "Following Prevenar 13, Vaxneuvance and the 15-valent vaccine have been introduced to South Korea. We must consider clinically if a vaccine with more serotypes is appropriate for South Korea. Pneumococcal serotypes tend not to mix, and serotypes common in South Korea must be accounted for vaccination." Ma added, "In other words, the vaccines were developed towards what's needed in the United States. We must look into common serotypes in South Korea and discuss whether the latest vaccines are necessary for immunizations in Korean citizens, including infants and children." Gwak Kyung-Keun, the President of Seoul Physicians' Association, said, "In my opinion, there is no difference between the 15-valent and the 20-valent vaccines. There is no basis for which one is more superior." Gwak anticipated, "In the end, it will be the sales‧marketing competition between companies. One would argue that the 20-valent vaccine has more serotypes but will be a marketing fight without a comparison basis."
- Company
- Roche seeks digital solutions for its healthcare ecosystem
- by Whang, byung-woo Nov 06, 2024 05:52am
- With the importance of personalized healthcare being emphasized more than ever with the emergence of innovative new drugs, Roche Diagnostics Korea is aiming to lead the market by focusing on digital solutions. The company plans to lead the healthcare sector through digital transformation in line with the paradigm shift in treatment, from early diagnosis and precision medicine to predictive medicine. (From the left) Tae-Hyun Um (Director of Insurance Policy Affairs, KSLM), Yeo-Min Yun, Director of Scientific Affairs, KSLM), Sail Chun (CEO&Chaiarman, KSLM), Kit Tang (General Manager of Roche Diagnostics Korea), Muhwan Yun (head of Digital Insights, Roche Diagnostics Korea), Sungho Cho (Head of Core Lab & POC, Roche Diagnostics Korea) On the 5th, Roche Diagnostics Korea held a press conference on the theme of “Future Healthcare and Innovation Presented by Diagnostic Tests” and emphasized the importance and opportunities of digital transformation in the healthcare sector. With the launch of the Digital Insights division, the company has been focusing on providing data-driven insights and marking a new turning point in personalized medicine. “Digital transformation in healthcare is the new normal, and we have already entered the era of artificial intelligence (AI) transformation beyond digital transformation,” said Muhwan Yun, head of Digital Insights at Roche Diagnostics Korea. ”Roche Diagnostics has set its own ethical standards for applying AI to healthcare, and is aiming to expand and innovate its digital portfolio, including continuous R&D investment and collaboration.” One representative project is Smart Lab, a digital transformation of diagnostic laboratories. The goal is to make existing laboratory operations more efficient, flexible, and data secure, to ultimately increase insight from analyzing data. For this, Roche Diagnostics has been emphasizing 'digital solutions' and building an enabling ecosystem. It focuses on incorporating significant data of a certain scale, advanced analytics, and digital technologies to support medical decisions and improve the treatment experience. The company’s representative technology, NAVIFY, is a cloud-based data platform that analyzes vast amounts of healthcare data in a standardized format to enable precision medicine. “In practice, we have seen significant improvements in time, manpower, and cost metrics for testing and analysis after implementing Roche Diagnostics Digital Insight Solution’s NAVIFY portfolio,” said Yun, ”and the satisfaction rate among healthcare providers who have used them is over 90%.” “With South Korea set to enter a super-aged society next year, the role of diagnostic test data for efficient and effective treatment is expected to increase further,” said Kit Tang, General Manager of Roche Diagnostics Korea. ”Roche Diagnostics Korea will continue to strive to contribute to an efficient healthcare system and improved patient outcomes with innovative diagnostic solutions covering a wide range of disease areas.” The hurdle to building a digital ecosystem for diagnostic tests is in the “maturity” of the digital transformation In the long run, digital transformation in diagnostics is essential, but there are hurdles. Not only are institutional improvements required for the digital transformation of the existing system, but the nature of healthcare is such that it is important to integrate the different perspectives of each stakeholder, including hospitals, manufacturers, patients, and academic societies. Yeo-Min Yun (Director of Scientific Affairs, KSLM)In response to this, Yeo-Min Yun, Director of Scientific Affairs at KSLM of the Korean Society for Laboratory Medicine (Konkuk University Hospital), emphasized that the emergence of technology should be supported by measures that can encourage their use in practice, such as by assigning service fees. “If you look at imaging, the technology that analyzes the images through AI technology is being applied and utilized to create values that have never existed before,” said Director Yun. ”Referring to this precedent, I think that efforts should be made to apply a service fee for such diagnostic tests and recognize their value in clinical practice.” Ultimately, Roche Diagnostics' challenge is to collect and utilize fragmented data. To this end, the company expects the newly launched division to serve as a base to build the ecosystem. “In Korea, data compatibility is relatively poor, so we are striving to collect and utilize data well,” said Director Yun, adding, ”As the implementation of digital ecosystems is not fully mature yet, it will be important for each component, including the company, to be integrated.”
- Company
- CKD’s CKD-508 receives approval to initiate P1T in the U.S.
- by Son, Hyung Min Nov 06, 2024 05:52am
- Chong Kun Dang announced on the 4th that it has received approval from the U.S. Food and Drug Administration (FDA) for the Phase I clinical trial of CKD-508, its new drug candidate for dyslipidemia. In the trial, Chong Kun Dang will confirm the safety and lipid-improving effects of CKD-508 and explore the optimal dose for a Phase II trial. CKD-508 is a treatment for dyslipidemia that works by inhibiting the activity of cholesteryl ester transfer protein (CETP), which facilitates the transport of cholesteryl esters (CE) and triglycerides (TG) between lipoproteins in the blood, thereby lowering low-density cholesterol (LDL-C) levels and increasing high-density cholesterol (HDL-C) levels. In a non-clinical efficacy trial conducted by Hyosung Research Center, Chong Kun Dang confirmed the LDL-C-lowering and HDL-C-raising effects of CKD-508 and demonstrated a significant reduction in apolipoprotein (Apo-B), a key marker of dyslipidemia. “CKD-508 is an innovative drug that is expected to be effective at low doses by solving the problems of previous CETP inhibitors that were discontinued due to drug accumulation and blood pressure increase based on its strong binding to CETP,” said Chong Kun Dang. ”If successful, it is expected to become a new treatment option for patients with statin-resistant dyslipidemia that cannot be controlled with statins.”
- Company
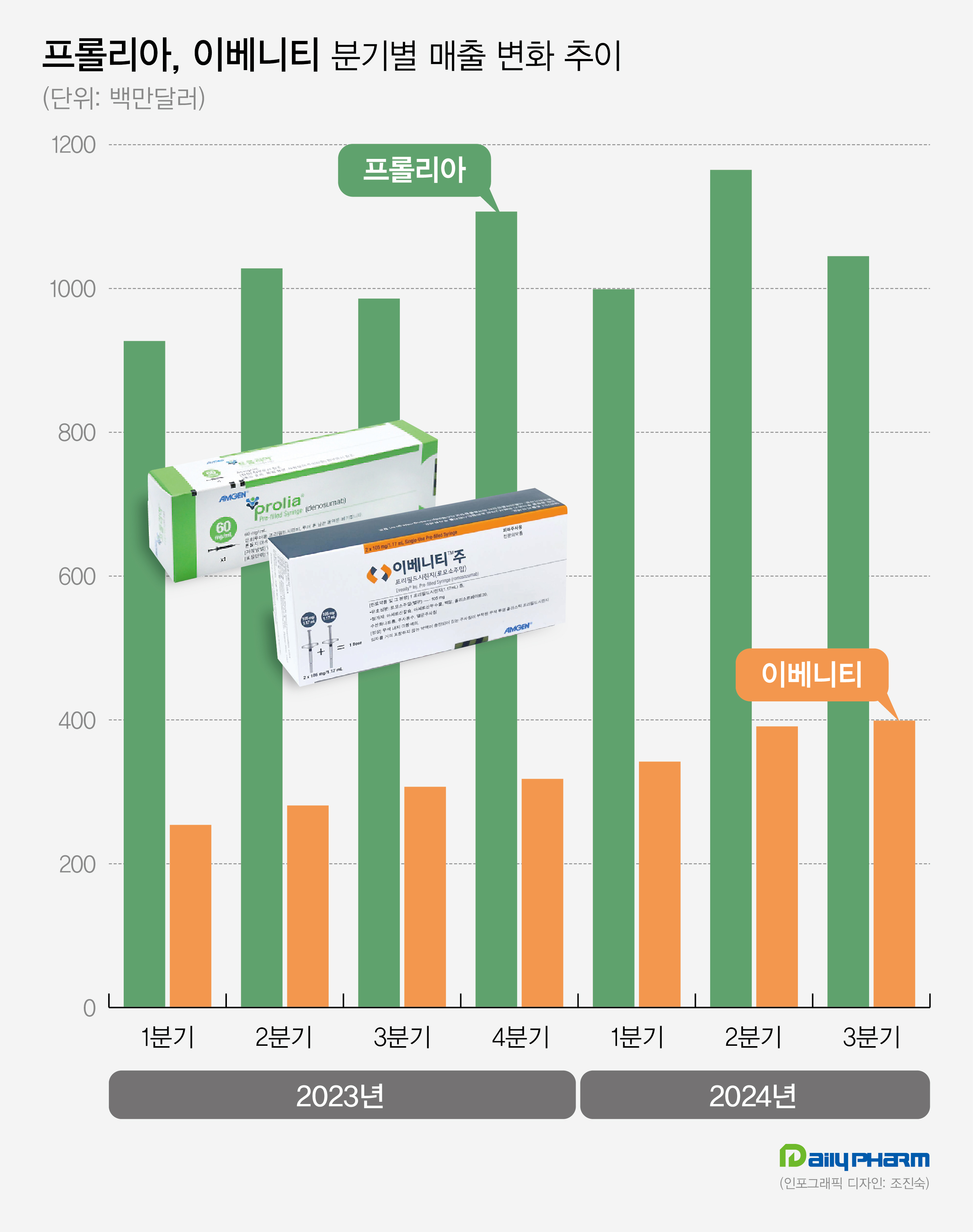
- Amgen's Prolia·Evenity generate KRW 2T in quarterly sales
- by Son, Hyung Min Nov 06, 2024 05:52am
- Amgen Amgen's Prolia and Evenity for the treatment of osteoporosis continue to show sales growth. Prolia and Evenity led Amgen's performance, generating sales of KRW 2 trillion in Q3. Analysis suggests that sequential therapy, using Evenity followed by Prolia for the treatment of osteoporosis with high-risk fractures, has been widely used, and it has led to continued increases in sales. Sources said on November 6th, Amgen's sales in Q3 were US$ 8.50 billion (approximately KRW 11.72 trillion), up 23.1% year-over-year (YoY). Operating profit for the same period was US$ 2.047 billion, up 1.3% from 2022. Prolia generated the highest sales among Amgen's products. Prolia's sales in Q3 were US$1.045 billion (approximately KRW 1.44 trillion), up 6.0% YoY. Prolia recorded US$3.20 billion (approximately KRW 4.42 billion) for the nine months this year, up 9.1%. Last year, Prolia's annual sales were US$40.48 billion. Developed by Amgen, Prolia is a treatment for osteoporosis. It was approved in the United States, and after that, Amgen successfully received Prolia's approval in major countries, including South Korea, Europe, and Japan. Prolia sales nearly doubled to US$352 million in Q1 of 2016 in two years from US$196 million (approximately KRW 270 billion) in Q1 of 2014. Prolia's sales continue to grow, generating US$852 million (approximately 1.2 trillion) in Q1 of 2022. In Q2 of 2023, its quarterly sales topped US$1 billion. In Q1 of this year, it dropped to US$9.99 billion, but then successfully rebounded in Q2, recording US$1.165 billion. Prolia's strength lies in having secured real-world data. Based on its FREEDOM and FREEDOM Extension clinical studies spanning 10 years, Prolia effectively reduced the risk of fractures than the conventional osteoporosis treatment, Alendronate. Unlike other medications, Prolia continues to increase bone density. Prolia is categorized as a medication that can be administered for long-term. As a result, pharmaceutical companies are developing Prolia biosimilars. Earlier this year, U.S.-based Sandoz received approval for a biosimilar of Prolia and Korean pharmaceutical companies such as Celltrion and Samsung Bioepis also plan to complete clinical trials and prepare for approval. Prolia's patent is set to expire in 2025. Quaterly sales performance of Prolia (green) and Evenity (oranage) in 2023 and 2024 (unit: US$1 million). Evenity records KRW 550 billion in Q3 sales…continues to increase The success of the osteoporosis treatment Evenity has contributed to Amgen's sales performance. Evenity's Q3 sales amounted to US$399 million (approximately KRW 550 billion), up 30.0% from US$307 million in 2022. Evenity is a bone-forming agent that provides dual effects of promoting bone formation and inhibiting bone absoption. It was approved in the United States in 2019. Evenity recorded sales of US$100 million for the first time in Q1 2020, and it continues to grow. In Q3 2022, Evenity recorded sales of US$200 million, more than doubling its initial launch. In Q3 of Last year, Evenity surpassed US$300 million and showed steady growth, reaching sales of US$400 million in Q3 of this year. Evenity's increase in sales is mainly due to its osteoporosis treatment strategy. As part of its marketing strategy, Amgen has strategized a sequential therapy of using the bone-forming agent Evenity followed by the bone resorption inhibitor, Prolia. Based on their approved indications, Bone-forming agents have a maximum duration of use of 2 years. Therefore, after a certain period of treatment, switching to bone resorption inhibitors such as Prolia or bisphosphonate agents are mainly used. According to Evenity's ARCH study or a study comparing teriparatide and risedronate, a sequential therapy using bone-forming agents first followed by bone resorption agents is more effective in preventing the risk of bone fractures in patients. Korea-based or overseas guidelines for osteoporosis treatment recommend bone-forming agents, such as Evenity, as first-line treatments for high-risk individuals.
- Company
- Chong Kun Dang speeds up dyslipidemia drug development
- by Son, Hyung Min Nov 05, 2024 05:46am
- Chong Kun Dang is accelerating the development of new drugs for dyslipidemia. The company has received approval to initiate a second global clinical trial in 10 years for its new drug candidate 'CKD-508' since the company began its research in 2014. According to industry sources on the 4th, Chong Kun Dang recently received approval from the U.S. Food and Drug Administration (FDA) to initiate a Phase 1 clinical trial on its dyslipidemia drug candidate, 'CKD-508'. Through the clinical trial, Chong Kun Dang plans to verify the safety and lipid-improving effect of CKD-508 and explore the optimal dose for its future Phase 2 trial. CKD-508 is a second-generation drug that overcomes the challenges of first-generation cholesteryl ester transfer protein (CETP) inhibitors, including limitations such as off-target effects (unexpected side effects caused by the drug), accumulation of fat cells, and poor stability. The drug candidate works by inhibiting the activity of CETP, which facilitates the transport of cholesteryl esters (CE) and triglycerides (TG) between fatty proteins in the blood, thereby lowering low-density lipoprotein cholesterol (LDL-C) levels and raising high-density cholesterol (HDL-C) levels. More than 6 years after initiating the CKD-508 study in 2014, Chong Kun Dang has now initiated global clinical trials. In June 2020, the company received approval from the UK's MHRA for a Phase 1 clinical trial of CKD-508 and is advancing its research and development. Preclinical results showed that CKD-508 significantly reduced LDL-C and LDL-C-containing apoprotein (Apo-B) and increased HDL-C in animal models with dyslipidemia. In addition, CKD-508 did not cause drug accumulation in adipose tissue or an increase in blood pressure, which were observed in clinical trials of anacetrapib and torcetrapib. Anacetrapib and torcetrapib were candidates for dyslipidemia developed by Merck and Pfizer, but the companies discontinued their development in 2017 due to safety concerns in the clinical trial stage. Chong Kun Dang believes that CKD-508 has the potential to be a breakthrough drug that could provide a new treatment option for patients with dyslipidemia that is not controlled by existing drugs such as statins. CKD-508 can be administered once weekly and may offer improved convenience over existing therapies. Along with CKD-508, Chong Kun Dang is also conducting 5 clinical trials in the synthetic drug category, including CKD-943 for uremic pruritus, CKD-950 (namodenoson) for hepatocellular carcinoma, CKD-951 for metabolic dysfunction-associated steatohepatitis (MASH), and CKD-510 for rare diseases. The company completed clinical trials for CKD-943 in the U.S. in 2015 and is currently in Phase III clinical trials. Chong Kun Dang has been developing CKD-943 since 2012 after signing a license agreement with Cara Therapeutics in the US for the exclusive development and sales of CKD-943 in Korea. CKD-950 recently entered Phase II clinical trials in Israel. In 2016, Chong Kun Dang entered into an exclusive domestic license agreement with Israel's Can-fite Biopharma for CKD-950, a novel liver cancer drug candidate. The company also acquired a domestic patent for CKD-950 in 2020. CKD-951, which is being developed as a treatment for MASH, has been recruiting patients since its IND approval in 2020. CKD-951 has a mechanism of action that improves inflammatory and fibrotic responses by acting on A3AR, which is overexpressed in liver inflammatory/fibrosis-promoting cells. CKD-510 was licensed out to Novartis in November of last year. CKD-510 is a histone deacetylase 6 (HDAC6) inhibitor that uses the company’s highly selective, non-hydroxamic acid platform technology. The drug candidate has demonstrated safety and tolerability in Phase I clinical trials in the U.S. and Europe.
- Company
- ‘Prevent MI recurrence through efficient LDL-C control'
- by Whang, byung-woo Nov 05, 2024 05:45am
- With the rise of metabolic diseases such as hypertension, diabetes, and hyperlipidemia increase in Korea, the prevalence of myocardial infarction and atherosclerotic cardiovascular diseases are also on the rise. The mortality rate of myocardial infarction is in the 20-30% range when it occurs for the first time, but the mortality rate increases sharply to 68-85% when it recurs, which is why efforts to prevent recurrence are being stressed now. In particular, one of the hot topics in treatment is how to manage LDL cholesterol, which is known to be an important factor in preventing the recurrence of atherosclerotic cardiovascular disease (ASCVD). In recent years, treatment options have become more diverse and multiple approaches have been proposed. Dr. Dong-Oh Kang, Professor of Cardiology and Cardiovascular Center at Korea University Guro Hospital, emphasized the need to effectively lower LDL cholesterol levels in high-risk patients. Dong-Oh Kang, Professor, Department of Cardiology, Korea University Guro Hospital “New drugs have changed the approach to LDL cholesterol management in high-risk patients” In severe cases of acute myocardial infarction, stenting or balloon angioplasty is performed to open up the blood vessel, as it is an emergency treatment for blocked blood vessels or low blood flow. However, these procedures are reactive, and it is important to use medications to prevent the same event from happening again. “It is important for patients who have had a myocardial infarction to use drugs to prevent further accumulation of atherosclerotic plaque and narrowing of the artery,” said Professor Kang. ”Lowering cholesterol to inhibit the progression of atherosclerotic plaque and preventing blood clots has become a key treatment.” This is why one of the most important topics in recent guidelines is to what level LDL cholesterol should be lowered in very-high-risk patients. Both domestic and international academic societies have proposed a strict management standard for patients with a history of atherosclerotic cardiovascular disease, with LDL cholesterol targets of less than 55 mg/dL and at least 50% lower than baseline. “The past guidelines suggested that LDL cholesterol levels could be as low as 100 mg/dL, but more potent drugs have come in a variety of combinations.” said Professor Kang, “As lowering LDL cholesterol levels has been shown to reduce the risk of atherosclerotic cardiovascular disease, even lower levels are now being recommended.” According to Kang, the suggested LDL cholesterol level for high-risk patients was less than 70 mg/dL in the 2010s, but by the late 2010s, patients with coronary artery disease or at very-high-risk were advised to lower their LDL cholesterol level to less than 55 mg/dL and at least 50% from baseline. In particular, the European guidelines suggest lowering LDL cholesterol levels to less than 40 mg/dL for patients with acute coronary syndrome who have had a recurrent event within the last 2 years. “Cardiologists who see patients with more severe acute myocardial infarction or patients undergoing procedures seem to be in agreement with the lower LDL cholesterol targets. However, some have concerns about lowering LDL cholesterol levels below 55 mg/dL or 70 mg/dL.” Diversification of treatment options, including PCSK9 inhibitors...“Strategy will change depending on reimbursement status” As Professor Kang noted, the lower LDL cholesterol target levels have been accompanied by the emergence of drugs that can effectively lower the levels to such targets. In the past, statins, which inhibit the synthesis of cholesterol in the liver, were the only drugs available to lower LDL cholesterol levels, but more strategies became available with the introduction of ezetimibe, which inhibits cholesterol absorption in the intestine, including statin and ezetimibe combinations. Then, the entry of monoclonal antibody drugs such as Repatha (evolocumab), a PCSK9 inhibitor, into the reimbursement system has transformed the clinical landscape. Currently, PCSK9 inhibitors are used in patients with myocardial infarction whose LDL cholesterol levels have not dropped sufficiently despite the use of high-intensity statins and ezetimibe. “It's important to monitor the dose escalation during initial therapy,” said Kang. “If LDL-C targets are not met, the dose should be increased and the patient reevaluated. If the maximum dose is not effective, a PCSK9 inhibitor such as Repatha, which has a faster LDL cholesterol lowering rate and is more potent, may be considered.” “In terms of Repatha’s effect, 19 out of 20 people will have lower LDL cholesterol level maintained, even at 30 mg/dL. In patients who had low LDL cholesterol, to begin with, we see reductions to less than 10 mg/dL.” In the long term, the introduction of oral bempedoic acid and injectable siRNA therapies is expected to further expand treatment options. In addition to access to treatments based on patient condition, Professor Kang predicts that treatment approaches will change based on the drug’s reimbursement status. “As more effective treatments will continue to be developed, we expect more and more combination options to emerge, and it is necessary to prescribe them considering the patient's condition and the characteristics of each drug,” said Kang. ”Since there are various drugs, their use will likely be determined by how reimbursement is applied in high-risk patients.” In addition to secondary prevention, Kang emphasized the need for policy promotion to screen and manage patients before they become high-risk. “Even though people are sufficiently screened and informed about their risk factors through health screenings, they often overlook them and look back in retrospect after they become ill. It is necessary to always receive screening and make efforts to properly treat or improve lifestyle habits from the primary prevention stage.”
- Company
- New oHCM drug 'Camzyos' nearing approval for reimb in KOR
- by Eo, Yun-Ho Nov 05, 2024 05:45am
- Product photo of Camzyos. 'Camzyos,' a new drug to treat obstructive hypertrophic cardiomyopathy (oHCM), is nearing 90% approval for insurance reimbursement listing. Sources said that BMS Pharmaceutical Korea and the National Health Insurance Service (NHIS) concluded drug pricing negotiations for Camzyos (mavacamten), a new drug for obstructive hypertrophic cardiomyopathy (oHCM). The drug had previously faced a delay in the decision, but the company quickly reached an agreement this time. As a result, Camzyos is likely to be listed within this year. This drug received a re-assessment status during the Drug Reimbursement Evaluation Committee (DREC) review of the Health Insurance Review and Assessment Service (HIRA). After that, it passed the DREC review and entered a drug pricing negotiation in August, but the drug did not receive a decision during the negotiation period (60 days). Camzyos is the only drug that selectively inhibits cardiac myosin-actin cross-bridge formation, which is the cause of oHCM. Camzyos' mechanism involves dissociating myosin from actin, relaxing overstimulated heart muscle, and thereby improving left ventricular outflow tract (LVOT) structure and LVOT outflow obstruction. Due to the lack of available treatments for oHCM for a long time, off-label medications have been used to manage symptoms. After Camzyos launched, the European Society of Cardiology (ESC) updated its guidelines for managing cardiomyopathy for the first time in about nine years. Previously, the guidelines for HCM were based on evidence limited to small-scale monitoring data, retrospective analysis results, and consensus opinion. However, Camzyos has completely changed this situation. Two large-scale, phase 3 clinical trials conducted as randomized controlled trial (RCT) have confirmed the significant effect of Camzyos. Consequently, ESC guidelines recommend Camzyos with the highest evidence level A for the first time in treatment options. American College of Cardiology (ACC) and the American Heart Association (AHA) are preparing to update their guidelines. Furthermore, based on this phase 3 trial evidence, the U.S. FDA granted Camzyos Breakthrough Therapy Designation (BTD) and approval. Meanwhile, the efficacy of Camzyos was demonstrated through Phase 3 EXPLORER-HCM trials. In this trial, Camzyos improved primary endpoints, which were the patient’s symptoms (NYHA classification) and exercise capacity measured with peak oxygen uptake (pVO2), more than twofold compared to the placebo. 20% of the patients treated with Caymzyos met the NYHA classification and pVO2 improvements. It also reduced the LVOT outflow obstruction index by four-fold after exercise. 7 out of 10 patients who received Camzyos treatment had improved indexes and ended up not considering surgery, and they maintained the effects for 30 weeks.