- LOGIN
- MemberShip
- 2025-12-22 07:38:28
- Company
- ‘Use of Prevenar 20 will rise to address the unmet need'
- by Son, Hyung Min Nov 20, 2024 06:08am
- Dr. Su-Eun Park, professor of pediatrics at Pusan National University Children Prevenar 20 has become the pneumococcal vaccine that covers the most serotypes in Korea. Experts believe that its use will increase to address the unmet need for a treatment that protects against serotypes not targeted by existing vaccines. Pfizer Korea held a press conference at the Lotte Hotel in Jung-gu, Seoul on the 19th to celebrate the approval of Prevenar 20 in South Korea. The Ministry of Food and Drug Safety approved Prevenar 20 as a new pneumococcal vaccine on March 31st. With the approval, Prevenar 20 is now available for ▲ the prevention of invasive disease, pneumonia and acute otitis media caused by 20 Streptococcus pneumoniae strains (1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F) in individuals 6 weeks and older; ▲ For the prevention of invasive disease and pneumonia caused by pneumococci (serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F) in persons 18 years of age and older. Prevenar 20 is Pfizer's first new pneumococcal vaccine in 14 years, which adds 7 serotypes (serotypes 8, 10A, 11A, 12F, 15B, 22F, and 33F) to the existing Prevenar 13 vaccine. In a clinical trial, the company confirmed Prevenar 20’s immunogenicity and tolerability against 20 serotypes in healthy infants after 4 doses. The results were also similar in another clinical trial that was conducted on adults. “Prevenar 20 demonstrated immunogenicity and safety against all 13 serotypes it shared with Prevenar 13, and 7 additional serotypes compared to the existing 13-valent pneumococcal polysaccharide vaccine,” said Dr. Sunju Kim, Medical Lead at Pfizer Korea. “More serotypes, more protection with Prevenar 20” Pneumococcus pneumoniae is an infectious disease caused by the bacterium Streptococcus pneumoniae, of which there are approximately 100 serotypes. In the United States, more than 150,000 adults are hospitalized for pneumococcal pneumonia each year. Streptococcus pneumoniae is the leading cause of bacterial pneumonia, meningitis, and sepsis in children worldwide. While the incidence of invasive pneumococcal disease is declining due to the availability of various vaccines, including Prevenar, the disease burden due to serotypes not covered by existing vaccines still remains. In a surveillance study on Korean pediatric invasive pneumococcal disease conducted from January 2014 to December 2019, frequent serotypes isolated from 168 cases included 10A (40 cases). 10A is one of the serotypes not targeted by existing vaccines. In addition, among the serotypes (67 strains) of invasive pneumococcal disease in children and adolescents in Korea that occurred between 2018 and July 2021, serotypes targeted by Prevenar 20 accounted for 54%. Dr. Su-Eun Park, professor of pediatrics at Pusan National University Children's Hospital, said, “The serotypes that were of an issue in Korean children have been added to Prevenar 20. It is expected to provide an additional 40% protection against pediatric invasive infection, 30% against otitis media, and 20% protection in adults.”
- Company
- Drug switching policy for atopic dermatitis fails to address
- by Whang, byung-woo Nov 20, 2024 06:08am
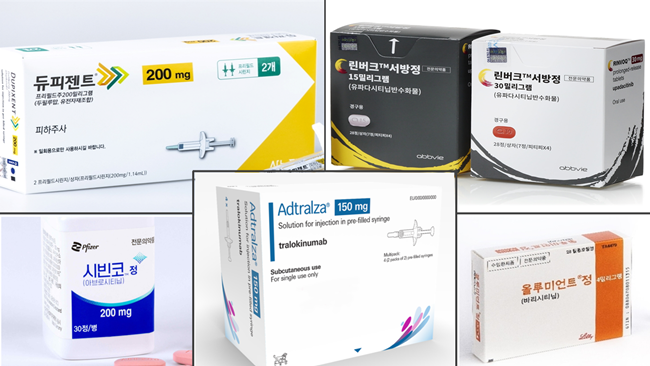
- A discussion has been advancing to allow drug switching between a biological agent and a JAK inhibitor for treating atopic dermatitis (hereafter referred to as atopy), and there have been further suggestions for the revision. The Health Insurance Review and Assessment Service (HIRA) has already established reimbursement criteria, but concerns have been raised about potential challenges in the effective use of the drug, including drug switching between drugs of the same class. (clockwise from top left) Product photos of Dupixent, Rinvoq, Olumiant, Adtralza, and Cibinqo The Embassy of Denmark in Korea met with the Severe Atopic Dermatitis Association (SADA) to discuss potential improvements to atopy treatment settings and systems. The HIRA has exchanged opinions with experts about allowing drug switching between a biological agent and a JAK inhibitor since September, and they have established the reimbursement criteria based on the latest documents and clinical practices. According to the pharmaceutical industry, pharmaceutical companies have submitted measures to voluntarily cut drug prices as part of financial allotment following expanded reimbursement. Attention has been drawn to whether it will pass the Drug Reimbursement Evaluation Committee (DREC) review, which is the final step during the appropriateness review process. However, the Severe Atopic Dermatitis Association (SADA) emphasizes that, while they have positive views towards the ongoing discussion about drug switching between treatments, an additional review of the revision to reimbursement criteria is necessary. Their chief suggestion for the draft of revision is recognizing the need for drug switching within the same class of drugs in addition to drug switching between different classes. Currently, drug switching for atopy is approved for drug switching between a biological agent and a JAK inhibitor. As a result, drug switching between drugs of the same class is not allowed. For instance, drug switching between biological agents such as Dupixent and Adtralza or JAK inhibitors such as Rinvoq and Cibinqo has been limited. However, drug switching between the same classes must also be considered considering many underlying mechanisms of atopy. In fact, SADA's '2024 Guidelines for the Treatment of Atopic Dermatitis in Korea,' which has been updated after 9 years, does not provide detailed recommendations for switching drugs between treatments due to the different properties of atopy. A SADA member said, "We do not have a set order of treatments because it is difficult to decide which drug is more suitable. The associations hope that drug switching becomes possible regardless of the classes." "Still limited drug switching between atopy treatments, we must consider all aspects" During the meeting with Daily Pharm, SADA President Joeun Park said, "Due to the nature of atopy, patients have different characteristics of atopy. When treatment options for drug switching are limited, patients may have difficulty in switching drugs." Park added, "Since the discussion about improvements to treatment setting is ongoing, in my opinion, there should be no limitations to the classes of drugs and the number." Currently, patients are more likely to choose drugs with the greatest market presence within the same class based on their experiences. Therefore, they may not significantly benefit from the treatment. In other words, Park is concerned that the current revision for atopy drug switching may not produce effective outcomes and could remain merely an administrative action. However, Park states that the government's draft for revision of six months for switching is sufficient. Park emphasized the need for the government to pay more attention to patients who are unable to receive JAK inhibitors. "In my opinion, patients typically do not respond to atopy treatment until around four months, with noticeable effects starting to appear around six months. Therefore, I support continuing treatment for up to six months," Park stated. "However, in a few cases, patients become pregnant while undergoing JAK inhibitor treatment. As a result, the government should implement more flexibility concerning these situations." (from left) Joachim Arup-Fischer, Commercial Counselor at the Embassy of Denmark in Korea, and Mads Friborg, Health & Medical Counselor at the Embassy of Denmark in Korea. & Medical Counselor at the Embassy of Denmark in Korea Regarding this issue, the Embassy of Denmark in Korea plans to collaborate on healthcare issues, including atopy. "In Denmark, the importance and influence of patient advocacy groups are growing. We are considering including this aspect in next year's business plan when collaborating with the Ministry of Health and Welfare (MOHW)," Mads Friborg, Health & Medical Counselor at the Embassy of Denmark in Korea, said. "We started to pay attention to atopy when HIRA's agenda was hotly debated. Additionally, we are considering discussing and bringing suggestions about the disease," Joachim Arup-Fischer, Commercial Counselor at the Embassy of Denmark in Korea, said. "Drug pricing was the chief discussion point during the recent meeting with the HIRA. From now on, we expect to discuss various aspects."
- Company
- Returned out-licensing, Daewoong's autoimmune disease drug
- by Son, Hyung Min Nov 19, 2024 06:13am
- Daewoong Pharmaceutical. Daewoong Pharmaceutical's new drug candidate, 'DWP213388,' for the treatment of autoimmune diseases is set to return after a year and a half. DWP213388, a new drug candidate developed as the First-in-Class, has entered the Phase 1 clinical trial. According to the Financial Supervisory Service (FSS) on November 16, Daewoong Pharmaceutical announced the U.S.-based biotech company Vitalli Bio has notified of out-licensing agreement termination for DWP213388, a treatment for autoimmune diseases. Both companies will undergo 60 days of negotiation and finalize the contract termination. Daewoong's upfront payment amounting to US$1.1 million is non-refundable. Daewoong Pharmaceutical had signed a global out-licensing agreement with Vitalli Bio, a subsidiary of the biotech investment firm, for its DWP213388 during the 'Korea-U.S. Digital and Bio-Health Business Forum' held on April 2023 in Boston, U.S. DWP213388 has a bispecific mechanism of inhibiting both Bruton's Tyrosine Kinase (BTK) and Interleukin-2-inducible T-cell Kinase (ITK), which are involved in activating immune cells such as B cells and T Cells. This new drug candidate received Phase 1 approval from the U.S. Food and Administration Agency (FDA) in 2022. In a preclinical trial, DWP213388 confirmed efficacy and safety in an autoimmune disease animal model. The Graft vs Host Disease (GvHD) mouse model study showed that DWP213388 effectively suppresses symptoms and improves survival rate. In a rheumatoid arthritis mouse model, DWP213388 demonstrated efficacy with a lower dosage than existing treatment and effects protecting bone damage. Additionally, an animal model study of cytomegalovirus (CMV) infections confirmed that DWP213388 effectively killed the virus. Most BTK inhibitors work selectively on B cells, suppressing BTK overexpression in B cells. The most prominent diseases caused by B-cell overexpression are blood cancers such as leukemia and lymphoma. DWP213388 also selectively targets ITK, another type of protein that regulates the immune function of T cells. BTK and ITK overexpression can result in the development of autoimmune diseases, including psoriasis, lupus, and inflammatory bowel disease. DWP213388 is different from existing BTK inhibitors in that it has high ITK-selectivity and low EGFR-selectivity, providing benefits of lower risk of side effects such as liver toxicity. BTK inhibitor may secure new indication…will it be a new opportunity for Daewoong? Daewoong Pharmaceutical's return of a new drug candidate is highly possible. Yet, attention is drawn to whether the company would obtain a new opportunity similar to the case of Hanmi Pharm. Hanmi Pharmaceutical had 'Poseltinib' returned from the global pharmaceutical company Eli Lily. However, Hanmi Pharm continues to develop the drug after changing indications. Poseltinib is a new drug candidate developed by Hanmi Pharm in 2010 for the first time, and it was out-licensed to Eli Lily in 2015. After that, Elily Lily failed Poseltinib's Phase 2 clinical trial for patients with autoimmune diseases and returned the development rights in 2019. After that, in collaboration with Korean bioventure NOBO Medicine (previously Genome Opinion), Hanmi Pharm discovered a new possibility of Poseltinib in diffuse large B-cell lymphoma (DLBCL) in a clinical trial. Both companies confirmed the safety and effectiveness of the combination therapy containing Poseltinib+Columvi+Revlimid. Based on the clinical results presented to date, of the 14 patients who can be assessed for response, the percentage of patients who met the objective response rate (ORR) recorded was 79%. Although it was early data, 36% of the patients reported complete response (CR) with a lack of all signs of cancer cells. No adverse reactions were reported in a cohort assessed for safety. In October 2021, Hanmi Pharmaceutical signed a joint development agreement with the Korean bioventure NOBO medicine for Poseltinib and jumped into the market for blood cancer treatments. Genome Opinion has been conducting an Investigator Initiated Trial (IIT) for triple-combination therapy containing Poseltinib, Roche's Columvi, and BMS' Revlimid for patients with relapse/refractory DLBCL at Seoul National Hospital.
- Company
- Three months after Pluvicto launch
- by Moon, sung-ho Nov 19, 2024 06:13am
- Novartis Korea's prostate cancer treatment, Pluvicto, which has garnered attention since its approval by the Ministry of Food and Drug Safety (MFDS), has now been administered to Korean patients for three months. Pluvicto is a blockbuster drug that generated over KRW 1 trillion in global sales last year and is recognized as a product initiating the so-called "radioactive missile" era. Pluvicto can primarily be prescribed in large hospitals in South Korea, leading to a growing number of patients receiving the treatment. However, its high cost and non-reimbursable status remain significant barriers to patient access. As the use of radiopharmaceuticals becomes more widespread in domestic clinical settings, competition among local pharmaceutical‧biotech companies developing similar treatments is intensifying once again. According to the medical community on November 14, since the MFDS approved Novartis Korea's prostate cancer treatment Pluvicto (Lutetium (177Lu) vipivotide tetraxetan injection) at the end of May, six medical institutions, including the National Cancer Center, have administered or plan to administer the treatment as of November. Pluvicto is a radioligand therapy that targets prostate-specific membrane antigen (PSMA), which is overexpressed in prostate cancer, by binding the radioactive isotope Lutetium-177 (177Lu) to PSMA to eliminate cancer cells. Radioligands are therapies that combine a ligand (a targeting molecule) with a therapeutic radioactive isotope. When the radioligand binds to the target cell, it emits therapeutic radiation, which suppresses cancer cell proliferation. The basis of domestic approval was the Phase 3 VISION clinical tiral. The trial involved 831 patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). It was designed to compare the efficacy and safety of Pluvicto combined with standard therapy versus standard therapy alone. The clinical trial results showed that the Pluvicto group recorded a median radiographic progression-free survival (PFS) of 8.7 months, significantly longer than 3.4 months in the control group. The median overall survival (OS) was 15.3 months for the Pluvicto group, compared to 11.3 months for the control group. Administration of Pluvicto reduced the risk of disease progression or death by 60%. To introduce Pluvicto, medical institutions must have dedicated PSMA PET-CT equipment for prostate cancer and facilities for preparation, quality control, and separate spaces for patient administration. Additionally, multidisciplinary collaboration involving nuclear medicine, oncology, and urology specialists is essential. The treament requires significant investment in medical institutions' facilities, equipment, and personnel. Currently, 15 medical institutions nationwide, including major hospitals in South Korea, have adopted PSMA PET-CT for diagnostic purposes. As of November, six of these institutions are providing treatment using Pluvicto. The remaining issue is the cost. The recommended dose of Pluvicto is 7.4 GBq (200 mCi), administered intravenously every six weeks (±1 week) for up to six doses. Each dose costs KRW 30-40 million out-of-pocket in clinical practices, bringing the total treatment cost to approximately KRW 200 million. However, despite the high cost, the clinical efficacy of Pluvicto has been proven to surpass existing treatments. Nearly 20 patients have completed or are undergoing Pluvicto therapy within three months after launch. Considering the expense, this is a significant number of patients. Dr. Inkeun Park from the Oncology Department at Asan Medical Center in Seoul said, "From a medical professional's perspective, the introduction of Pluvicto sheds a positive light as it provides an additional effective treatment option with manageable side effects." Park added, "The challenge lies in its high cost, the limited number of institutions equipped to perform PSMA PET-CT, and the restricted availability of facilities capable of administering the treatment." Novartis, fully aware of these challenges, has set reimbursement for Pluvicto as a chief objective for next year. A representative from Novartis Korea said, "We are currently conducting internal reviews to secure reimbursement for Pluvicto." Adding, "Given its role in transforming the prostate cancer treatment paradigm, we aim to improve patient accessibility by obtaining reimbursement approval next year."
- Company
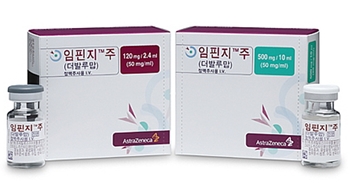
- Imfinzi passes first step to reimb in KOR… key is the price
- by Whang, byung-woo Nov 19, 2024 06:13am
- With the reimbursement standard set for the immuno-oncology drug Imfinzi (durvalumab), which is under review for reimbursement extensions, how the discussions will develop thereafter is gaining attention. Although the first step has been taken, attention is being paid to the future process as the matter was mentioned during the National Assembly’s Health Insurance Review and Assessment Service National Audit. The drug price is the key issue. Consensus on the cost is expected to be crucial amid the rising health insurance expenses being spent on anticancer drugs. Pic of Imfinzi According to industry sources on the 18th, the Health Insurance Review and Assessment Service recently held the 8th Cancer Disease Deliberation Committee (CDDC) meeting in 2024 to deliberate on whether to establish reimbursement standards for major anticancer drugs. As a result, Imfinzi was set reimbursement standards in combination with gemcitabine and cisplatin for the first-line treatment of patients with locally advanced or metastatic biliary tract cancer. In addition, AstraZeneca's Imjudo (tremelimumab) has also been set reimbursement standards in combination with Imfinzi in liver cancer, raising expectations for further coverage of the drug. In a response to a written inquiry during the NA Audit, HIRA had said, “As a result of the CDDC review that was held in November last year, the committee decided Imfinzi’s cost should fully be borne by patients due to its high price and high financial spending compared to clinical benefits.” However, the CDDC changed its decision and recognized Imfinzi’s clinical benefits this time around. In fact, a Korean subgroup analysis of TOPAZ-1, Imfinzi’s Phase 3 trial in biliary tract cancer, which was presented at the Korean Society of Medical Oncology (KSMO) International Conference in September, showed improved median overall survival (mOS) and 3-year OS rate in Korean patients. Korean patients treated with the Imfinzi combination showed an mOS of 16.6 months, compared to the 11.3 months found in Korean patients treated with conventional therapy, which is a 5.3-month extension in overall survival. The OS rate at 3 years also improved over twofold, being 21.0% in the Imfinzi combination arm and 8.8% in the conventional therapy arm. ▲ Key Results of the TOPAZ-1 overall patient and Korean subgroup analysis The industry also believes that AstraZeneca's improved financial proposal to the government for Imfinzi would have played a role during the CDDC review. The key question is whether it will pass the next stage, the Drug Reimbursement Evaluation Committee review. Although the company has succeeded in expanding the reimbursement standard for Imfinzi and setting the reimbursement standards for Imjudo in biliary tract cancer and liver cancer, respectively, there is also the burden of increased use for Imfinzi that would affect the drug’s price. Currently, Imfinzi costs around KRW3.34 million per bottle, and the cost of administering the drug is even higher for liver cancer patients who receive the drug in combination with Imjudo. In the end, it will be important to find a compromise between the fact that Imfinzi has become the global standard of care for biliary tract cancer in 12 years and its increasing burden on health insurance finances. In the U.S., U.K., and Japan, Imfinzi was reimbursed within a year of its approval. The industry believes the U.K.’s case would provide clues for Korea’s implementation. During reimbursement discussions, the U.K. used a quality-adjusted life years (QALYs) weight of 1.2 and a flexible ICER in recognition of the fact that Imfinzi was the first immuno-oncology drug approved for the first-line treatment of biliary tract cancer. However, as Imfinzi’s ICER does not meet the criteria for the Korean government's 'preferential treatment for innovative new drugs', it remains to be seen how the discussions will unfold. The industry analyzes that it will come down to what the pharmaceutical companies will propose in terms of financial sharing and whether the government will accept the proposal. “AstraZeneca Korea is committed to providing innovative treatment options for biliary tract cancer patients in Korea. We will continue to do our best in the coming process,” said an AstraZeneca official.
- Company
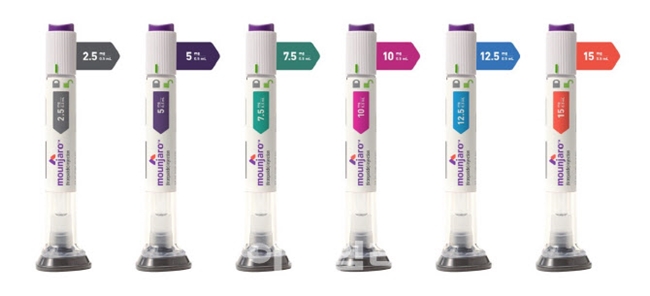
- Mounjaro aims for 'diabetes·obesity' synergy
- by Whang, byung-woo Nov 19, 2024 06:12am
- Eli Lily's Mounjaro (tirzepatide), the first once-weekly GIP·GLP-1 receptor agonist, will enter the market with its diabetes and obesity indications. Unlike already launched Wegovy (semaglutide), Mounjaro has two indications: diabetes and obesity. Experts anticipate that Mounjaro's sales strategy will be focused on diabetes treatment although a separate indication is not a chief factor for medication choice in clinical practices. Product photo of Mounjaro. Mounjaro is a single molecule that activates both GIP and GLP-1 receptors, which are primary incretin hormones that stimulate insulin secretion in the body. Mounjaro received the Ministry of Food and Drug Safety (MFDS) approval for the treatment of type 2 diabetes in June 2023. In August, it added an indication to treat chronic weight management, resulting in two indications. Mounjaro generated significant sales in the global market, so it has high expectations for the Korean market. Mounjaro's sales in Q3 amounted to US$3.11 billion (KRW 4.29 trillion), an increase from US$1.41 billion (KRW 1.9683 trillion) last year. Zepbound recorded US$1.26 billion (KRW 1.73 trillion). In South Korea, 73.6% of patients with diabetes are overweight and obesity patients. Therefore, the analysis suggests that Mounjaro has a high potential. Hyuk-Sang Kwon, Korean Diabetes Association (Endocrinology at The Catholic University of Korea Yeouido St. Mary's Hospital), said that "Type 2 diabetes patients with obesity must be treated with a goal of improving glycated hemoglobin and weight loss." Kwon explained, "Still, many type 2 diabetes in South Korea have difficulty managing glycated hemoglobin (HbA1c) and body mass index (BMI). We need a proactive treatment to improve treatment prognosis." SungHee Choi, Korean Diabetes Association (Endocrinology at Seoul National University Bundang Hospital), said, "Mounjaro is administered as an adjuvant treatment to dietary therapy and physical therapy to improve regulation of blood sugar in adult patients with type 2 diabetes." Choi said, "Wegovy with semaglutide was recently launched. It is said that Mounjaro is positioned as the treatment for obesity rather than diabetes, but in my opinion, it must be focused as a diabetes treatment." Lily Korea says that the company is preparing for reimbursement before announcing the launch date. "We are closely discussing with the Ministry of Health and Welfare (MOHW) for reimbursement, and we have invested over US$20 billion in the past four years to expand production and distribution capacities," Lily's representative said. "In South Korea, we are putting our best efforts into supplying Mounjaro stably." Clinical practices point out high costs as a barrier. The company is expected to determine a launch date after weighing the options for reimbursement strategy and the possibility of a non-reimbursement. As the domestic distributor candidates for Mounjaro are being discussed, the industry anticipates the launch date of Mounjaro in the first half of 2025. It is said that Mounjaro will enter the market before Wegovy launches and has a market-dominating effect. Meanwhile, the latest Mounjaro's SURMOUNT-1 study results will positiviely affect obtaining reimbursement. Sub-group analysis of adults who participated in the randomized Phase 3 SURMOUNT-1 trial showed that the risk of diabetes progression was reduced by 94% after patients were treated with Mounjaro for about 3 years. The average rate of weight loss during the treatment was 15.4-22.9% for the Mounjaro group, and the average weight was reduced by 34.6~54.2lb from about 236.8lb. Mounjaro's weight loss effects observed in the early treatment stage were maintained throughout the study period.
- Company
- Academia pushes for Tagrisso’s reimbursement expansion
- by Moon, sung-ho Nov 19, 2024 06:12am
- Less than a year after AstraZeneca's lung cancer drug Tagrisso (osimertinib) was granted expanded reimbursement coverage, another round of discussions on its further expansion has begun, drawing attention to its background. As the expansion request came from the medical community, not pharmaceutical companies, it will be interesting to see if the experts' request will be granted. #On the 18th, according to the pharmaceutical industry and medical community, the Health Insurance Review and Assessment Service recently held the 8th Cancer Disease Deliberation Committee meeting in 2024 to deliberate on whether to set a reimbursement standard for Tagrisso. The committee discussed the need to set a reimbursement standard for ‘Tagrisso in combination with pemetrexed and platinum-based chemotherapy’ as a first-line treatment for patients with locally advanced or metastatic non-squamous non-small-cell lung cancer with EGFR exon 19 deletion or exon 21 (L858R) substitution mutation. In other words, less than a year after the drug’s reimbursement was expanded to its use as a monotherapy, the authorities are reviewing extending its reimbursement as a combination therapy as a first-line treatment for NSCLC. The review is based on the results of the FLAURA2 study, which compared the efficacy of the Tagrisso+chemotherapy combination therapy with Tagrisso monotherapy. Study results showed that the Tagrisso+chemotherapy combination reduced the risk of disease progression or death by 38% compared to Tagrisso alone. Median investigator-assessed PFS was 25.5 months, an 8.8-month extension compared to 16.7 months with Tagrisso monotherapy. Median PFS by blinded independent central review (BICR) was 29.4 months, compared with 19.9 months in the Tagrisso monotherapy arm. This led to a reimbursement discussion, but the outcome was a 'reimbursement standard not established’ decision However, it is worth noting the background of why CDDC decided to discuss Tagrisso's reimbursement extension. Usually, a “pharmaceutical company” applies to expand the reimbursement of anticancer drugs, but this time, the Korean Lung Cancer Society applied for the reimbursement expansion upon which the cancer review was held. The Korean Association For Lung Cancer applied to expand the reimbursement of the Tagrisso+chemotherapy combination, and AstraZeneca supported it. This means that the need to use the Tagrisso+chemotherapy combination has been recognized in clinical practice. “Pemetrexed plus cisplatin or carboplatin, which is used as combination therapy, is relatively well tolerated by patients,” said Professor Sang-We Kim (Medical Oncology) from Seoul Asan Medical Center. ”It can be relatively difficult because you have to receive chemotherapy for four cycles, but from the fifth cycle, you receive only pemetrexed. Given the positive outcomes, including PFS in the FLAURA2 study, the combination may be a viable first-line treatment option compared to Tagrisso monotherapy.” As such, AstraZeneca is expected to further review the data and decide whether to reapply for reimbursement expansions. However, there is a possibility that AstraZeneca may not pursue the reimbursement expansions, given that any additional reimbursement would require further price cuts for Tagrisso. A HIRA official said, “The regulations allow medical societies to apply for reimbursement of drugs. This is what happened to Tagrisso. There have been many such cases before. However, even if a medical society applies for a reimbursement expansion, it may not be possible to discuss it without the cooperation of the pharmaceutical company because the drug’s cost-effectiveness must be verified.” “For new drugs that are applied the risk-sharing agreement, it is more often the pharmaceutical company that applies for reimbursement coverage, as they have to prove the drug’s cost-effectiveness. However, it has always been possible for academic societies to apply for reimbursement expansions, like in this case.”
- Company
- Premium meningitis vaccine Bexsero lands in hospitals in KOR
- by Eo, Yun-Ho Nov 18, 2024 05:49am
- The next-generation meningococcal vaccine, ‘Bexsero’ can now be prescribed in general hospitals in Korea. According to industry sources, GSK Korea’s Bexsero, the first meningococcal B vaccine introduced to Korea, has passed the drug committees (DCs) of the Big 5 tertiary hospitals, including Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary's Hospital, Seoul Asan Medical Center, and Sinchon Severance Hospital. Kyungpook National University Hospital, Korea University Anam Hospital, Korea University Ansan Hospital, Inje University Paik Hospital, Seoul National University Bundang Hospital, Seogwipo Medical Center, Ajou University Hospital, and Pusan National University Yangsan Hospital. Meningococcal infections can cause invasive meningococcal infections, meningitis, and sepsis. Invasive meningococcal infection progresses rapidly and can cause death within 24 to 48 hours of the onset of symptoms. Even with treatment, the disease is deadly, with a fatality rate of 8-15%. Typical serogroups of meningococci that cause invasive meningococcal infections in humans include A, B, C, W, X, and Y. The most predominant meningococcal serogroup in Korea, the United States, and Europe are serogroup B, with high levels found in infancy and adolescence. From 2010 to 2016, the proportion of meningococcal B serogroup cases identified in Korea was 28%, but from 2017 to 2020, the rate increased significantly to 78%. Meningococcal B's capsular polysaccharide is structurally similar to human tissue, which has made vaccine development challenging due to the risk of autoimmune damage. GSK developed Bexsero by applying novel technologies that employ genome sequencing. Since its initial European approval in 2013, GSK has accumulated over a decade of experience in preventing meningococcal B infections, conducting 17 studies on subjects aged 2 months to adults. Professor Hyunmi Kang, Department of Pediatrics at St. Mary's Hospital in Seoul, said, "The prevalence of meningococcal serogroups varies across countries and time periods, so it is not easy to predict. In Korea, serogroup B meningococcal infection cases have increased in recent years, increasing the need for its prevention.”
- Company
- 'Tevimbra' can be prescribed at general hospitals
- by Eo, Yun-Ho Nov 18, 2024 05:49am
- 'Tevimbra,' a type of immune checkpoint inhibitor, is becoming more widely available for prescription at general hospitals. According to industry sources, BeiGene's PD-1 inhibitor Tevimbra (tislelizumab), which has applied for reimbursement for its esophageal cancer indication, has passed the drug committees (DC) of tertiary general hospitals, including Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary's Hospital, and Asan Medical Center. Tevimbra is a PD-1 inhibitory immune checkpoint inhibitor that demonstrated clinical usefulness as the second-line therapy for esophageal squamous cell carcinoma. It was approved in South Korea in November 2023. The drug passed the Cancer Disease Review Committee (CDRC) of the Health Insurance Review and Assessment Service (HIRA) in August in its second attempt and is awaiting consideration for the Drug Reimbursement Evaluation Committee (DREC) review. Since there haven't been any immune checkpoint inhibitors with reimbursement for esophageal cancer, it remains to be watched whether Tevimbra will become available as the new treatment option. Currently, seven immune checkpoint inhibitors have been approved or authorized for marketing in South Korea, including ▲Keytruda ▲Opdivo ▲Tecentriq ▲Imfinzi ▲Bavencio ▲Jemperli ▲Tevimbra. The number of indications for these drugs totals 64. However, only 21 therapies are reimbursement-listed (about 33%). None of these drugs are included on the reimbursement list for the treatment of esgophageal cancer. In South Korea, platinum-based anticancer chemotherapy is approved for reimbursement as both first-line therapy and second-line therapy or higher for esophageal squamous cell carcinoma. The low reimbursement rate for immune checkpoint inhibitors in each indication, such as esophageal cancer, is due to drug price and finance. It has been known that after drugs were reimbursed for treating several cancer types, such as lung cancer, the overall claim amount for immune checkpoint inhibitors and the percentage of anticancer agents under the National Health Insurance significantly increased, resulting in a financial burden. Based on the 2023 report, the claim amount of anticancer agents was KRW 2.4 trillion. The claim amount for immune checkpoint inhibitors was about KRW 500 billion, accounting for 20% of the anticancer agents' claims. Patients have high hopes for BeiGene's Tevimbra because the company announced the drug supply at a relatively lower price. BeiGene has already demonstrated its 'Fair pricing of innovative new drugs' philosophy to eliminate potentially excluded patients through the reimbursement procedure for the blood cancer treatment, 'Brukinsa (zanubrutinib).' Meanwhile, the global Phase 3 clinical RATIONALE-302 study showed Tevimbra prolonged the median overall survival (OS) by 2.3 months compared to chemotherapy (8.6 months vs. 6.3 months), statistically reducing the death risk by 30%. The response rate was twofold higher in Tevimbra compared to chemotherapy (20% vs. 10%), and Tevimbra extended the median duration of response (DOR) by approximately three months, from 4.0 months to 7.1 months. As a result, the National Comprehensive Cancer Network (NCCN) Guidelines were revised, detailing Category 1 recommendation for Tevimbra as a favorable option for second-line therapy for esophageal squamous cell carcinoma.
- Company
- Demand rises for reform of the cancer drug reimb system
- by Moon, sung-ho Nov 18, 2024 05:49am
- With the presence of new anticancer drugs increasing in the clinical field, the pharmaceutical industry has been demanding the authorities apply a new reimbursement model in Korea. In addition to the existing immuno-oncology drugs, the emergence of antibody-drug conjugates (ADCs), which have proven effective in various cancers, has led to calls for different drug prices for each 'indication.’ # These calls have been mainly coming from multinational pharmaceutical companies that own new anticancer drugs, but they have been met with a lukewarm response in the field. The reason is that the system they requested considers the profitability of companies rather than the patients. According to industry sources on the 9th, as immuno-oncology drugs or ADCs with indications for various cancer types have recently been introduced to the field, the opinion has been raised on how different drug prices should be applied to the same drug by indication. The system, indication-based pricing (IBP), is a further subdivision of value-based pricing (VBP), which states that drug prices should reflect the actual value of drugs. Currently, the single-price policy used by Korea’s health insurance system bases a drug’s price on the initial indication. For each additional indication that is covered, the existing drug’s price must be reduced to reflect the expanded scope of coverage. For example, if an immuno-oncology drug called A is initially approved for lung cancer, and then expands its indication to gastric and breast cancer, the existing price must be reduced through negotiation to reflect the increased use in practice. The problem is that an increasing number of drugs have indications for multiple cancers, including major immuno-oncology drugs and ADCs, and as demand for their reimbursement is rising, the current single-price system cannot accommodate all the indications. One such treatment is MSD Korea's immuno-oncology drug Keytruda (pembrolizumab). As of August, Keytruda was approved in Korea for 33 indications in 17 different cancers. Since last year company has been pushing to expand reimbursement for Keytruda. It has applied for reimbursement of a total of 17 indications in Korea but has not been able to cross the Cancer Disease Deliberation Committee’s threshold. After applying for reimbursement for 13 of its indications last year, the company has added 4 more indications this year: ▲MSI-H gastric cancer, ▲MSI-H biliary tract cancer, ▲HER2-positive gastric cancer, and ▲HER2-negative gastric cancer. MSD Korea recently submitted an additional financial-sharing proposal that included a gastric cancer indication and is reportedly making every effort to cross the CDDC threshold. The multinational pharmaceutical industry is of the opinion that the government should consider treatments with multiple indications, including immuno-oncology drugs such as Ono Pharmaceutical’s Opdivo (nivolumab), Roche’s Tecentriq (atezolizumab), AstraZeneca’s Imfinzi (durvalumab), and ADCs represented by Enhertu (trastuzumab deruxtecan), and the autoimmune disease treatment, Sanofi’s Dupixent (dupilumab). “In the current system, we have to apply for the reimbursement of each indication separately, which means that we have to continuously conduct negotiations with the government,” said one multinational pharmaceutical industry official. ”I think the need for indication-specific pricing, which is being adopted in some countries overseas, is a reference to the need that we need to find a more efficient way to work the system.” In fact, major countries such as the United Kingdom, France, Germany, Italy, Japan, Switzerland, and the United States have adopted indication-specific pricing, said the official. “The use of a single drug for multiple indications will be increasing not only in cancer but also in other diseases,” said the official. “We need a more comprehensive approach.” Then how do clinicians who actually use the drugs on-site feel about the need? First of all, the pharmaceutical industry’s dominant opinion is that IBP is necessary for 'multinational pharmaceutical companies,' but believes in the need for a cautious approach in implementing the system. This means that while the need is acknowledged, patient consent should be prioritized. For now, the demand is more focused on the profit logic of pharmaceutical companies than an improvement on the patient’s part. “With the rising number of anticancer drugs that hold multiple indications, I do agree on the need to calculate drug prices by indication,” said Dr. Shin-kyo Yoon, Professor of Oncology at Asan Medical Center, ”but the opinions of patients are more important than ours. It is not easy because the entire system would need to be reorganized.” Another professor of oncology at a university hospital said, “It's not an easy problem, as the price cuts in the current system are entirely borne by the drug companies. The IBP seems to have come about because they have to apply for reimbursement while avoiding drug price reductions and receive approval for the additional indications. In the end, it's a problem that the government should need to address.” As such, introducing IBP has been mentioned during the National Assembly's audit, which ended last month. However, the Ministry of Health and Welfare and the Health Insurance Review and Assessment Service did not specifically mention the need for such a system, saying that the necessity of the system should be reviewed first. At the same time, the authorities stated that it is time to think about how too much of Korea’s health insurance finances are being spent on reimbursing anticancer drugs. The MOHW said, “We will review the necessity of introducing a Korean-style IBP system that reflects the value of each indication by comprehensively considering various factors such as expanding access to new drugs and the impact on health insurance finances. However, we would need to conduct literature reviews, explore domestic and foreign cases, evaluate its operability within the current drug pricing system and the national health insurance system, and calculate the benefits of introducing the system comprehensively.” It added, “The post-settlement method, in which the actual price is set differently for each indication and then settled between the payer and pharmaceutical companies, requires sufficient review and public discussion on the method of calculating drug prices by indication and the reimbursement method between the payer and pharmaceutical companies.” “In the case of anticancer drugs, many good drugs are being released, but at a higher than we expected,” said Kook-hee Kim, Director of the Pharmaceutical Management Division at HIRA. ”Recently, we have been managing finances by adjusting the reimbursement standards for artificial tears and choline alfoscerate drugs, but there is a concern on whether such saved finances should be solely invested in anticancer drugs.”