- LOGIN
- MemberShip
- 2025-12-20 21:13:17
- Company
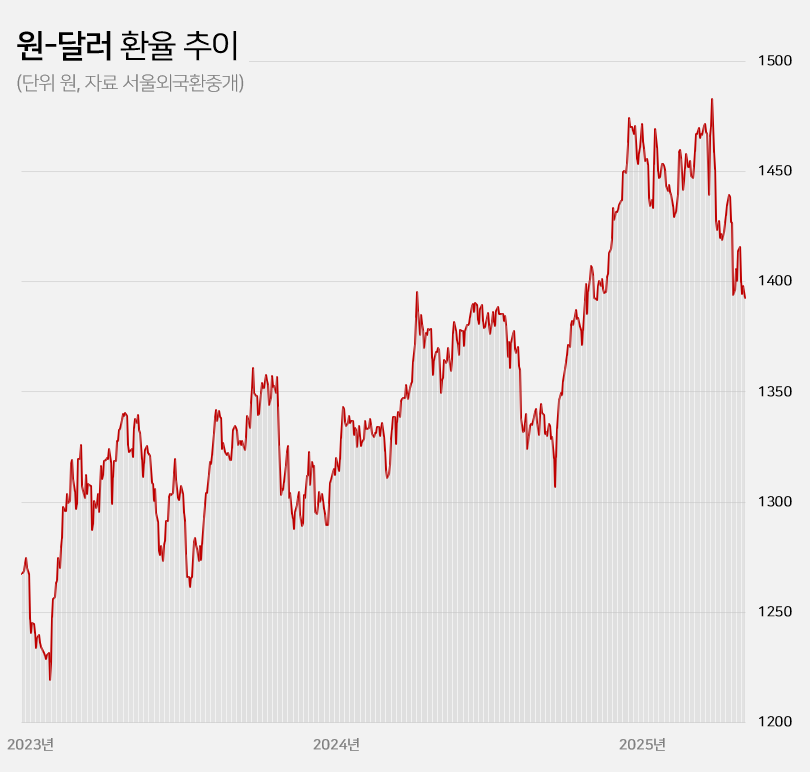
- Won-dollar rate lowest in 6mths... industry mixed
- by Kim, Jin-Gu May 23, 2025 05:51am
- With the won-dollar exchange rate falling below KRW 1,400, pharmaceutical and biotech companies are experiencing a mix of anticipation and concern. If the prolonged high exchange rate returns to previous levels, API imports and overseas clinical trial costs are expected to decrease, leading to an improvement in the cost structure. On the other hand, some predict that the asset value of pharmaceutical and biotech companies with a high proportion of drug exports will decline. Won-dollar exchange rate falls below KRW 1,400... Will the burden of API purchases and overseas clinical trials decrease? According to industry sources on the 22nd, the won-dollar exchange rate closed at KRW 1,392.60 on the 21st, down KRW 5.40 from the previous trading day. This is a 6.1% (KRW 90.30) decrease from the KRW 1,482.90 on the 10th of last month when concerns over the U.S.’s mutual tariffs were at their peak. The won-dollar exchange rate has remained high for a long time since entering the KRW 1,300 range in March 2023. It rose further to over KRW 1,350 from the middle of last year and broke through KRW 1,400 after the martial law crisis at the end of last year. With mutual tariffs by the US adding to the concern, the exchange rate soared to exceed KRW 1,480 at the beginning of last month. However, the won-dollar exchange rate has been falling this month amid expectations that trade tensions between the US and China will ease. In particular, the exchange rate has remained in the KRW 1,300 range for 4 consecutive trading days since the 16th, raising expectations that the prolonged high exchange rate trend will return to normal levels. KRW to USD exchange rate The pharmaceutical industry is hopeful that the decline in the won-dollar exchange rate will continue, leading to an improvement in the cost structure. Over the past 2 years, the rise in the won-dollar exchange rate has had a negative impact on the cost structure of domestic pharmaceutical and biotechnology companies. Due to their high dependence on imported APIs, the exchange rate increase directly led to higher manufacturing costs. Additionally, since these companies purchase APIs from China and India, with a high import dependency, they were significantly affected by the rise in the won-dollar exchange rate. As of 2023, the self-sufficiency rate for domestically produced APIs for pharmaceuticals stands at 25.4%. Among these, imports of APIs from China account for 30.5% of the total, while those from India make up 15.2%. Together, these two countries account for nearly 50% of the total API imports. Although manufacturing costs rose due to the increase in the won-dollar exchange rate, unlike other consumer goods, the price of finished drugs cannot be arbitrarily set by companies. As a result, the deterioration of the cost structure in the pharmaceutical industry has become more pronounced over the past 2 years. Additionally, the burden of clinical trial costs conducted overseas has steadily increased during the prolonged high exchange rate environment. Most clinical trials targeting the US and European markets are conducted locally. When the won-dollar exchange rate rises, global clinical trial costs also increase accordingly. In this situation, there are expectations that a decline in the won-dollar exchange rate will reduce the cost of importing APIs and the burden of global clinical trials, ultimately improving cost burdens. In the long term, improvements in the cost structure are expected to contribute positively to performance recovery. Asset value decline is inevitable for companies with high export ratios... Samsung Biologics to lose KRW 91.6 billion if exchange rate falls by 10% However, companies with high export ratios, such as Samsung Biologics and Celltrion, are expected to see their asset values decline due to the decline in the won-dollar exchange rate. These companies earn a large amount of assets in dollars overseas, so when the exchange rate rises, their asset values increase. Conversely, when the exchange rate falls, their asset values decline. Samsung Biologics had an overseas sales ratio of 96.5% in the first quarter. Of the KRW 1.2983 trillion in sales rendered in the first quarter, KRW 1.2528 trillion came from overseas. As the proportion of overseas sales is high, its performance is expected to be greatly affected by fluctuations in the exchange rate. Samsung Biologics explained in its quarterly report that a 10% increase or decrease in the won-dollar exchange rate would result in a KRW 91.6 billion increase or decrease in pre-tax income. Considering how the current won-dollar exchange rate has fallen by 1.5% compared to the average exchange rate (KRW 1,452.66) at the time of the quarterly report, the recent decline in the exchange rate is estimated to have reduced asset value by approximately KRW 14 billion. Samsung Biologics has seen an increase in cash and cash equivalents over the past 2 years due to the impact of high exchange rates. The figures were KRW 12.2 billion in 2023 and KRW 41.6 billion last year. This was due to the average won-dollar exchange rate rising from KRW 1,291.95 in 2022 to KRW 1,307.90 in 2023 and KRW 1,363.09 in 2024. The same goes for Celltrion and SK Biopharm. Given their high proportion of overseas sales, they are relatively more affected by fluctuations in the exchange rate. Celltrion saw its cash and cash equivalents increase by KRW 52.4 billion last year due to exchange rate changes. The company explains that, assuming all other variables remain constant, an 8% change in the won-dollar exchange rate would result in a KRW 33.8 billion increase or decrease in pre-tax profit. For SK Biopharm, the change in cash and cash equivalents due to exchange rate fluctuations last year amounted to KRW 3.6 billion. Based on the exchange rate at the end of last year, a 10% change in the exchange rate would result in an increase or decrease of KRW 14.1 billion in the company's pre-tax profit. It is expected that the fluctuation in pre-tax profit due to exchange rate changes will widen further this year if sales of Xcopri increase in the United States.
- Company
- Will Tibsovo be reimbursed for cholangiocarcinoma this time?
- by Eo, Yun-Ho May 22, 2025 06:10am
- Attention is focused on whether the targeted anticancer drug “Tibsovo” for cholangiocarcinoma and acute myeloid leukemia will be successful in its attempt to be reimbursed by health insurance in Korea. Servier Korea’s IDH1 (isocitrate dehydrogenase 1) genetic mutation targeting therapy recently passed the Health Insurance Review and Assessment Service's Cancer Disease Review Committee. The drug is indicated for use in IDH1-mutation-positive patients in combination with azacitidine for newly-diagnosed acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation in adults aged ≥75 years, or who have comorbidities that preclude use of intensive induction chemotherapy, or as monotherapy for locally advanced or metastatic cholangiocarcinoma in previously treated adults with IDH1 mutation. The dual AML indication passed the Health Insurance Review and Assessment Service's Cancer Disease Review Committee’s review in October last year, but the cholangiocarcinoma indication had not been approved until now. It remains to be seen whether Tibsovo will be submitted to the Drug Reimbursement Evaluation Committee and complete the reimbursement process in the field of cholangiocarcinoma, where treatment options are limited. Cholangiocarcinoma is a highly aggressive cancer with a poor prognosis, with a 5-year relative survival rate of just 28.9%. In particular, 65% of patients with intrahepatic cholangiocarcinoma are diagnosed at an advanced stage where surgery is not feasible. Tibsovo is the only targeted therapy recommended by the National Comprehensive Cancer Network (NCCN) in the highest category (Category 1) as a second-line treatment for bile duct cancer. According to the Phase III ClarlDHy trial, Tibsovo reduced the risk of disease progression by 63% compared to placebo, with a median progression-free survival (PFS) of 2.7 months (1.4 months in the placebo group). Additionally, the median overall survival (mOS) was 10.3 months in the Tibsovo group, more than twice as long as the 5.1 months in the placebo group. Do-Youn Oh, Professor of Hematology-Oncology at Seoul National University Hospital, stated, “The development of drugs for cholangiocarcinoma has accelerated significantly in recent years. As new drugs are being developed, many companies are also actively working on the development of treatments for cholangiocarcinoma. Patients with cholangiocarcinoma must follow the doctors’ guidance and receive appropriate treatment rather than feeling discouraged, so they can participate in clinical trials and access new treatment opportunities.” Meanwhile, in the Phase III AGILE study that enrolled patients with acute myeloid leukemia, Tibsovo demonstrated improved event-free survival (EFS) when used in combination with azacitidine, and also significantly improved overall survival (OS). The median OS in the Tibsovo-treated group was 24.0 months (7.9 months in the placebo group), and long-term follow-up results showed that the median OS with Tibsovo combination therapy was 29.3 months, over 3.7 times longer than with placebo combination therapy. Kyu-Pyo Kim, Professor of Oncology at Asan Medical Center, said, “Tibsovo is indicated for IDH1 mutation-positive cholangiocarcinoma and acute myeloid leukemia, and has demonstrated excellent efficacy and safety in clinical studies. Considering that both studies allowed crossovers, the significant improvement in survival is a very meaningful result.” He added, “Given the limited treatment options for cholangiocarcinoma and acute myeloid leukemia, the emergence of a new targeted therapy with promising efficacy is expected to contribute to improving treatment outcomes and quality of life for patients with these diseases in Korea.”
- Company
- SK Bioscience wins the '8-year Prevenar patent dispute'
- by Kim, Jin-Gu May 22, 2025 06:09am
- In a patent infringement trial against Pfizer involving SK Bioscience's export of 'SKYPneumo' raw solution, SK Bioscience has won after eight years. The latest ruling is expected to enable SK Bioscience's global export of its pneumococcal conjugate vaccine 'SKYPneumo' raw solution. Ruling that 'Exporting SK Bioscience's SKYPneumo raw solution does not infringe on the patent'…an opportunity has been presented to export According to SK Bioscience, on May 21, the Supreme Court of Korea ruled in favor of SK Bioscience in a legal dispute filed by Pfizer for 'Cessation of infringement of Pfizer's Prevenar 13 (PCV13) pneumococcal conjugate vaccine patent.' The Supreme Court recently upheld the second trial ruling, which ruled in favor of SK Bioscience. SK Bioscience and Pfizer long fought over whether the overseas supply of SKYPneumo's raw solution infringes on Pfizer's 'Prevenar 13.' This eight-year legal battle began in 2017 when Pfizer filed a patent infringement lawsuit. The Supreme Court ruled that "Producing and supplying PCV13 finished products for research purposes does not infringe on the patent," adding, "Individual conjugates that make up PCV13 do not fall within the scope of the patent claim." It is analyzed that the Supreme Court's final ruling favoring SK Bioscience is expected to open the door for SK Bioscience to export SKYPneumo raw solution overseas. SK Bioscience says, "While supplying PCV13 finished products is not permitted, the latest ruling presents an opportunity for supplying individual conjugates overseas," and added, "We plan to initiate a new project to supply raw solution of individual conguates to regions with high vaccine demand, such as Southeast Asia and Central and South America. We also intend to pursue technology transfer through local partnerships." SK Bioscience vs. Pfizer: Patent Invalidation·Infringement Lawsuits…3 out of 4 cases transferred to the Supreme Court In addition to this case, the dispute between SK Bioscience and Pfizer surrounding the Prevenar 13 patent has been ongoing for a long time, including 'patent invalidation' lawsuits filed by SK Bioscience and 'cessation of patent infringement' lawsuits counter-filed by Pfizer. First, SK Bioscience challenged two related patents after developing SKYPneumo, a follow-up product to Prevenar 13. SK Bioscience challenged two of Pfizer's Prevenar 13-related patents: ▲Expiring in March 2026, 'a novel formulation that stabilizes immunogenic compositions and prevents precipitation' patent ▲Expiring in April 2027, 'a multivalent pneumococcal polysaccharide-protein conjugate composition' patent. SK Bioscience filed invalidation trials for both patents. Among these, the patent expiring in 2026 was successfully invalidated. The Intellectual Property Trial and Appeal Board (IPTAB) ruled in favor of SK Bioscience in January 2020, and the decision was finalized without an appeal from Pfizer. On the other hand, the patent expiring in 2027 ultimately failed to be invalidated. SK Bioscience argued for the invalidation of this patent, but received a dismissal from IPTAB in June 2015. SK Bioscience also lost in the second trial, which proceeded with SK Bioscience's appeal, in November 2017. SK Bioscience decided to appeal to the Supreme Court. However, they again received a losing ruling from the Supreme Court in 2018. In conclusion, the patent expiring in March 2026 was successfully invalidated, while the patent expiring in April 2027 was not. Consequently, domestic sales of SKYPneumo are prohibited until April 2027. Pfizer counter-filed a patent infringement prohibition lawsuit against SK Bioscience during this process. In 2017, Wyeth LLC, the patent holder of Prevenar 13, and Pfizer Korea, the domestic distributor, filed a lawsuit claiming that SKYPneumo infringed on their patent rights. This lawsuit also went to the third trial. Ultimately, the Supreme Court issued a recommendation for settlement in 2019, essentially siding with Pfizer. The Supreme Court ruled that Pfizer's patent was valid and prohibited the production and launch of SK Bioscience's SKYPneumo until 2027. SK Bioscience voluntarily withdrew the product approval for SKYPneumo. However, they re-obtained approval for SKYPneumo in June 2021. The conflict between the two companies continued thereafter. Facing difficulties with the domestic launch, SK Bioscience signed a license agreement to transfer related technology to a Russian pharmaceutical company to develop vaccines in that region. SK Bioscience supplied raw solution for its self-developed pneumococcal vaccine and finished pharmaceutical products for research. Pfizer and Wyeth LLC also interfered with this, filing a cession of patent infringement lawsuit in 2020. SK Bioscience countered, arguing that supplying raw solution for research and testing purposes, not finished products, to overseas markets was outside the scope of patent infringement. In August 2023, Pfizer won in the first trial. SK Bioscience appealed, and ultimately, the Patent Court overturned the first trial ruling and sided with SK Bioscience. This time, Pfizer appealed to the Supreme Court after losing in the second trial. Finally, the Supreme Court upheld the original ruling in the appeal. The two companies engaged in a long-term battle, going to the Supreme Court for three out of four cases, including patent invalidation lawsuits and patent infringement lawsuits. With this, most disputes between the two companies concerning the Prevenar 13 patent have been concluded. However, a trade commission dispute remains. The Korea Trade Commission under the Ministry of Trade, Industry and Energy is investigating SK Bioscience's unfair trade practices. This is because Pfizer and Wyeth LLC have filed a complaint requesting a cessation on exporting SK Bioscience's pneumococcal raw solution. In the pharmaceutical industry, there is an outlook that the trade commission dispute surrounding the export of pneumococcal raw solution will also be concluded soon due to this Supreme Court ruling.
- Company
- KPBMA proposes policies ahead of the presidential election
- by Kim, Jin-Gu May 22, 2025 06:08am
- The Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA) proposes the "Pharmaceutical and Biotech Policies for Korea Ahead of South Korea's 21st presidential election, the Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA) has proposed "The Ten Pillars of Pharmaceutical and Biotechnology Policies." KPBMA's proposal can be summarized in three keywords: supporting R&D, establishing an AI-based new drug development platform, and improving the drug pricing system. The KPBMA proposed initiatives, such as establishing performance-based R&D policies, creating a compensation system for domestically developed novel drugs, and supporting domestically-produced active pharmaceutical ingredients (APIs). The KPBMA also asked the government to develop an AI-driven drug discovery big data platform and cultivate specialized professionals in this field. Furthermore, they asked to establish a reinvestment cycle where drug price reduction exemptions based on R&D investment ratios are reinvested. They also urged the creation of a predictable drug pricing roadmap according to the balanced post-market management policies. In its policy report published on May 19, the KPBMA proposed its "Pharmaceutical and Biotech Policies for Korea's Healthy Future for the 21st Presidential Election." ◆Performance-Based R&D Policy Establishment = According to the KPBMA, the Korean government's 2023 R&D budget for the pharmaceutical and biotech sector was KRW 2.5826 trillion. However, only KRW 347.7 billion, or 13.5%, was allocated to direct corporate support. This differs significantly from the IT sector, where the proportion of corporate support reached 44.5%. In response, the KPBMA called for the establishment of a strategic R&D investment system. They stressed the need for institutional improvements, including expanding the 'Korean ARPA-H' project (a reference to the U.S. Advanced Research Projects Agency for Health) and applying exemption from preliminary feasibility studies. Furthermore, they urged for the continuous expansion of mega-funds to foster the creation of blockbuster novel drugs and to broaden tax credits for pharmaceutical and biotech companies' investments in bio-ventures. The KPBMA also stressed a shift in R&D budget policy towards an 'performance-based' approach. They emphasized the need to increase the corporate support ratio of government R&D budgets to over 30%. Additionally, they stressed the importance of strengthening support for Phase 2 and 3 clinical trials and global market entry to enhance the success rates of new drug development and commercialization. ◆Domestically Produced Vaccines·Treatments for Infectious Disease = The KPBMA asked for expanded support for research and development into next-generation vaccine platforms, such as mRNA and synthetic antigen technologies. They stressed the urgent need for swift countermeasures to the infectious disease crisis, citing initiatives like a '100-Day Vaccine Development Project' or 'Large-scale Rapid Global Clinical Projects.' Furthermore, the KPBMA proposed introducing a 'loss compensation system' and establishing pre-purchase schemes and long-term procurement contract systems to accelerate the development of national essential vaccines and treatments. For instance, countries like the United States and Japan actively utilized pre-purchase systems during the COVID-19 pandemic to shorten development timelines. ◆Establishing a Compensation System for Domestically Developed Novel Drugs = The KPBMA urged an improved drug pricing compensation system for domestically developed innovative new drugs. Currently, preferential drug pricing is only applied to novel drugs produced by innovative pharmaceutical companies or those that conduct domestic clinical trials. The KPBMA argued this should be expanded to pharmaceutical and biotech companies that contribute to the supply of essential medicines or job creation. The KPBMA also called for expanding the scope of the refund system, also known as a dual pricing system, to enhance global competitiveness. They anticipate that the refund system could be applied when the market launch plans for new drugs developed overseas or those subject to technology export are identified. ◆Supporting domestically produced APIs = According to the KPBMA, the number of domestic API manufacturers in Korea decreased significantly over the last decade, from 381 in 2013 to 296 in 2023. During the same period, API items sharply declined from 10,341 to 6,244. This trend is attributed to the lower price competitiveness of domestically produced raw materials, leading to an increased reliance on APIs from China·India. The KPBMA also pointed out the severe lack of policy support, such as preferential reimbursement drug pricing and tax incentives, as a reason for the lack of revitalization in API development. While the government introduced a system for preferential reimbursement pricing (68%, 5 years + 5 years) for using domestically produced raw materials, its application is limited to newly listed national essential medicines. Concerns remain regarding potential drug price reductions due to post-market management. In response, the KPBMA urged the establishment of a control tower to build a stable drug supply system and called for real-time investigation, analysis, and monitoring of drug supply and demand. In the mid-to-long term, they recommended developing a supply stability plan and establishing a government preferential procurement system for domestically produced essential medicines. Furthermore, they proposed expanding tax benefits for APIs under Korea's 'Act on Restriction on Special Cases Concerning Taxation.' They also emphasized the need for incentives, such as preferential drug pricing or manufacturing facility support, when diversifying supply sources or achieving self-sufficiency. ◆Accelerating AI-Driven Drug Discovery Initiatives = Korea's AI-driven drug discovery technology is estimated to be at 74% of the level of the United States, indicating a technology gap of approximately five years. While over 50 domestic AI drug discovery specialized companies demonstrate capabilities, a diagnosis suggests limitations in competing with global companies due to insufficient infrastructure, including data, specialized personnel, and computing resources. Accordingly, the KPBMA called for expanding federated learning and establishing a collaborative AI drug discovery acceleration project (AIDA). They proposed expanding the concept of the 'K-MELLODDY Project' to foster a nationwide data-driven open innovation ecosystem. ◆Developing a New Drug Discovery Big Data Platform = The KPBMA recommended establishing a government-funded 'New Drug Discovery Big Data Platform.' This platform would collect 'bio R&D public data' tailored to the specific needs of each drug discovery area and support industry access. Additionally, they called for developing an 'AI-based intelligent autonomous laboratory optimization model' to generate and accumulate high-quality experimental data for new drug development purposes. ◆Training AI-based Biotech Specialized Talent = Currently, the government is running an 'AI-based New Drug Development Education and Promotion Project.' The KPBMA proposed expanding this initiative to develop an 'AI Drug Discovery Industry-Academia Cooperative Convergence Talent Training Program,' where industry and universities jointly produce talent. They anticipate that establishing this program would significantly alleviate the shortage of AI talent in Korea. ◆Operating Industry-Led Advanced Industry Academies = The KPBMA proposed designating and operating an industry-led 'Pharmaceutical and Bio-Pharma Advanced Industry Academies.' This initiative would involve leading industry organizations coordinating government, industry, and academia to support practical education and employment linkages. Through this, they emphasized the importance of building a sustainable talent supply system and nurturing talent to prepare for future new technology demands. ◆Reinvestment of Drug Price Reduction Exemptions Based on R&D Ratios = The KPBMA pointed out that existing post-market drug price management systems, such as the drug price-volume agreement (PVA) program and actual transaction price-based drug price reductions, operate inefficiently. They highlighted that drug price reduction risks frequently arise, leading to adverse effects such as a decrease in companies' R&D investment capacity. Therefore, they called for strengthening compensation, including expanding exemptions based on R&D investment ratios during drug price reductions. They argued for establishing a virtuous cycle where the reduced drug prices are reinvested into R&D, leading to new drug creation. Specifically, they suggested Belgium's 'R&D Incentive Drug Pricing Model' as an alternative. ◆Establishing a Predictable Drug Pricing Roadmap = The KPBMA proposed establishing an intuitive and predictable long-term roadmap through balanced post-market management policies. They argued for rationalizing the fragmented post-market management systems and integrating the implementation schedules for drug price reductions to enhance predictability for the pharmaceutical industry. Furthermore, they also urged that pediatric and orphan drugs should be excluded from post-market management systems. The KPBMA argued for excluding pediatric and rare disease drugs, which are difficult to produce and have low profitability, from preferential drug pricing and post-market management selection.
- Company
- "Aiming the Alzheimer's market with ultrasound"
- by Whang, byung-woo May 22, 2025 06:07am
- Korea's biotech company Deepsonbio is gaining interest for its ultrasound-based brain disorder treatment technology. The company's recent exploratory clinical trials demonstrated the effect of improving cognitive function for patients with Alzheimer's disease and those with normal pressure hydrocephalus. Deepsonbio plans to enter the market on a full scale. Based on its multiple-low intensity pilant ultrasound (M-LIPUS) technology, Deepsonbio is projected to revolutionize treatments for brain disorders, such as Alzheimer's disease. DeepsonbioDaily Pharm met with Deepsonbio, a member of the KoreaBIO organization, to listen to the company's potential and vision. Deepsonbio is led by CEO Lee Dong-hyuk, who is currently a professor at Gachon University of Medicine and Science's Department of Biomedical Engineering. The company's key technology is its proprietary M-LIPUS. Unlike existing high-intensity focused ultrasound (HIFU) or transcranial magnetic stimulation (TMS), M-LIPUS uses safe, low-intensity ultrasound to non-invasively clear waste from the brain. Notably, the technology utilizes multiple ultra-sound generating devices, stimulating brain areas with ultrasound energy and promoting the cerebrospinal fluid (CSF) flow, ultimately increasing clearance of amyloid-β (Aβ), known as the pathogenesis of Alzheimer's disease. Deepsonbio CEO Lee said, "Disorders like Alzheimer's disease are caused by the accumulation of waste in the brain due to decreased CSF flow," and added, "M-LIPUS has been demonstrated to promote the elimination of Aβ and tau proteins, which are key waste in the brain. Clinical results showed that M-LIPUS improved cognitive function and neural functions." The company's recently finalized exploratory clinical results demonstrated Deepsonbio's technological advancement. It was found that within a month of ultrasound treatments, patients' cognitive function significantly improved. Furthermore, clinical evaluation showed a clear improvement in cognitive function with the trail making test (TMT) performance time, which is used to evaluate cognitive function in patients with Parkinson's disease, reduced by up to half. Additionally, biological indicators observed positive changes, including activated glucose metabolism in the brain and a significant reduction in beta-amyloid levels. When asked, "Are there any barriers to entering the brain disease treatment market using ultrasound technology?" CEO Lee emphasized that a shift in perspective led to the company's unique technology. Lee said, "Previous research focused more on opening the blood-brain barrier (BBB) rather than improving CSF fluidity. The ultrasound used in those studies was also less effective in humans, so no cases showed the same efficacy as Deepsonbio's low-intensity ultrasound." Global market entry and strategy to achieve 'KRW 100 Billion' mark Deepsonbio is targeting the global Alzheimer's treatment market. The global Alzheimer's treatment market is valued at approximately KRW 20 trillion as of 2023, showing rapid annual growth of over 20%. Deepsonbio aims to complete confirmatory clinical trials by the end of this year and secure additional medical device indications both domestically and internationally by early next year, thereby officially entering the global market. The company's strategy, led by CEO Lee, a regulatory expert, is expected to facilitate rapid market penetration. Considering that current Alzheimer's new drugs focus on early treatment to slow cognitive decline rather than fundamentally improving symptoms, there is an expectation that Deepsonbio's role could expand significantly. The company is actively pursuing domestic and international patent applications to enter the global market, including the United States. The company plans to secure a unique global medical device market position. Specifically, assuming a 6.4% market share of the domestic dementia population after receiving approval this year, Deepsonbio aims for approximately KRW 80 billion in revenue by 2028. CEO Lee stated, "Deepsonbio's device is not large, so there's no need to secure a separate space, which we predict will allow us to expand our influence in the non-reimbursable market rapidly." He added, "Our goal is to quickly generate revenue through the commercialization of our ultrasound technology." Expected to expand indications beyond Alzheimer's disease to normal pressure hydrocephalus Another area where Deepsonbio anticipates treating with its ultrasound technology is normal pressure hydrocephalus (NPH). The company has demonstrated that even with ultrasound treatment alone, patients with NPH significantly improved walking ability within a week, especially when existing treatments are limited. These results are evaluated to suggest the possibility of future indication expansion. Lee described Deepsonbio's technology as 'the technology of happiness.' In a reality where brain diseases like dementia and hydrocephalus severely impair the quality of life for entire families, he believes it is an innovative and valuable technology that can restore happiness to both patients and their families. Finally, Lee presented a vision to expand indications beyond the Alzheimer's disease treatment market to various brain disease treatment areas and to establish strong competitiveness in the global medical device market. Lee remarked, "Deepsonbio's technology can be a game-changer in the Alzheimer's disease treatment market," and added, "We will overcome the limitations of existing treatments, and our medical device will provide real and sustainable happiness to patients and their families."
- Company
- Dyslipidemia drug 'Leqvio' enters drug price negotiatiation
- by Eo, Yun-Ho May 21, 2025 06:37am
- Product photo of Leqvio Leqvio, a new drug for dyslipidemia administered once yearly, has recently entered into negotiations with the National Health Insurance Service (NHIS). In February, Novartis Korea's siRNA drug Leqvio (inclisiran) had initially failed at setting criteria for 'reducing cardiovascular events in patients with atherosclerotic cardiovascular disease (ASCVD)' indication at the Health Insurance Review and Assessment Service (HIRA)'s Drug Reimbursement Evaluation Committee (DREC) review, then conditionally passed the HIRA's DREC review with its 'heterozygous familial and non-familial, or mixed dyslipidemia' indication. Novartis Korea accepted the conditional decision of the DREC and has recently begun drug pricing negotiations. Accordingly, it is to be watched when Leqvio will be listed for the first time. Leqvio gained attention as it can be administered twice-yearly by medical practitioners at hospitals. In addition to reduced number of administration, it has the advantage for medical practitioners administering the drug at hospitals rather than self-administration. In fact, 78.4% of the patient group, including ASCVD patients who received Leqvio up to 6.8 years, reached the LDL-c goal. In a real-world study conducted in the United States, the fully adherent ASCVD patient group, including heart attack, had a 27% lower major adverse cardiovascular events (MACE) than the low adherent group. Additionally, analysis suggests that the fully adherent group also had less yearly medical cost than the low adherent group. Therefore, Leqvio was found to lower the risk of cardiovascular disease relapse and financial burden in ASCVD patients. Leqvio's administration convenience provides a significant therapeutic benefit. The market for Statins and ezetimibe is worth KRW 1 trillion. Including the spending on Statins and PCSK9 inhibitors, the fund for lowering LDL-c is estimated to be approximately KRW 1.5 trillion to KRW 2 trillion. However, LDL-c achievement rate remains at 24% in Korean ASCVD patients. Meanwhile, Leqvio recently acquired additional study data demonstrating significant improvement in the lipidemia profile of patients with dyslipidemia. The meta-analysis results were published in the American Journal of Cardiology last month, including five randomized comparative clinical trials evaluating Leqvio in patients with dyslipidemia. 4072 participants were included in the five trials. 2129 participants were assigned to the Leqvio group and 1943 to the control group. The analysis suggests that the Leqvio group had 51.25% lower LDL-c than the control group.
- Company
- ‘Bispecific antibodies gain attention as option for DLBCL'
- by Whang, byung-woo May 21, 2025 06:36am
- Diffuse large B-cell lymphoma (DLBCL) is considered a difficult disease to treat due to its aggressive nature, but the emergence of new drugs has brought about a paradigm shift in its treatment. As efforts for DLBCL progress towards a cure beyond extending survival, the role of bispecific antibody therapies is garnering attention. Georg Lenz, Professor at Münster University Hospital in Germany, and Jung-ok Lee, Professor of Hematology and Oncology at Seoul National University Bundang Hospital, emphasized the diversified DLBCL treatment options and the importance of timely treatment for maximizing treatment efficacy during their interview with Daily Pharm. Professor Georg Lenz (Münster University Hospital) Approximately 60% of DLBCL patients can expect a cure with first-line treatment, but around 30–40% of the remaining patients are resistant to first-line treatment or experience relapse. Although no two patients are in the same situation, the two professors explained that achieving complete remission remains the primary goal, even for patients with relapsed or refractory (r/r) disease or those who have undergone third-line or later-line treatments. Innovative new drugs such as “bispecific antibodies” and “antibody-drug conjugates (ADC)” are playing a major role in this process. However, DLBCL, which is characterized by rapid progression and aggressive characteristics among non-Hodgkin's lymphomas, is one disease that is difficult to cure. Overseas, particularly in countries such as Germany, CAR-T therapy and bispecific antibody therapies have become clear treatment options depending on the recurrence period and health status of DLBCL patients. According to Professor Georg, Germany uses various bispecific antibody therapies, including Epkinly, glofitamab, and odronextamab in the third-line treatment stage. Professor Georg explained, “For r/r DLBCL patients receiving third-line treatment, bispecific antibody therapies become a treatment option. If CAR-T therapy was not administered in previous treatment stages, CAR-T therapy can also be considered as a treatment option in the third-line treatment stage.” In contrast, in South Korea, the only CAR-T therapy available after second-line treatment is Kymriah, and bispecific antibody therapies are not reimbursed. Regarding this, Professor Lee stated, “Polatuzumab vedotin in combination with R-CHP improved progression-free survival (PFS) compared to R-CHOP in the first-line treatment of DLBCL, and it is being used as a first-line treatment in several countries overseas. However, in Korea, R-CHOP remains the standard of care, so the rate of recurrence after first-line treatment will be relatively high.” In particular, the difference in treatment access between Korea and other countries is evident after second-line treatment. This is because while three CAR-T therapies have been approved in the United States, only one, Kymriah, has been approved in Korea. Professor Lee emphasized, “The domestic treatment environment falls short of global standards, and patients are missing out on real treatment opportunities. In addition to Kymriah, it is urgent to introduce additional CAR-T therapies such as Axi-cel and Liso-cel and apply reimbursement to bispecific antibodies such as Epkinly.” With Epkinly prescriptions on the rise, the challenge is to overcome the remaining “treatment hurdles” The reason why it is important for new drugs such as Epkinly, a bispecific antibody, to enter the regulatory system is because the ultimate goal of r/r DLBCL treatment is a complete cure. Although bispecific antibodies lack long-term data compared to CAR-T therapies, which were developed earlier, they are considered to be a useful treatment option due to their relatively lower toxicity for elderly patients and others who cannot receive CAR-T therapy. Professor Jeong-Ok Lee (Seoul National University Bundang Hospital) Professor Georg said, “I believe that approximately 3 years of long-term data accumulation will be sufficient. Considering the rapid changes in the treatment environment and the various data, I think there is a high possibility that Epkinly will firmly establish itself in the second-line treatment area in the near future.” He added, “Based on the clinical data disclosed to date, the results of Epkinly combination therapy are showing positive trends. We optimistically anticipate that Epkinly may advance into an earlier line treatment option or even become a first-line treatment option used before the r/r stage, rather than completely replacing CAR-T therapy.” According to three-year follow-up data presented at the American Society of Hematology (ASH) 2024 Annual Meeting, Epkinly achieved an overall response rate (ORR) of 59%, with 41% achieving complete remission. Professor Lee explained, “It is encouraging that the 36-month survival rate of patients that reached complete remission was 63%. Additionally, the fact that the progression-free survival (PFS) curve showed a stable ‘plateau’ pattern after a certain point is a positive signal that the treatment effect may persist in the long term.” Although direct comparison is difficult as long-term follow-up data has not yet been accumulated as much as for CAR-T therapy, Professor Lee believes that Epkinly is also expected to achieve high response rates, complete remission, and sustained response. Clear differences exist in the global and domestic treatment environments… reimbursement needs to be realized There are various conflicting opinions regarding the current position of CAR-T therapy and bispecific antibody therapy, including their complementary positions as a 'bridge therapy' before CAR-T therapy. Both professors stated that it is difficult to determine the exact order considering reimbursement conditions and their clinical experience, but mentioned that Epkinly may be considered over CAR-T therapy in elderly patients in terms of adverse reaction management. Professor Georg explained, “Epkinly has shown meaningful clinical efficacy in elderly or refractory patients who are difficult to treat with CAR-T therapy. In particular, it has a low and predictable incidence of cytokine release syndrome (CRS) and has been reported to be associated with minimal neurotoxicity in the form of Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), making it highly safe.” He added, “CAR-T therapy takes about a month to manufacture, so Epkinly may be considered first when time is limited. Although there are no age restrictions for either Epkinly or CAR-T, Epkinly may be a more appropriate choice for patients who are in poor overall health or are elderly.” The problem remains that the reimbursement situation in Korea is still lacking in terms of a global standard of care. He emphasized the need to improve the reimbursement system and access to treatment so that DLBCL patients in Korea can receive the latest global standard treatment. Professor Lee said, “In South Korea, there is a gap between the global standard and Korea’s treatment in all lines of treatment, from the first to the third. Therefore, other effective CAR-T therapies besides Kymriah should be introduced as soon as possible, and bispecific antibody therapies, including Epkinly, should be included in the health insurance reimbursement system.” Finally, he added, “As a hematologist treating DLBCL patients and a member of the Korean Society of Hematology, I hope that a medical environment is established where patients can receive bispecific antibody therapy as soon as possible. I feel a sense of responsibility as an expert and plan to actively voice my opinions and fulfill my role in both my academic society and as an individual physician.”
- Company
- Trodelvy may be the 1st drug reimbursed with ICER benefits
- by Eo, Yun-Ho May 21, 2025 06:36am
- ADC breast cancer drug Trodelvy may soon be listed for insurance reimbursement in Korea. Gilead Sciences recently finalized price negotiations with the National Health Insurance Service for its triple-negative breast cancer (TNBC) treatment Trodelvy (sacituzumab govitecan). As a result, Trodelvy is scheduled to be presented at the Health Insurance Policy Deliberation Committee meeting this month, and if approved, the drug could be listed as early as June. Last August, the detailed evaluation criteria for new drugs subject to price negotiations were revised, and Trodelvy has met the criteria for innovative new drugs, marking the first case where the incremental cost-effectiveness ratio (ICER) threshold was applied flexibly for reimbursement in Korea. While there have been previous cases where an exceptional ICER threshold was applied to an antibody-drug conjugate (ADC) like Trodelvy, such as “Enhertu (trastuzumab deruxtecan),” the fact that Trodelvy is the first drug to be subject to the revised criteria after the revised guidelines were implemented is significant. Although there are no clearly documented figures, the generally accepted maximum ICER threshold for insurance reimbursement in Korea is KRW 50 million. Even cases where the KRW 50 million threshold was approved are said to be extremely rare. Trodelvy's threshold is known to exceed KRW 70 million. With the first tape cut, it will be worth watching how many more drugs will qualify for such ICER benefits following Trodelvy. Triple-negative breast cancer is an aggressive form of breast cancer that recurs and metastasizes rapidly. Patients with metastatic triple-negative breast cancer who have metastasized despite treatment have a life expectancy of only a few months, even with chemotherapy. However, chemotherapy has long been the standard of care due to the lack of targets that can effectively kill cancer cells. Trodelvy, the first Trop-2-targeted antibody-drug conjugate (ADC), is the only treatment for metastatic triple-negative breast cancer in the second-line or higher setting that has been shown to prolong survival compared to chemotherapy and has settled as the global standard of care since its introduction. Currently, major guidelines in the U.S. and Europe specify Trodelvy as the preferred agent for patients with previously treated metastatic triple-negative breast cancer. In a Phase III study, the overall survival of the chemotherapy arm was 6.9 months, compared to a nearly one-year survival (11.8 months) in the Trodelvy arm. In addition, Troldelvy demonstrated an effect in controlling symptoms and pain caused by cancer and improvement in patients' quality of life by improving their overall health status. Trodelvy was awarded the highest possible score of 5 points on ESMO-MCBS, the European Society for Medical Oncology's (ESMO) scale used to rate the value of anticancer drugs. A score of 5 indicates that a drug is effective not only in prolonging patient survival but also in improving quality of life, and Trodelvy is the only treatment for metastatic triple-negative breast cancer to receive a score of 5 on ESMO-MCBS.
- Company
- MSD expands domestic clinical trial cooperation
- by Whang, byung-woo May 20, 2025 06:00am
- MSD Korea has broken its record for the most clinical trial approvals in Korea and is now in full swing, developing innovative new drugs for Koreans. With open innovation playing an increasingly important role in new drug research and development (R&D), MSD is expanding its ties with Korea, which plays a pivotal role in its global clinical trials. On the 19th, MSD Korea held R:IM (Notification) DAY and highlighted changes in R&D trends under the theme of “A New Paradigm in the Pharmaceutical Industry: Global Clinical Trends and MSD's Vision.” Korea plays a pivotal role in global MSD cancer research The reason why MSD Korea's performance in the domestic R&D field is attracting attention is that it received the most clinical trial approvals last year. In 2024, MSD Korea received 36 clinical trial approvals from the Ministry of Food and Drug Safety, the most among domestic pharmaceutical companies. During the same period, AstraZeneca Korea (22), AD pharma (19), AbbVie Korea (17), and Boehringer Ingelheim Korea (15) received clinical trial approvals from the MFDS. In particular, MSD Korea has been leading Korea’s medical ecosystem by investing more than KRW 70 billion for 4 consecutive years since 2021, with a cumulative total of KRW 290 billion in research and development costs. This proves the company’s R&D competitiveness in terms of the number of clinical trials, costs, as well as its quality and quantity. Hyunjoo Lee, Executive Director of Clinical Research at MSD Korea Hyunjoo Lee, Executive Director of Clinical Research at MSD Korea, who made a presentation on that day, said, “We are focusing on research and development of new drugs that have been proven to be effective and safe for Koreans in cooperation with domestic research institutes and academic societies.” MSD Korea has also been playing a central role in MSD's global anticancer drug clinical trials. Currently, the company is conducting over 180 clinical trials in collaboration with more than 640 domestic research institutions, with the largest portion (161 studies) focused on oncology. Although Korean institutions account for only 3% (518) of the 14,770 global institutions conducting anticancer drug clinical trials, these institutions are responsible for 73% of MSD's global cancer drug trials, demonstrating the concentrated utilization of Korea's research capabilities. Major hospitals in Korea, such as Seoul National University Hospital, Samsung Medical Center, Asan Medical Center, and Severance Hospital, are among the top institutions leading MSD's global clinical trials, which is considered a testament to Korea's internationally recognized clinical research capabilities. In addition, Korea ranks fourth in the world in terms of the number of patients enrolled in MSD's global anticancer drug clinical trials, following the United States, China, and Japan. Accelerating digitalization... challenge remains on establishing a sustainable cooperation model Another trend in the global pharmaceutical industry's clinical trials is the integration of digital technology. MSD is also actively promoting the introduction of digital technology and artificial intelligence (AI) to keep pace with this trend. A representative example is the increased use of innovative clinical designs such as umbrella, basket, and adaptive protocols in clinical trial design. “MSD is systematically introducing AI and machine learning technologies into the drug discovery and development process to accelerate pipeline diversification,” said Lee. ”MSD has developed AI tools that enable more accurate evaluation of the safety and efficacy of the active substances in its pipeline during the preclinical development stage.” MSD Korea In the long term, MSD Korea aims to become a trusted R&D partner, a bridgehead for domestic pharmaceutical and biotech companies to gain global research experience and enter the market, and foster an ecosystem for innovative new drug development. This includes improving the regulatory environment in Korea, expanding clinical trial infrastructure, and building more active partnerships with domestic research institutions and biotech companies. Currently, MSD Korea is expanding its partnership by conducting joint clinical trials with major domestic biotech companies for the combination therapy of the immuno-oncology drug Keytruda. In the future, the company plans to expand this collaboration to various other fields to build a sustainable ecosystem that will enhance the global competitiveness of the Korean pharmaceutical industry. Lee stated, “To enhance the global competitiveness of Korea's pharmaceutical and biotechnology industry, it is essential for the government, academia, and industry to collaborate closely. We will strive to establish a sustainable collaboration model to position Korea as a global hub for pharmaceutical research.”
- Company
- Hanmi-MSD collaborate for R&D
- by Cha, Jihyun May 20, 2025 05:59am
- Hanmi Pharmaceutical entered into a clinical trial collaboration agreement with the U.S.-based Merck (MSD) for developing an immune anticancer drug candidate. The clinical collaboration between Hanmi Pharmaceutical (hereafter, Hanmi) and Merck has expanded to three cases. In addition to clinical trial collaboration, Hanmi continues to collaborate with MSD for efforts such as technology transfers. According to pharmaceutical sources on May 20, Hanmi recently signed a clinical collaboration and distribution agreement with MSD to evaluate the combination therapy containing 'HM16390,' a next-generation anticancer drug that is "LAPS interleukin-2 (IL-2) analog," and MSD's anti-PD-1 anticancer drug 'Keytruda (active ingredient name: pembrolizumab). According to the agreement, Hanmi will be responsible for conducting the Phase 1 trial to assess the safety and efficacy of HM16390+Keytruda combination therapy as a clinical trial sponsor. MSD will supply Keytruda used in clinical trials. HM16390 is a next-generation interleukin-based immuno-oncology candidate that activates immune cells. HM16390 induces T-cell proliferation and activation to enhance immune responses within the tumor microenvironment. By increasing the number of tumor-infiltrating lymphocytes (TIL) that respond to immune checkpoint inhibitors in the tumor microenvironment, it is designed to convert 'cold tumors' (with low immune activity) into 'hot tumors' (infiltrated by immune cells) and to maximize efficacy when used in combination with checkpoint inhibitors. 'Proleukin' is the only recombinant IL-2 therapy approved by the U.S. Food and Drug Administration (FDA). Still, its use is limited due to the risk of adverse events at high doses. Most IL-2 analogs in development focus on enhancing binding affinity to the IL-2 β-receptor to boost anti-tumor effects, which can trigger excessive systemic immune responses and lead to severe side effects such as cytokine release syndrome. (source: Hanmi Pharmaceutical) Hanmi has addressed these limitations by finely adjusting HM16390's binding affinity to the IL-2 α-receptor. According to Hanmi, this approach secures safety while maximizing efficacy. In particular, Hanmi is developing HM16390 as a long-acting therapeutic using its proprietary Labsccovery platform technology, enabling once-per-cycle subcutaneous (SC) administration alongside chemotherapy. HM16390 is currently in a multinational Phase 1 clinical trial. Earlier, Hanmi announced at the Society for Immunotherapy of Cancer (SITC) last November that preclinical studies of HM16390 demonstrated complete remission. Keytruda, MSD's immuno-oncology agent, is the world's top-selling pharmaceutical. It is an immune checkpoint inhibitor that blocks the interaction between PD-1 on T cells and PD-L1 on cancer cells, enabling immune cells to attack tumor cells. First approved by the U.S. FDA in September 2014 for malignant melanoma, Keytruda has continuously added new indications. It has over 40 indications to date, including breast, gastric, and lung cancers, making it the checkpoint inhibitor with the broadest range of cancer uses. However, the efficacy of Keytruda is limited to patients whose tumors express high levels of PD-L1. It is known that those with low PD-L1 expression derive minimal benefit. To address this, MSD is actively developing combination therapies to expand the responsive patient population and enhance efficacy. Hanmi expects that combining HM16390 with Keytruda will further improve treatment outcomes. Hanmi and MSD have expanded collaboration deals to three in total. Hanmi also conducts Keytruda combination therapy clinical trials with MSD's PD-L1/4-1BB bispecific antibody candidate 'BH3120' in combination with its oral CCR4 antagonist, 'Tivumecirnon.' BH3120, which is being co-developed by Hanmi and Beijing Hanmi Pharm, is a bispecific antibody immuno-oncology candidate in which a single antibody simultaneously binds to two different targets. It features Hanmi's proprietary Pentambody platform technology, combining an immuno-oncology mechanism that activates immune cells with the target-oncology characteristic of selectively attacking only cancer cells. In particular, Hanmi explains that BH3120 is designed to respond differently to each target, PD-L1 and 4-1BB, thereby enhancing therapeutic efficacy while reducing side effects. Hanmi plans to present the interim results of the Phase 1 clinical trial evaluating the BH3120 and Keytruda combination therapy in the second half of this year. Previously, Hanmi Pharmaceutical reported that in tumor-bearing mouse models refractory to immune-oncology agents, the combination of BH3120 and Keytruda demonstrated tumor growth inhibition that was at least comparable to that observed with a competing pipeline agent (GEN1046). R&D new pipeline: Preclinical trials, Phase 1 Trials, Phase 2 Trials, Phase 3 Trials, Approved (source: Hanmi Pharmaceutical) Tivumecirnon is an oral immuno-oncology candidate Hanmi acquired from RAPT Therapeutics in 2019. It blocks the CCR4 receptor protein, thereby inhibiting the migration of regulatory T cells that suppress immune responses into tumors. In January, at the ASCO Gastrointestinal Cancers Symposium held in San Francisco, Hanmi presented a poster on the Phase 2, Part 1 results for Tivumecirnon. In collaboration with RAPT and MSD, this trial treated ten Epstein-Barr virus (EBV)-positive gastric cancer patients and achieved an objective response rate (ORR) of 60%. ORR is a key efficacy metric representing the proportion of patients whose tumors have either disappeared entirely or shrunk by a defined amount following cancer treatment. Among these responses, there was one complete response and five partial responses. The median time to response (mTTR) was 2.7 months. The median duration of response (mDOR) was 17.3 months. In Cohort 2, the median progression-free survival (PFS) was 10.4 months. Hanmi explained that the treatment-related adverse events observed among the 20 patients enrolled in the trial were mostly manageable. In addition to its clinical collaboration with MSD, which is focused on Keytruda combination therapy, Hanmi is also maintaining partnerships via technology licensing agreements. For instance, it includes 'efpeglenatide,' which Hanmi licensed to MSD in 2020 in a deal valued at USD 860 million. Efpeglenatide is a dual-action agent that activates the glucagon-like peptide-1 (GLP-1) receptor, which enhances insulin secretion and suppresses appetite, and the glucagon receptor, which increases energy metabolism. Hanmi previously licensed efpeglenatid to Janssen in 2015 for obesity and diabetes indications, regained the rights in 2019, repurposed it for metabolic-associated steatohepatitis (MASH), and successfully licensed it again to MSD. MSD is currently conducting a Phase 2 clinical trial of efpeglenatide. The trial compares efpeglenatide with the comparator treatment, semaglutide from Novo Nordisk, and a placebo. According to a 2023 presentation at the European Association for the Study of the Liver (EASL) in Vienna, data from the Phase 2a analysis showed that at week 24 of treatment, efpeglenatide reduced liver stiffness by 72.7% compared to baseline. This result markedly outperformed semaglutide, which achieved a 42.3% reduction over the same period. Resolution of steatosis without fibrosis worsening and improvement of fibrosis without steatosis worsening are key evaluation endpoints defined by the FDA for NASH therapies. MSD aims to complete the Phase 2 trial of efpeglenatide by December of this year.